Richness and Elevation Patterns of a Stonefly (Insecta, Plecoptera) Community of a Southern Appalachian Mountains Watershed, USA
Abstract
1. Introduction
2. Materials and Methods
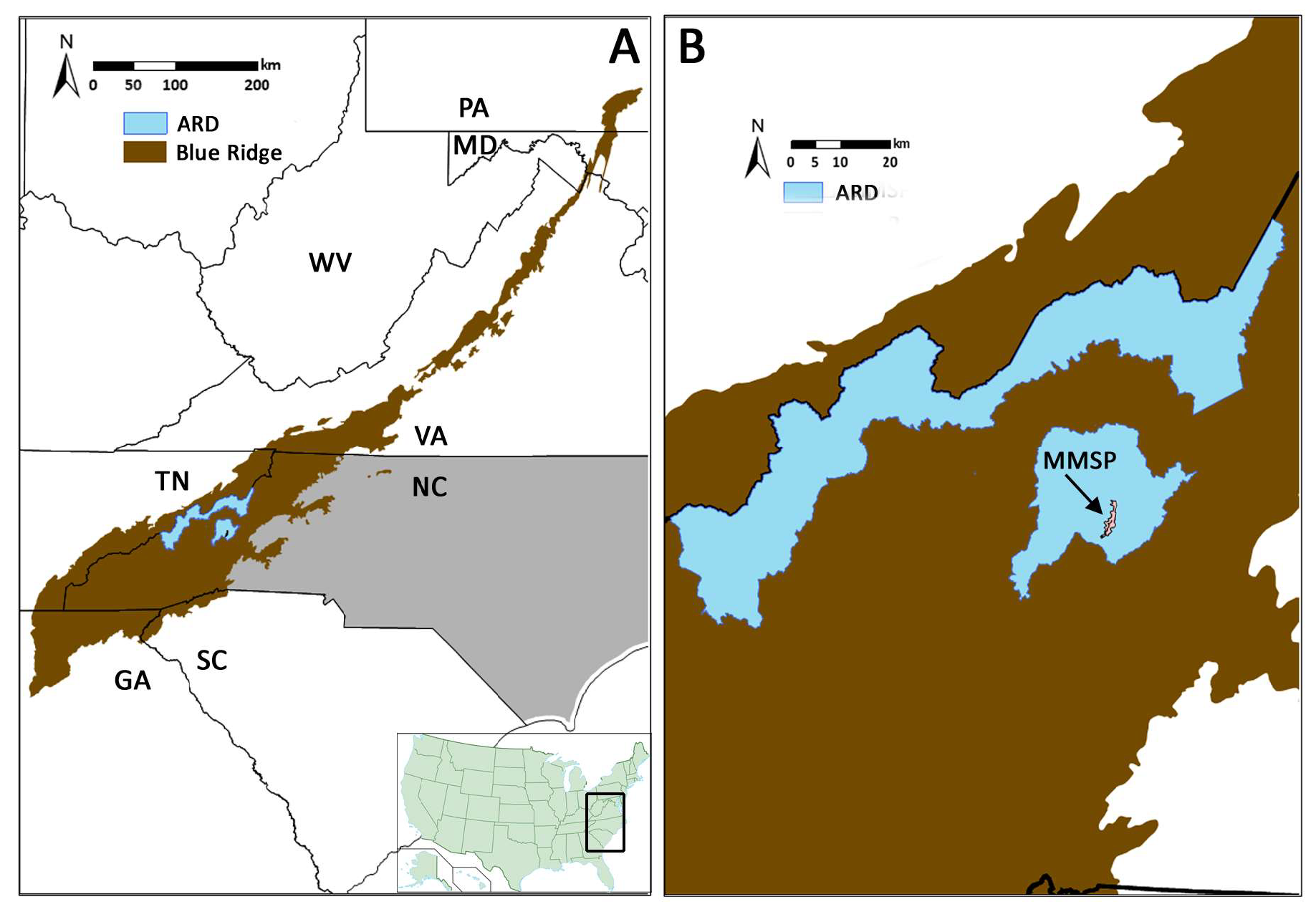
2.1. Blue Ridge Region
2.2. Field Methods
2.3. Microscopy and Molecular Methods
2.4. Data Analyses
3. Results and Discussion
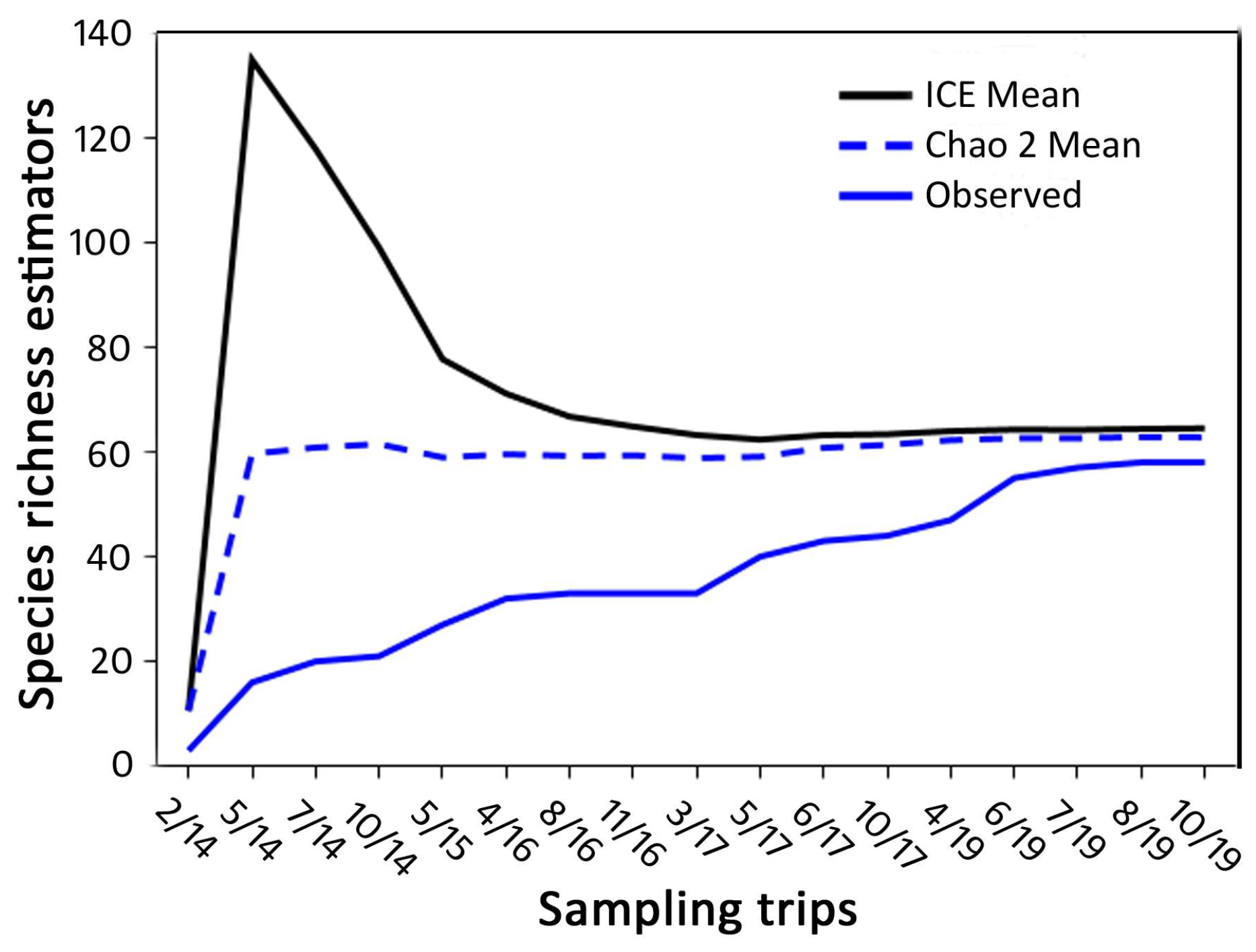
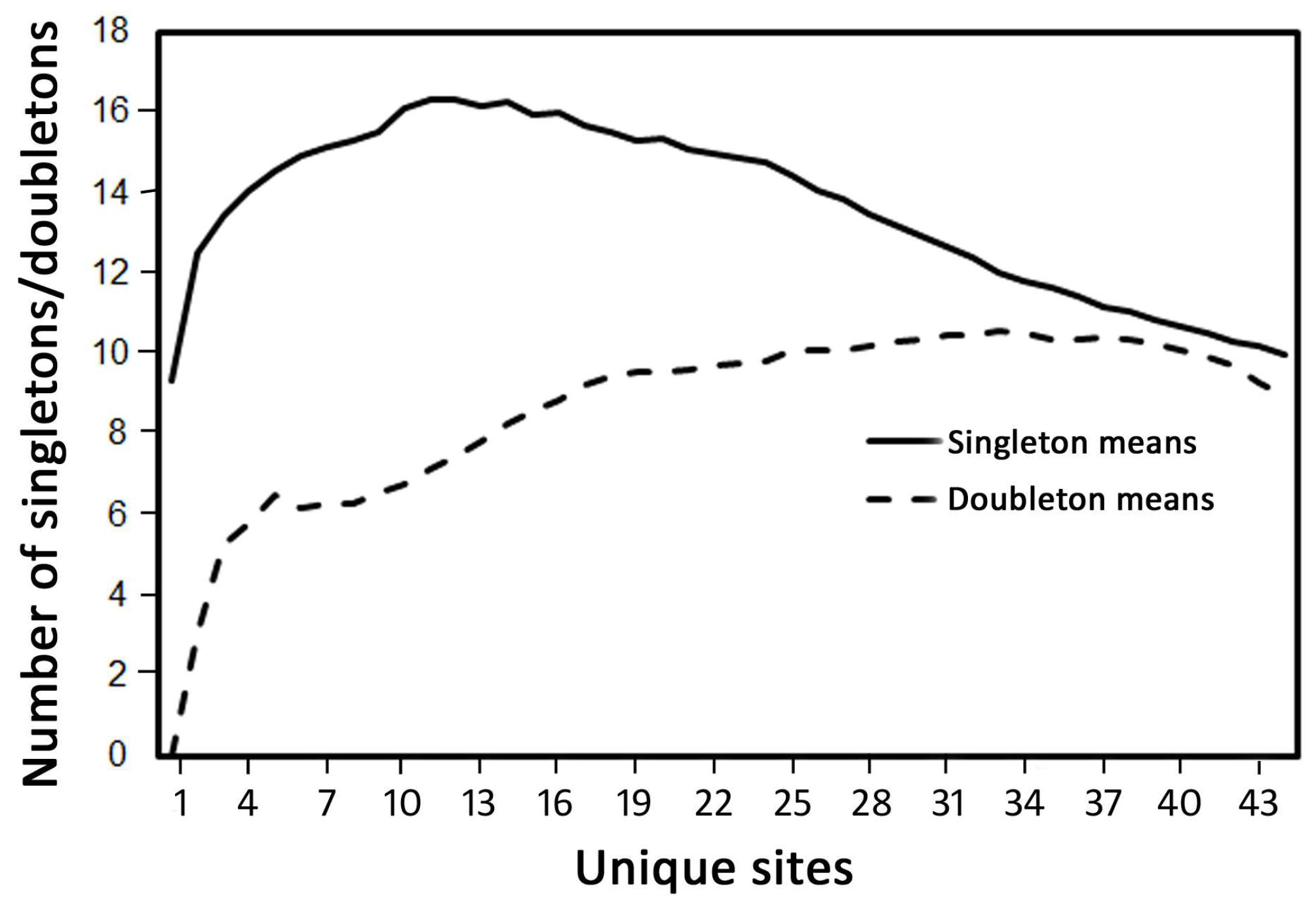
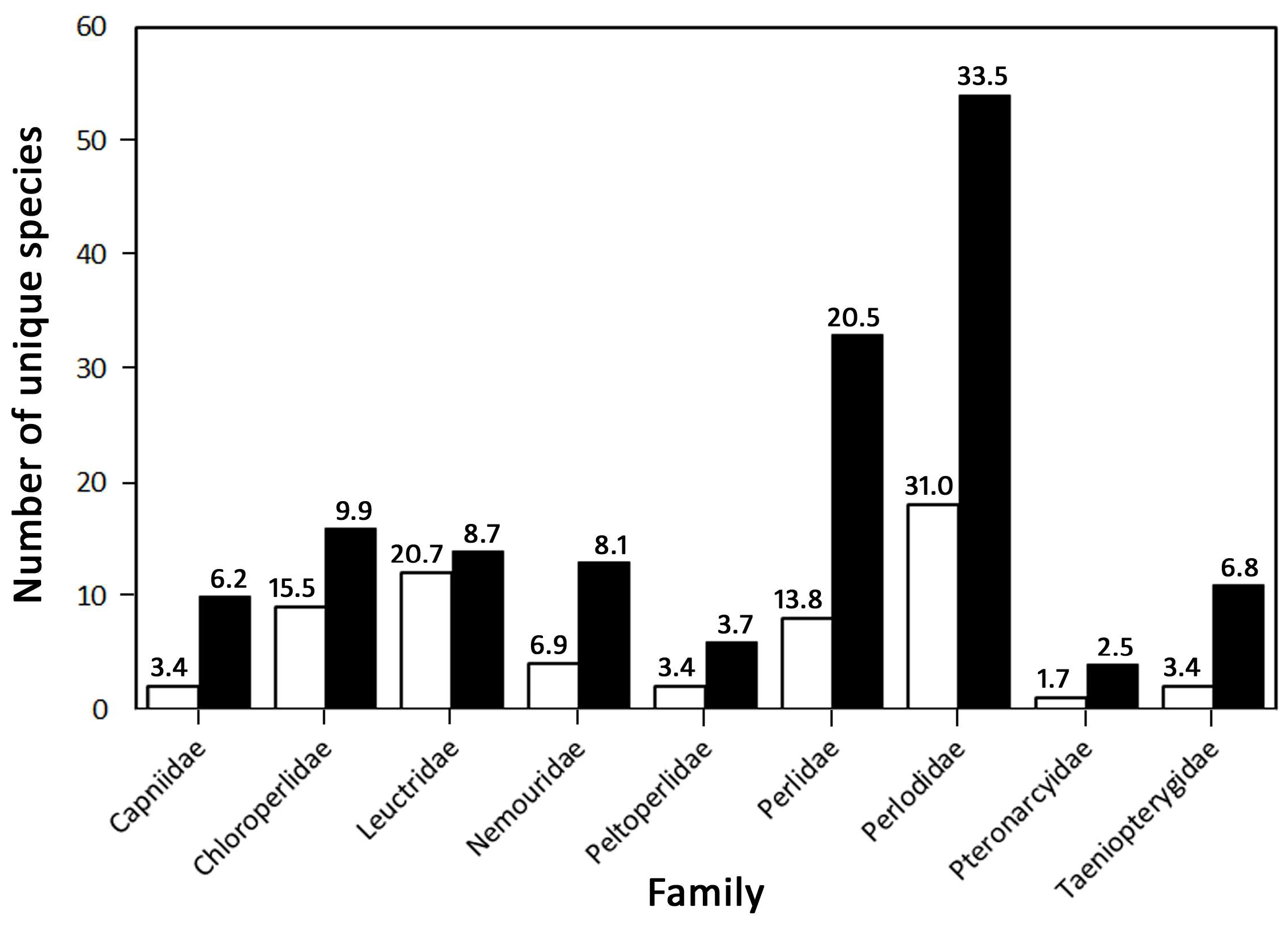
3.1. Species Richness and Community Composition
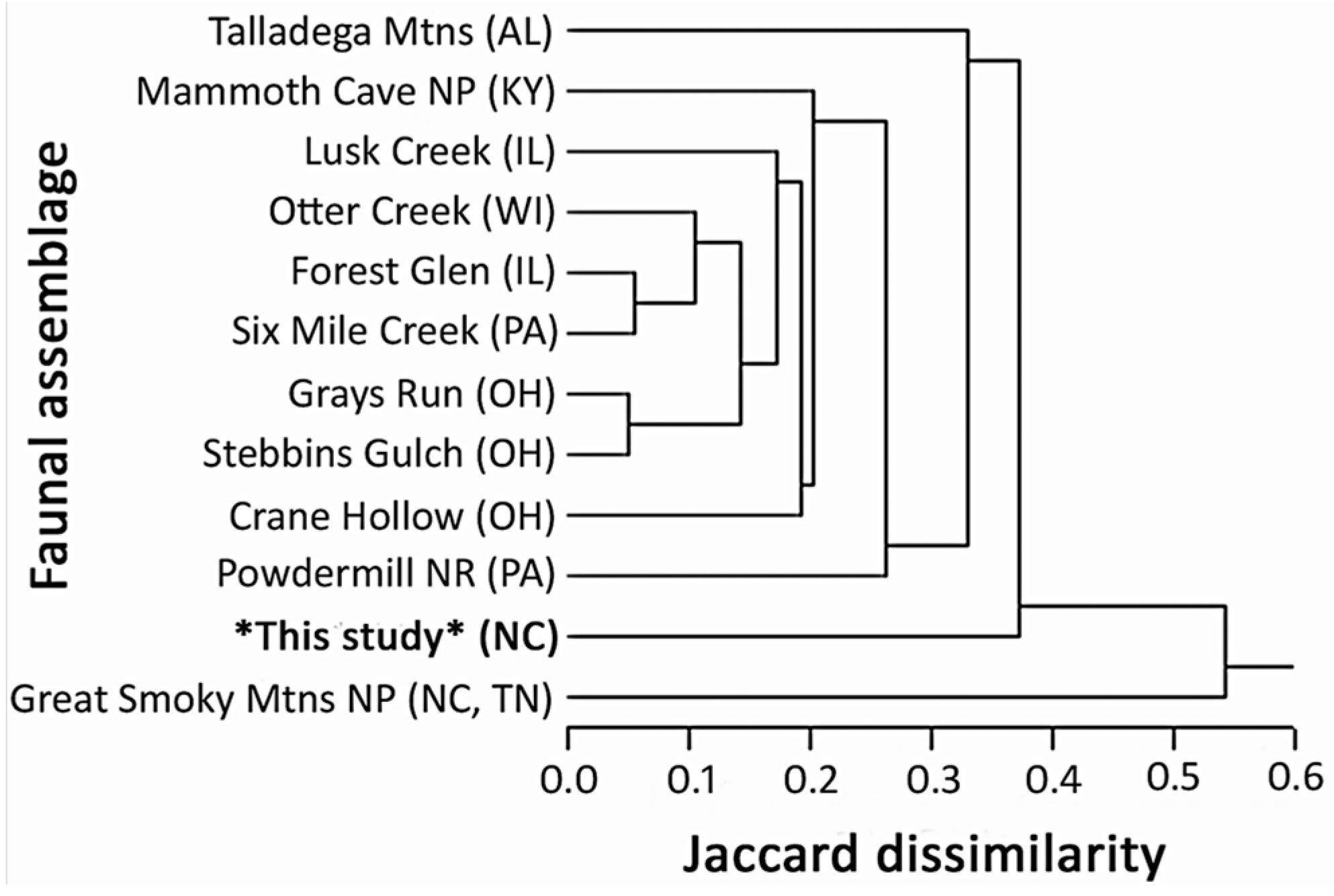
3.2. Proportionality and Comparisons to Other Fauna
3.3. Seasonal Trends
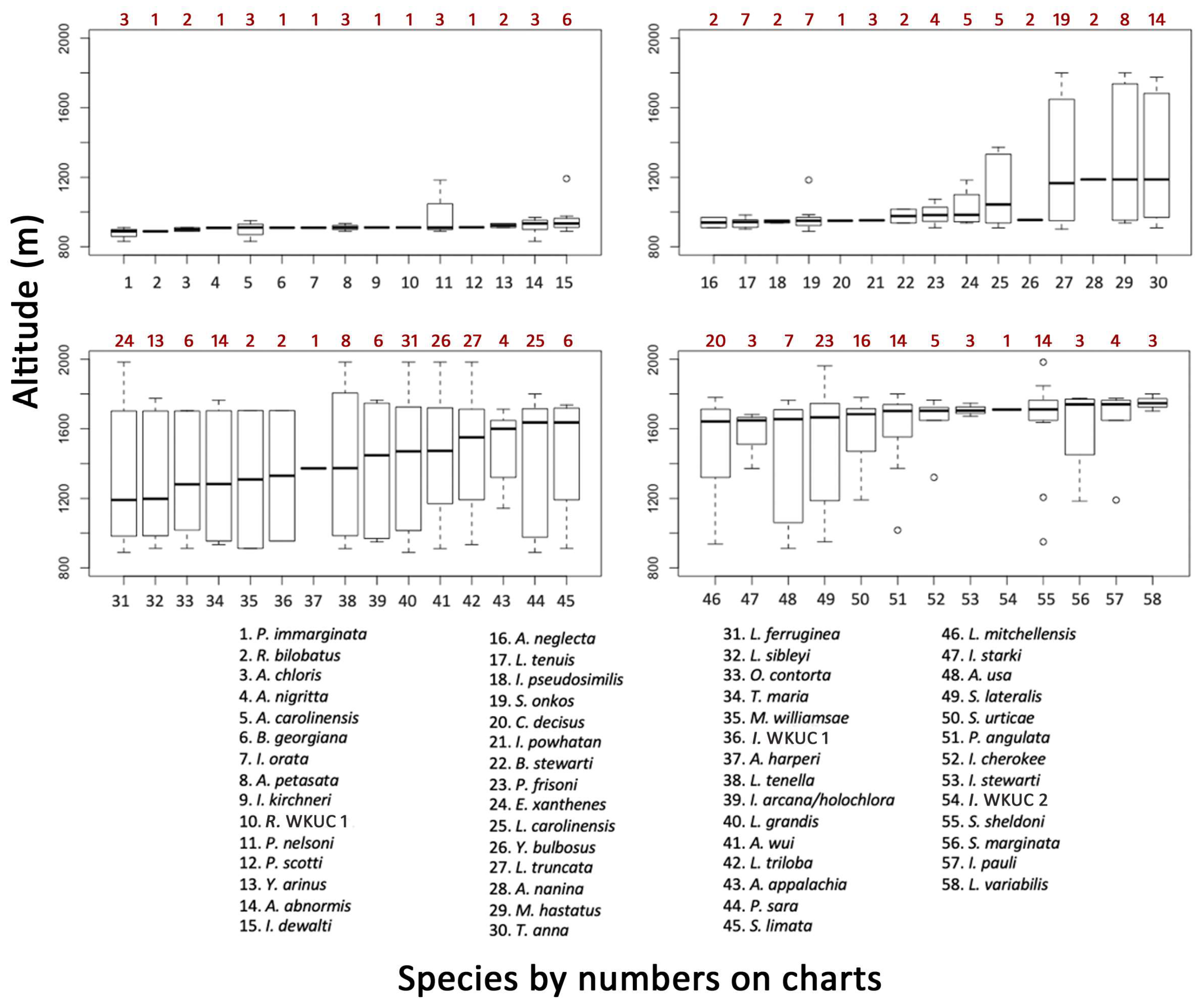
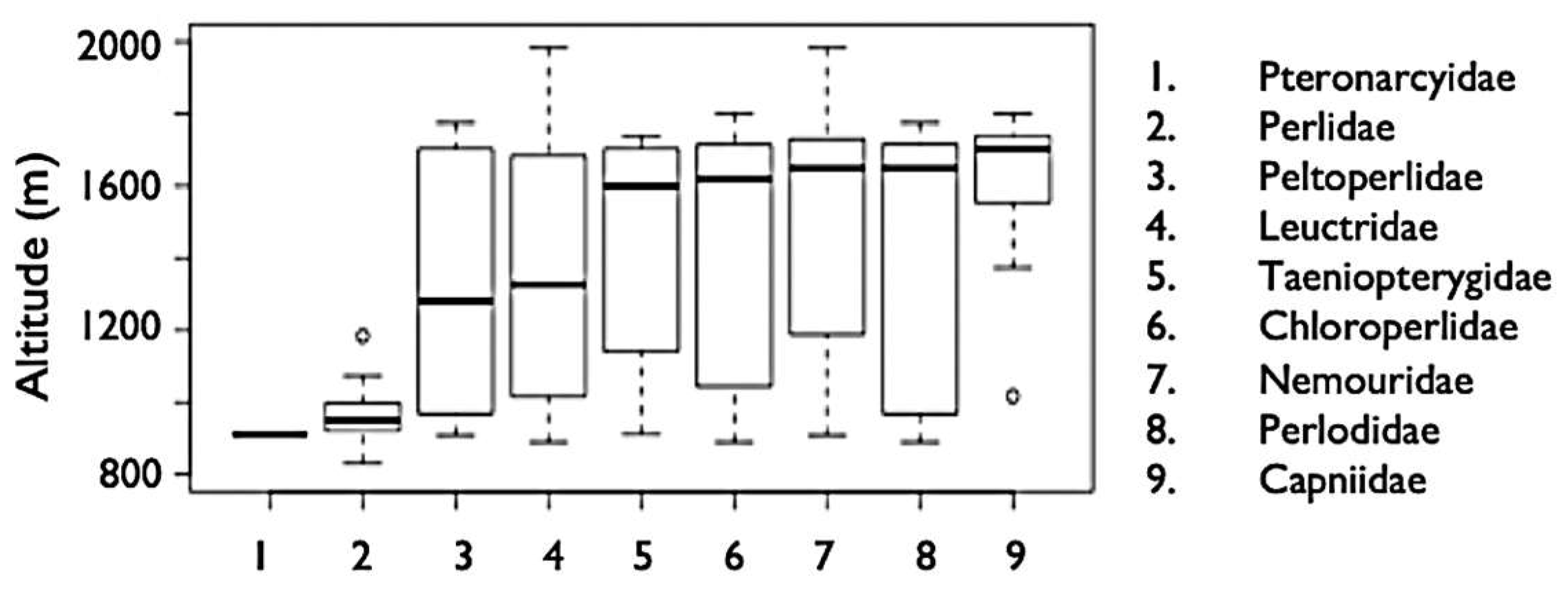
3.4. Elevation Trends
3.5. Rare and Regional Species
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Venter, O.; Fuller, R.A.; Segan, D.B.; Carwardine, J.; Brooks, T.; Butchart, S.H.M.; Di Marco, M.; Iwamura, T.; Joseph, L.; O’Grady, D.; et al. Targeting global protected area expansion for imperiled biodiversity. PLoS Biol. 2014, 12, e1001891. [Google Scholar] [CrossRef] [PubMed]
- Scott, D. Global environmental change and mountain tourism In Tourism and Global Environmental Change; Gössling, S., Hall, M.C., Eds.; Routledge: London, UK, 2006; pp. 54–75. [Google Scholar]
- Mainstone, C.P. The role of specially designated wildlife sites in freshwater conservation—An English perspective. Freshwat. Rev. 2008, 1, 89–98. [Google Scholar] [CrossRef]
- Nelson, G.C.; Rosegrant, M.W.; Koo, J.; Robertson, R.; Sulser, T.; Zhu, T.; Ringler, C.; Msangi, S.; Palazzo, A.; Batka, M.; et al. Climate Change: Impact on Agriculture and Costs of Adaptation; International Food Policy Research Institute Food Policy Report, International Food Policy Research Institute: Washington, DC, USA, 2009; 199p. [Google Scholar]
- Niño-García, J.P.; Ruiz-González, C.; del Giogrio, P.A. Interactions between hydrology and water chemistry shape bacterioplankton biogeography across boreal freshwater. IMSE J. 2016, 10, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Bojsen, B.H. Effects of deforestation on fish community structure in Ecuadorian Amazon streams. Freshwat. Biol. 2002, 47, 2246–2260. [Google Scholar] [CrossRef]
- Mainstone, C.P.; Clarke, S.J. Managing multiple stressors on sites with special protection for freshwater wildlife—The concept of limits of liability. Freshwat. Rev. 2008, 1, 175–187. [Google Scholar] [CrossRef]
- Nelson, K.C.; Palmer, M.A. Stream temperature surges under urbanization and climate change: Data, models, and responses. J. Am. Wat. Res. Assoc. 2007, 43, 440–452. [Google Scholar] [CrossRef]
- Treanor, H.B.; Giersch, J.J.; Kappenman, K.M.; Muhlfeld, C.C.; Webb, M.A.H. Thermal tolerance of meltwater stonefly Lednia tumana nymphs from an alpine stream in Waterton-Glacier International Peace Park, Montana, USA. Freshwat. Sci. 2013, 32, 597–605. [Google Scholar] [CrossRef]
- Jordan, S.; Giersch, J.J.; Muhlfeld, C.C.; Hotaling, S.; Fanning, L.; Tappenbeck, T.H.; Luikart, G. Loss of genetic diversity and increased subdivision in an endemic alpine stonefly threatened by climate change. PLoS ONE 2016, 11, e0157386. [Google Scholar] [CrossRef]
- Domisch, S.; Jähnig, S.C.; Haase, P. Climate-change winners and losers: Stream macroinvertebrates of a submontane region in Central Europe. Freshwat. Biol. 2011, 56, 2009–2020. [Google Scholar] [CrossRef]
- Muhlfeld, C.C.; Giersch, J.J.; Hauer, F.R.; Pederson, G.T.; Luikart, G.; Peterson, D.P.; Downs, C.C.; Fagre, D.B. Climate change links fate of glaciers and an endemic alpine invertebrate. Clim. Chang. 2011, 106, 337–345. [Google Scholar] [CrossRef]
- Webb, B.W.; Clack, P.D.; Walling, D.E. Water-air temperature relationships in a Devon River system and the role of flow. Hydrol. Proc. 2003, 17, 3069–3084. [Google Scholar] [CrossRef]
- Kundzewicz, Z.W.; Döll, P. Will groundwater ease freshwater stress under climate change? Hydrol. Sci. J. 2009, 54, 665–675. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, A.L. Possible climate-induced shift of stoneflies in a southern Appalachian catchment. Freshwat. Sci. 2012, 31, 765–774. [Google Scholar] [CrossRef]
- Shuter, B.J.; Post, J.R. Climate, population viability and the zoogeography of temperate fishes. Trans. Am. Fish. Soc. 1990, 119, 314–336. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Jackson, J.K.; Newbold, D.N.; Funk, D.H. Climate change and the life histories and biogeography of aquatic insects in eastern North America. In Global Climate Change and Freshwater Ecosystems; Firth, P., Fisher, S.G., Eds.; Springer: New York, NY, USA, 1992; pp. 143–176. [Google Scholar]
- Williams, S.E.; Shoo, L.P.; Isaac, J.L.; Hoffman, A.A.; Langham, G. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 2008, 12, e235. [Google Scholar] [CrossRef]
- Busby, J.R. Potential impacts of climate change on Australia’s flora and fauna. In Greenhouse: Planning for Climate Change; Pearman, G.I., Ed.; E.J. Brill: New York, NY, USA, 1988; pp. 387–398. [Google Scholar]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Nat. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Williams, L.R.; Taylor, C.M.; Warren, M.L., Jr.; Clingenpeel, J.A. Environmental variability, historical contingency, and the structure of regional fish and macroinvertebrate faunas in Ouachita Mountain stream systems. Trans. Amer. Fish. Soc. 2003, 132, 120–130. [Google Scholar] [CrossRef]
- Sauer, J.; Domsich, S.; Nowak, C.; Haase, P. Low mountain ranges: Summit traps for montane freshwater species under climate change. Biodivers. Conserv. 2011, 20, 3133–3146. [Google Scholar] [CrossRef]
- Downes, B.J. Back to the future: Little-used tools and principles of scientific inference can help disentangle effects of multiple stressors on freshwater ecosystems. Freshwat. Biol. 2010, 55, 60–79. [Google Scholar] [CrossRef]
- Statzner, B.; Bêche, L.A. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems? Freshwat. Biol. 2010, 55, 80–119. [Google Scholar] [CrossRef]
- Resh, V.H. Which group is best? Attributes of different biological assemblages used in freshwater biomonitoring programs. Env. Mon. Assess. 2008, 138, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Saltveit, S.J.; Brittain, J.E.; Lillehammer, A. Stoneflies and river regulation—A Review. In Regulated Streams; Craig, J.F., Kemper, J.B., Eds.; Springer: Boston, MA, USA, 1987; pp. 117–129. [Google Scholar]
- Master, L.L.; Stein, B.A.; Kutner, L.S.; Hammerson, G.A. Vanishing assets, conservation status of U.S. species. In Precious Heritage, The Status of Biodiversity in the United States; Stein, B.A., Kutner, L.S., Adams, J.S., Eds.; Oxford University Press: New York, NY, USA, 2000; pp. 93–118. [Google Scholar]
- DeWalt, R.E.; Favret, C.; Webb, D.W. Just how imperiled are aquatic insects? A case study of stoneflies (Plecoptera) in Illinois. Ann. Ent. Soc. Am. 2005, 98, 941–950. [Google Scholar] [CrossRef]
- Bojková, J.; Komprdova, K.; Soldán, T.; Zahrádková, S. Species loss of stoneflies (Plecoptera) in the Czech Republic during the 20th century. Freshwat. Biol. 2012, 57, 2550–2567. [Google Scholar] [CrossRef]
- DeWalt, R.E.; Ower, G.D. Ecosystem services, global biodiversity and rate of stonefly species descriptions (Insecta: Plecoptera). Insects 2019, 10, 99. [Google Scholar] [CrossRef]
- Figueroa, J.M.; López-Rodríguez, M.J.; Lorenz, A.; Graf, A.; Schmidt-Kloiber, A.; Hering, D. Vulnerable taxa of European Plecoptera (Insecta) in the context of climate change. Biodiv. Conserv. 2009, 19, 1269–1277. [Google Scholar] [CrossRef]
- Beaty, S.R. The Plecoptera of North Carolina: A Biologist’s Handbook for the Identification of Stonefly Nymphs with Standard Taxonomic Effort Levels; Version 4.0; North Carolina Department of Environmental Quality, Division of Water Resources, Biological Assessment Branch: Raleigh, NC, USA, 2015; iv + 93p. [Google Scholar]
- Keith, A. Description of the Mount Mitchell Quadrangle; North Carolina-Tennessee: U.S. Geological Survey Geological Atlas, Folio 124; U.S. Geological Survey: Washington, DC, USA, 1905; 9p. [Google Scholar]
- Moore, P.T.; van Miegroet, H.; Nicholas, N.S. Examination of forest recovery scenarios in Southern Appalachian Picea-Abies forest. Forestry 2008, 81, 183–194. [Google Scholar] [CrossRef]
- Lusk, L.; Mutel, M.; Walker, E.S.; Levey, F. Forest change in high-elevation forests of Mt. Mitchell, North Carolina: Re-census and analysis of data collected over 40 years. In Proceedings of the Conference on the Ecology and Management of High-Elevation Forests in the Central and Southern Appalachian Mountains, Snowshoe Mountain Resort, Slatyfork, WV, USA, 14–15 May 2009. [Google Scholar]
- North Carolina State Climate Office. Available online: https://climate.ncsu.edu/office/ (accessed on 15 July 2022).
- Deyton, J.B. The Toe River Valley to 1865. N. Car. Hist. Rev. 1947, 24, 423–466. [Google Scholar]
- Silver, T. Mount Mitchell and the Black Mountains: An Environmental History of the Highest Peaks in Eastern America; The University of North Carolina Press: Chapel Hill, NC, USA, 2003; 322p. [Google Scholar]
- Metzger, M.L. An Integrative Ecological and Taxonomic Assessment of the Stoneflies (Plecoptera) of the Black Mountains, North Carolina, USA. Master’s Thesis, Western Kentucky University, Bowling Green, KY, USA, 2020. Available online: https://digitalcommons.wku.edu/theses/3198 (accessed on 30 March 2023).
- Metzger, M.L.; Grubbs, S.A.; Johnson, J.J. An integrative phylogenetic analysis of eastern Nearctic Leuctra (Plecoptera: Leuctridae), with an emphasis on the fauna of southern Appalachian Highlands landscape. Can. Ent. 2022, 154, e15. [Google Scholar] [CrossRef]
- Stark, B.P.; Nelson, C.R. The Nearctic plecopteran families: Morphology and systematics. In Stoneflies (Plecoptera) of Eastern North America. Volume 1. Pteronarcyidae, Peltoperlidae, and Taeniopterygidae; Stark, B.P., Armitage, B.J., Eds.; Bulletin of the Ohio Biological Series; Ohio Biological Survey: Columbus, OH, USA, 2000; Volume 14, pp. 11–28. [Google Scholar]
- Ross, H.H.; Ricker, W.E. The classification, evolution, and dispersal of the winter stonefly genus Allocapnia. Ill. Biol. Monogr. 1971, 45, 1–167. [Google Scholar]
- Kirchner, R.F. A new Allocapnia from Virginia (Plecoptera: Capniidae). Ent. News 1980, 91, 19–21. [Google Scholar]
- Stark, B.P.; Baumann, R.W. The winter stonefly genus Paracapnia. Monogr. West. N. Am. Nat. 2004, 2, 96–108. [Google Scholar]
- Surdick, R.F. Chloroperlidae (The Sallflies). In The Stoneflies (Plecoptera) of Eastern North America. Volume II. Chloroperlidae, Perlidae, and Perlodidae (Perlodinae); Stark, B.P., Armitage, B.J., Eds.; Bulletin of the Ohio Biological Series; Ohio Biological Survey: Columbus, OH, USA, 2004; Volume 14, pp. 1–60. [Google Scholar]
- Grubbs, S.A.; Baumann, R.W. Alloperla clarki sp. nov. (Plecoptera: Chloroperlidae), a new species from the eastern Nearctic with discussion of a new species group. Zootaxa 2019, 4624, 241–255. [Google Scholar] [CrossRef]
- Kondratieff, B.C.; Kirchner, R.F. A new species in the Sweltsa onkos Complex (Plecoptera: Chloroperlidae). In A Lifetime Contributions to Myriapodology and the Natural History of Virginia: A Festschrift in Honor of Richard, L. Hoffman’s 80th Birthday; Roble, S.M., Mitchell, J.C., Eds.; Virginia Museum of Natural History Special Publication: Martinsville, VA, USA, 2009; Volume 16, pp. 295–300. [Google Scholar]
- Hitchcock, S.W. Guide to the insects of Connecticut. Part VII. The Plecoptera or stoneflies of Connecticut. Bull. State Geol. Nat. Hist. Surv. 1974, 107, 1–262. [Google Scholar]
- Claassen, P.W. New species of North American Plecoptera. Can. Ent. 1923, 55, 257–263, 281–293. [Google Scholar] [CrossRef]
- Grubbs, S.A. Leuctra schusteri, a new stonefly species (Plecoptera: Leuctridae) of the L. tenuis (Pictet) group from the southeastern USA. Illiesia 2015, 11, 147–166. [Google Scholar]
- Hanson, J.F. Studies on the Plecoptera of North America, II. Bull. Brook. Ent. Soc. 1941, 36, 57–66. [Google Scholar]
- Hanson, J.F. Records and descriptions of North American Plecoptera. Part I. New species of Leuctra of the eastern United States. Am. Mid. Nat. 1941, 26, 174–178. [Google Scholar] [CrossRef]
- Harper, P.P.; Harper, F. The genus Leuctra Stephens in North America: A preliminary report. In Ephemeroptera & Plecoptera: Biology-Ecology-Systematics; Landolt, P., Sartori, M., Eds.; MTL: Fribourg, Switzerland, 1997; pp. 467–472. [Google Scholar]
- Baumann, R.W.; Stark, B.P. The genus Megaleuctra Neave (Plecoptera: Leuctridae) in North America. Illiesia 2013, 9, 65–93. [Google Scholar]
- Stark, B.P.; Kyzar, J.W. Systematics of Nearctic Paraleuctra with description of a new genus (Plecoptera: Leuctridae). Tijd. Ent. 2000, 144, 135–199. [Google Scholar] [CrossRef]
- Baumann, R.W. Revision of the stonefly family Nemouridae (Plecoptera): A study of the world fauna at the generic level. Smithson. Cont. Zool. 1975, 211, 1–74. [Google Scholar] [CrossRef]
- Grubbs, S.A.; Baumann, R.W. The Nemourinae (Insecta, Nemouridae) of the eastern Nearctic. Zootaxa 2023, 5306, 1–53. [Google Scholar] [CrossRef]
- Grubbs, S.A.; Baumann, R.W. Soyedina Ricker, 1952 (Plecoptera: Nemouridae) in the eastern Nearctic: Review of species concepts, proposed morphology-based species groups, and description of a new species from North Carolina. Zootaxa 2019, 4658, 223–250. [Google Scholar] [CrossRef]
- Stark, B.P. Peltoperlidae (The Roachflies). In The Stoneflies (Plecoptera) of Eastern North America. Volume I. Pteronarcyidae, Peltoperlidae, and Taeniopterygidae; Stark, B.P., Armitage, B.J., Eds.; Bulletin of the Ohio Biological Series; Ohio Biological Survey: Columbus, OH, USA, 2000; Volume 14, pp. 41–54. [Google Scholar]
- Stewart, K.W.; Stark, B.P. Nymphs of the North American Stonefly Genera (Plecoptera), 2nd ed.; Caddis Press: Columbus, OH, USA, 2002; 510p. [Google Scholar]
- Stark, B.P. Perlidae (The Stones). In The Stoneflies (Plecoptera) of Eastern North America. Volume II. Chloroperlidae, Perlidae, and Perlodidae (Perlodinae); Stark, B.P., Armitage, B.J., Eds.; Bulletin of the Ohio Biological Series; Ohio Biological Survey: Columbus, OH, USA, 2004; Volume 14, pp. 61–148. [Google Scholar]
- Stark, B.P.; Szczytko, S.W. Contributions to the systematics of Paragnetina (Plecoptera: Perlidae). J. Kansas Ent. Soc. 1981, 54, 625–648. [Google Scholar]
- Kondratieff, B.C. Perlodidae—Perlodinae (The Springflies). In The Stoneflies (Plecoptera) of Eastern North America. Volume II. Chloroperlidae, Perlidae, and Perlodidae (Perlodinae); Stark, B.P., Armitage, B.J., Eds.; Bulletin of the Ohio Biological Series; Ohio Biological Survey: Columbus, OH, USA, 2004; Volume 14, pp. 149–190. [Google Scholar]
- Beaty, S.R.; Holland, V.B.; Lenat, D.R. Isoperla arcana and Isoperla borisi (Plecoptera: Perlodidae), two new stonefly species from North Carolina, USA with notes on the distribution of Isoperla powhatan. Illiesia 2017, 13, 140–166. [Google Scholar]
- Szczytko, S.W.; Kondratieff, B.C. A review of the eastern Nearctic Isoperlinae (Plecoptera: Perlodidae) with the description of twenty-two new species. Monogr. Illiesia 2015, 1, 1–289. [Google Scholar]
- Verdone, C.J.; Kondratieff, B.C. A new species of Isoperla Banks (Plecoptera: Perlodidae) from the Appalachian Mountains, Virginia & West Virginia, USA. Illiesia 2016, 12, 74–85. [Google Scholar]
- Verdone, C.J.; Kondratieff, B.C. A new species of Isoperla Banks (Plecoptera: Perlodidae) from the Appalachian Mountains, with notes on the I. montana group. Illiesia 2017, 13, 111–126. [Google Scholar]
- Verdone, C.J.; Kondratieff, B.C. Holomorphology and systematics of the eastern Nearctic stonefly genus Remenus Ricker (Plecoptera: Perlodidae). Illiesia 2018, 14, 81–125. [Google Scholar]
- Myers, L.; Kondratieff, B.C. Larvae of North American species of Pteronarcys (Plecoptera: Pteronarcyidae). Illiesia 2017, 13, 192–224. [Google Scholar]
- Stewart, K.W. Taeniopterygidae (The Willowflies). In The Stoneflies (Plecoptera) of Eastern North America. Volume I. Pteronarcyidae, Peltoperlidae, and Taeniopterygidae; Stark, B.P., Armitage, B.J., Eds.; Bulletin of the Ohio Biological Series; Ohio Biological Survey: Columbus, OH, USA, 2000; Volume 14, pp. 55–98. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef]
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. 2013. Version 9. User’s Guide and Application. Available online: http://purl.oclc.org/estimates (accessed on 2 June 2023).
- Gotelli, N.J.; Colwell, R.K. Estimating species richness. In Biological Diversity: Frontiers in Measurement and Assessment; Magurran, A.E., McGill, B.J., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 39–54. [Google Scholar]
- Chao, A. Estimating the population size of for capture-recapture data with unequal catchability. Biometrics 1987, 43, 789–791. [Google Scholar] [CrossRef]
- Lee, S.M.; Chao, A. Estimating population size via sample coverage for closed capture-recapture models. Biometrics 1994, 50, 88–97. [Google Scholar] [CrossRef]
- DeWalt, R.E.; Maehr, M.D.; Hopkins, H.; Neu-Becker, U.; Stueber, G.; Plecoptera Species File Online. Version 5.0/5.0. 2023. Available online: http://Plecoptera.SpeciesFile.org (accessed on 28 June 2022).
- DeWalt, R.E.; Snyder, E.D. Plecoptera of Crane Hollow Nature Preserve, Ohio, comparison to similar efforts. Illiesia 2017, 13, 70–81. [Google Scholar]
- Parker, C.R.; Flint, O.S., Jr.; Jacobus, L.M.; Kondratieff, B.C.; McCafferty, W.P.; Morse, J.C. Ephemeroptera, Plecoptera, Megaloptera, and Trichoptera of Great Smoky Mountains National Park. Southeast. Nat. Spec. Iss. 2007, 1, 159–174. [Google Scholar] [CrossRef]
- Grubbs, S.A.; Sheldon, A.L. The stoneflies (Insecta, Plecoptera) of the Talladega Mountain region, Alabama, USA: Distribution, elevation, endemism, and rarity patterns. Biodivers. Data J. 2018, 6, e22839. [Google Scholar] [CrossRef]
- McRoberts, T.C.; Grubbs, S.A. Effects of stream permanence on stonefly (Insecta, Plecoptera) community structure at Mammoth Cave National Park, Kentucky, USA Biodivers. Data J. 2021, 9, e62242. [Google Scholar] [CrossRef]
- Grubbs, S.A.; Kondratieff, B.C.; Baumann, R.W. A surprising rediscovery and description of a new species of Soyedina Ricker, 1952 (Plecoptera: Nemouridae) from Great Smoky Mountains National Park, USA. J. Ins. Biodiv. 2019, 13, 1–5. [Google Scholar] [CrossRef]
- Grubbs, S.A.; Baumann, R.W.; Sheldon, A.L. A review of eastern Nearctic Zapada (Plecoptera: Nemouridae) with a new species from the Great Smoky Mountains. Freshwat. Sci. 2015, 34, 1312–1323. [Google Scholar] [CrossRef]
- DeWalt, R.E.; South, E.J. Ephemeroptera, Plecoptera, and Trichoptera on Isle Royale National Park, USA, compared to mainland species pool and size distribution. ZooKeys 2015, 532, 137–158. [Google Scholar] [CrossRef]
- DeWalt, R.E.; South, E.J.; Robertson, D.R.; Marburger, J.E.; Smith, W.W.; Brinson, V. Mayflies, stoneflies, and caddisflies of streams and marshes of Indiana Dunes National Lakeshore, USA. ZooKeys 2016, 556, 43–63. [Google Scholar] [CrossRef]
- Bogan, M.T.; Carslon, S.M. Diversity and phenology of stoneflies (Plecoptera) from intermittent and perennial streams in Pinnacles National Park, California, USA. Illiesia 2018, 14, 144–154. [Google Scholar]
- Datry, T.; Larned, S.T.; Fritz, K.M.; Bogan, M.T.; Wood, P.J.; Meyer, E.I.; Santos, A.N. Broad-scale patterns of invertebrate richness and community composition in temporary rivers: Effects of flow intermittence. Ecography 2014, 37, 94–104. [Google Scholar] [CrossRef]
- Mazor, R.D.; Stein, E.D.; Ode, P.R.; Schiff, K. Integrating intermittent streams into watershed assessments: Applicability of an index of biotic integrity. Freshwat. Sci. 2014, 33, 459–474. [Google Scholar] [CrossRef]
- Schriever, T.A.; Bogan, M.T.; Boersma, K.S.; Cañedo-Argüelles, M.; Jaeger, K.L.; Olden, J.D.; Lytle, D.A. Hydrology shapes taxonomic and functional structure of desert stream invertebrate communities. Freshwat. Sci. 2015, 34, 399–409. [Google Scholar] [CrossRef]
- Bogan, M.T. Hurry up and wait: Life cycle and distribution of an intermittent stream specialist (Mesocapnia arizonensis). Freshwat. Sci. 2017, 36, 805–815. [Google Scholar] [CrossRef]
- Kerst, C.D.; Anderson, N.H. The Plecoptera community of a small stream in Oregon, USA. Freshwat. Biol. 1975, 5, 189–203. [Google Scholar] [CrossRef]
- Harper, P.P. Life histories of Nemouridae and Leuctridae in southern Ontario (Plecoptera). Hydrobiologia 1973, 41, 309–356. [Google Scholar] [CrossRef]
- Grubbs, S.A.; Thomas, C.M.; Hutchins, B.T.; Taylor, J.M. Life cycles of Allocapnia recta and Leuctra spp. (Plecoptera: Capniidae and Leuctridae) across a flow gradient in a central Kentucky karst headwater stream. Southeast. Nat. 2006, 5, 321–332. [Google Scholar] [CrossRef]
- DeWalt, R.E.; Cao, Y.; Tweddale, T.; Grubbs, S.A.; Hinz, L.; Pessino, M.; Robinson, J.L. Ohio USA stoneflies (Insecta: Plecoptera): Species richness estimation, distribution of functional niche traits, drainage affiliations, and relationships to other states. ZooKeys 2012, 178, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Harper, P.P.; Hynes, H.B.N. The Leuctridae of eastern Canada (Insecta; Plecoptera). Can. J. Zool. 1971, 49, 915–920. [Google Scholar] [CrossRef]
- North Carolina State Wildlife Action Plan. Wildlife Action Plan. Available online: https://ncwildlife.org/plan (accessed on 22 March 2023).




| Site # | Water Body | Longitude | Latitude | Altitude (m) | Drainage Area (km2) |
|---|---|---|---|---|---|
| 1 | South Toe River | 35.75176 | −82.22033 | 911 | 29.27 |
| 2 | Lower Creek | 35.75564 | −82.26803 | 1719 | 0.54 |
| 3 | Neals Creek | 35.74176 | −82.21492 | 954 | 2.93 |
| 4 | Lower Creek | 35.75803 | −82.26772 | 1764 | 0.39 |
| 5 | Left Prong South Toe River | 35.71130 | −82.25058 | 1184 | 1.79 |
| 6 | Right Prong South Toe River | 35.72900 | −82.28330 | 1648 | 0.22 |
| 7 | Big Lost Cove Creek | 35.74298 | −82.21127 | 950 | 0.21 |
| 8 | Hemphill Creek | 35.71024 | −82.25057 | 1192 | 1.35 |
| 9 | tributary Lower Creek | 35.75630 | −82.26460 | 1747 | 0.14 |
| 10 | Right Prong South Toe River | 35.73015 | −82.28414 | 1684 | 0.12 |
| 11 | South Fork Upper Creek | 35.73634 | −82.27946 | 1705 | 0.22 |
| 12 | South Toe River | 35.74417 | −82.22833 | 934 | 25.80 |
| 13 | Little Mountain Creek | 35.75202 | −82.22534 | 937 | 0.12 |
| 14 | unnamed seep | 35.74279 | −82.27468 | 1703 | 0.09 |
| 15 | Lower Creek | 35.75953 | −82.26683 | 1800 | 0.13 |
| 16 | Setrock Creek | 35.75561 | −82.24325 | 1471 | 0.28 |
| 17 | Setrock Creek | 35.75369 | −82.23779 | 1373 | 0.47 |
| 18 | tributary Setrock Creek | 35.75393 | −82.23773 | 1373 | 0.09 |
| 19 | tributary Right Prong South Toe River | 35.72718 | −82.28379 | 1636 | 0.54 |
| 20 | Thee Creek | 35.78222 | −82.25435 | 1741 | 0.12 |
| 21 | Setrock Creek | 35.75047 | −82.23009 | 1016 | 0.85 |
| 22 | tributary Hemphill Creek | 35.71005 | −82.24959 | 1206 | 0.06 |
| 23 | Thee Creek | 35.78013 | −82.25093 | 1600 | 0.62 |
| 24 | Upper Creek | 35.73135 | −82.23858 | 984 | 6.60 |
| 25 | Lower Creek | 35.73407 | −82.23466 | 969 | 4.14 |
| 26 | unnamed seep | 35.75298 | −82.26823 | 1704 | 0.08 |
| 27 | Balsam Spring | 35.76647 | −82.26406 | 1983 | 0.01 |
| 28 | tributary Little Mountain Creek | 35.75627 | −82.23494 | 1044 | 0.12 |
| 29 | (left) tributary South Toe River | 35.71342 | −82.24881 | 1154 | 3.55 |
| 30 | rock faces | 35.75592 | −82.23534 | 1320 | 0.14 |
| 31 | Right Prong South Toe River | 35.73095 | −82.28487 | 1723 | 0.12 |
| 32 | Middle Fork Rock Creek | 35.76408 | −82.25726 | 1781 | <0.01 |
| 33 | South Toe River | 35.74021 | −82.23237 | 960 | 23.36 |
| 34 | Right Prong South Toe River | 35.72194 | −82.24888 | 1074 | 4.12 |
| 35 | unnamed seep | 35.75655 | −82.23523 | 1320 | <0.00 |
| 36 | Lower Creek | 35.75694 | −82.26825 | 1746 | 0.47 |
| 37 | North Fork Rock Creek | 35.77470 | −82.25595 | 1775 | 0.09 |
| 38 | South Toe River | 35.74000 | −82.23166 | 950 | 23.36 |
| 39 | unnamed seep | 35.75369 | −82.23779 | 1373 | 0.47 |
| 40 | South Toe River | 35.80400 | −82.20570 | 831 | 84.95 |
| 41 | Rock Creek | 35.77315 | −82.21896 | 1017 | <0.00 |
| 42 | Spring | 35.74395 | −82.28172 | 1847 | 0.01 |
| 43 | Hemphill Creek | 35.71030 | −82.25784 | 1332 | 1.48 |
| Family | Genus (If Applicable) | References |
|---|---|---|
| All families | [43] | |
| Capniidae | Allocapnia | [44,45] |
| Paracapnia | [46] | |
| Chloroperlidae | all genera | [47] |
| Alloperla | [47,48] | |
| Sweltsa | [47,49] | |
| Leuctridae | all genera | [50] |
| Leuctra | [51,52,53,54,55] | |
| Megaleuctra | [56] | |
| Paraleuctra | [57] | |
| Nemouridae | all genera | [58,59] |
| Amphinemura | Grubbs and Baumann (unpublished key) | |
| Soyedina | [60] | |
| Peltoperlidae | all genera and species | [61] |
| Perlidae | all genera (larvae only) and species | [62,63] |
| Acroneuria (larvae only) | [50] | |
| Paragnetina (larvae only) | [64] | |
| Perlodidae | to Perlodinae genera (larvae only) and species) | [62,65] |
| Isoperla | [66,67,68,69] | |
| Remenus | [70] | |
| Pteronarcyidae | Pteronarcys (larvae only) | [71] |
| Taeniopterygidae | all genera/species | [72] |
| Species | Total No. Collections | No. Unique Sites | Species | Total No Collections | No. Unique Sites |
|---|---|---|---|---|---|
| Family Capniidae | Family Perlidae | ||||
| Allocapnia harperi * | 1 | 1 | Acroneuria abnormis | 9 | 3 |
| Paracapnia angulata | 19 | 14 | Acroneuria carolinensis | 5 | 3 |
| Beloneuria georgiae ** | 1 | 1 | |||
| Family Chloroperlidae | Beloneuria stewarti ** | 2 | 2 | ||
| Alloperla chloris | 3 | 2 | Eccoptura xanthenes | 5 | 5 |
| Alloperla nanina ** | 4 | 2 | Paragnetina immarginata | 5 | 3 |
| Alloperla neglecta ** | 3 | 2 | Perlesta frisoni * | 6 | 4 |
| Alloperla petasata | 3 | 3 | Perlesta nelsoni | 4 | 3 |
| Alloperla usa | 10 | 7 | |||
| Suwallia marginata | 9 | 3 | Family Perlodidae | ||
| Sweltsa lateralis | 33 | 23 | Isoperla arcana/holochlora | 6 | 6 |
| Sweltsa onkos * | 10 | 7 | Isoperla cherokee ** | 5 | 5 |
| Sweltsa urticae ** | 24 | 16 | Isoperla dewalti ** | 7 | 6 |
| Isoperla kirchneri | 1 | 1 | |||
| Family Leuctridae | Isoperla orata | 2 | 1 | ||
| Leuctra carolinensis | 6 | 5 | Isoperla pauli ** | 5 | 4 |
| Leuctra ferruginea | 64 | 24 | Isoperla powhatan | 3 | 3 |
| Leuctra grandis | 54 | 31 | Isoperla pseudosimilis ** | 2 | 2 |
| Leuctra mitchellensis ** | 23 | 20 | Isoperla starki ** | 3 | 3 |
| Leuctra sibleyi | 19 | 13 | Isoperla stewarti ** | 3 | 3 |
| Leuctra tenella | 8 | 8 | Isoperla WKUC 1 | 2 | 2 |
| Leuctra tenuis | 11 | 7 | Isoperla WKUC 2 | 1 | 1 |
| Leuctra triloba | 35 | 27 | Cultus decisus | 1 | 1 |
| Leuctra truncate * | 19 | 19 | Remenus bilobatus | 1 | 1 |
| Leuctra variabilis | 3 | 3 | Remenus WKUC 1 | 1 | 1 |
| Megaleuctra williamsae ** | 2 | 2 | Malirekus hastatus ** | 8 | 8 |
| Paraleuctra sara | 34 | 25 | Yugus arinus ** | 2 | 2 |
| Yugus bulbosus ** | 2 | 2 | |||
| Family Nemouridae | |||||
| Amphinemura appalachia | 6 | 4 | Family Pteronarcyidae | ||
| Amphinemura nigritta | 3 | 1 | Pteronarcys scotti ** | 1 | 1 |
| Amphinemura wui | 50 | 26 | |||
| Soyedina sheldoni ** | 16 | 14 | Family Taeniopterygidae | ||
| Oemopteryx contorta | 6 | 6 | |||
| Family Peltoperlidae | Strophopteryx limata | 6 | 6 | ||
| Tallaperla anna ** | 18 | 14 | |||
| Tallaperla maria | 15 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Metzger, M.L.; Grubbs, S.A. Richness and Elevation Patterns of a Stonefly (Insecta, Plecoptera) Community of a Southern Appalachian Mountains Watershed, USA. Ecologies 2023, 4, 442-460. https://doi.org/10.3390/ecologies4030028
Metzger ML, Grubbs SA. Richness and Elevation Patterns of a Stonefly (Insecta, Plecoptera) Community of a Southern Appalachian Mountains Watershed, USA. Ecologies. 2023; 4(3):442-460. https://doi.org/10.3390/ecologies4030028
Chicago/Turabian StyleMetzger, Madeline L., and Scott A. Grubbs. 2023. "Richness and Elevation Patterns of a Stonefly (Insecta, Plecoptera) Community of a Southern Appalachian Mountains Watershed, USA" Ecologies 4, no. 3: 442-460. https://doi.org/10.3390/ecologies4030028
APA StyleMetzger, M. L., & Grubbs, S. A. (2023). Richness and Elevation Patterns of a Stonefly (Insecta, Plecoptera) Community of a Southern Appalachian Mountains Watershed, USA. Ecologies, 4(3), 442-460. https://doi.org/10.3390/ecologies4030028






