Ecological Half-Life of 137Cs in Fish
Abstract
1. Introduction
1.1. 137Cs in Fish of the Cooling Pond (CP) of ChNPP
1.2. 137Cs in Fish of the Kaniv Reservoir (KR)
1.3. 137Cs in Fish after an Accident at Fukushima Dai-ichi Nuclear Power Plant
1.4. Ecological Half-Life of 137Cs in Fish
2. Materials and Methods
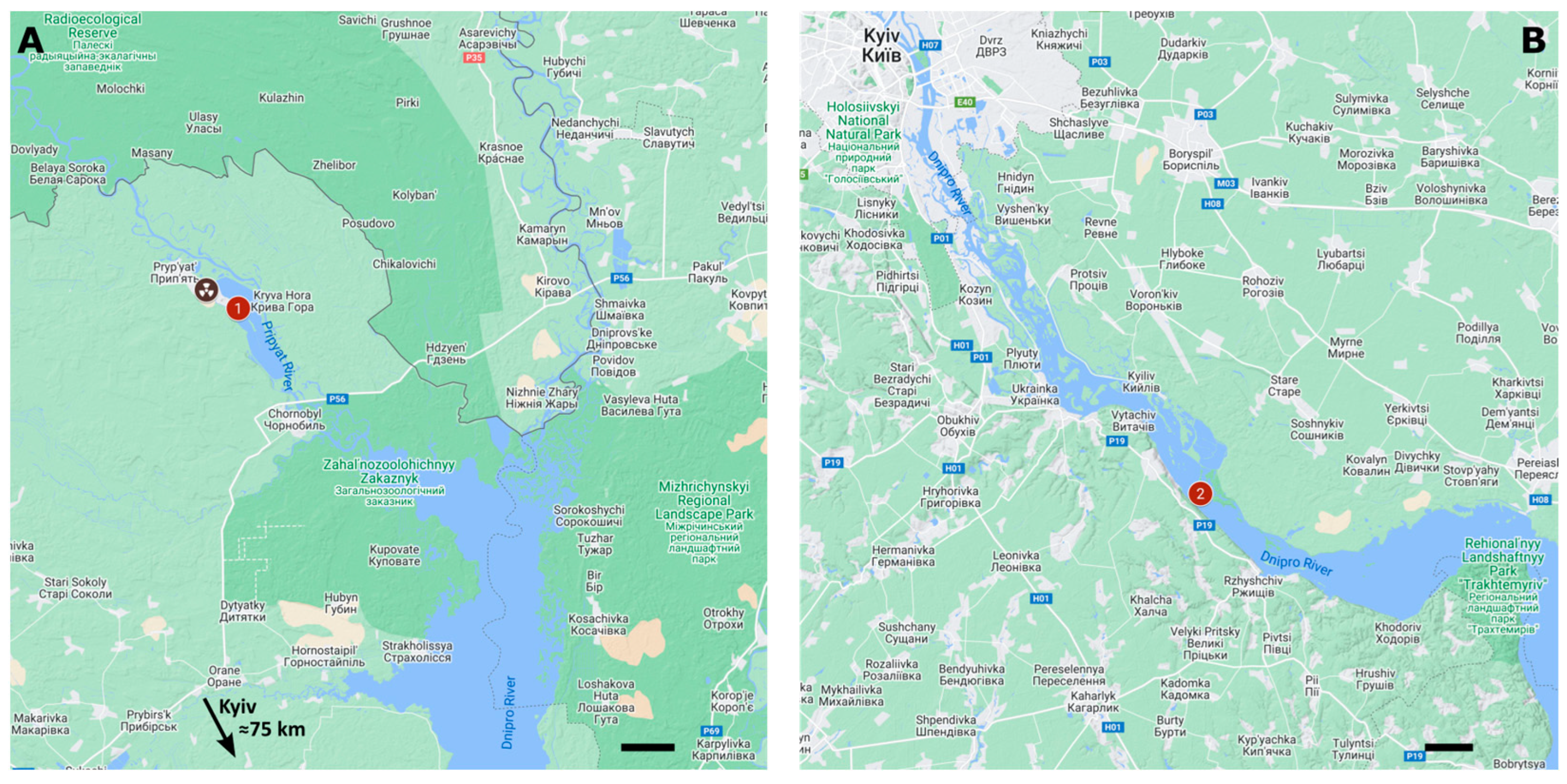
2.1. Sampling Sites
- The Cooling Pond of ChNPP: 30.1429120 E, 51.3732884 N, (point No. 1);
- Kaniv Reservoir: 30.9694639 E, 50.0493128 N, (point No. 2).
2.2. Preparation of the Fish Samples
- Benthophages: B. bjoerkna (Silver bream), A. brama (Bream), and R. rutilus (Roach) are characterized as benthic feeders. Their diet primarily comprises benthic invertebrates, such as mollusks, small crustaceans, mosquito larvae, worms, and organic detritus that settle on the bottom.
- Mixed type of nutrition (Omnivores): P. fluviatilis (Perch) and S. glanis (European Catfish) exhibit versatile dietary behavior. As they mature, these species have the capacity to adapt their feeding preferences, gradually incorporating other fishes into their diet. Their feeding habits can also be influenced by seasonal variations and the availability of food resources, allowing them to adjust and expand their feeding spectrum dynamically.
- Ichthyophages: S. lucioperca (Pikeperch) and A. aspius (Asp) are prominent piscivorous species, exhibiting a predatory feeding strategy focused on consuming fish.
2.3. Radiometry
2.4. Calculation of the Ecological Half-Life of 137Cs
3. Results
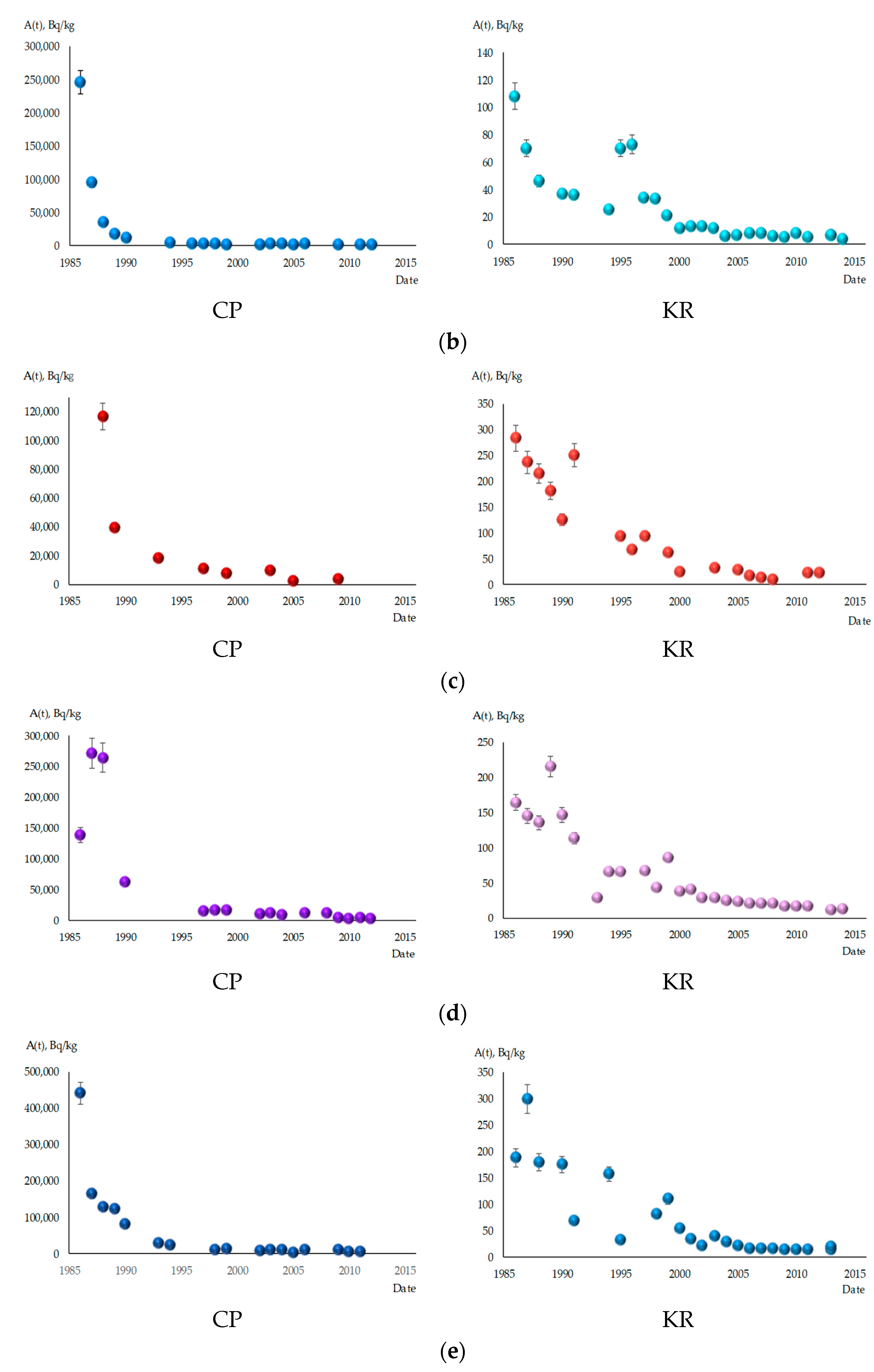
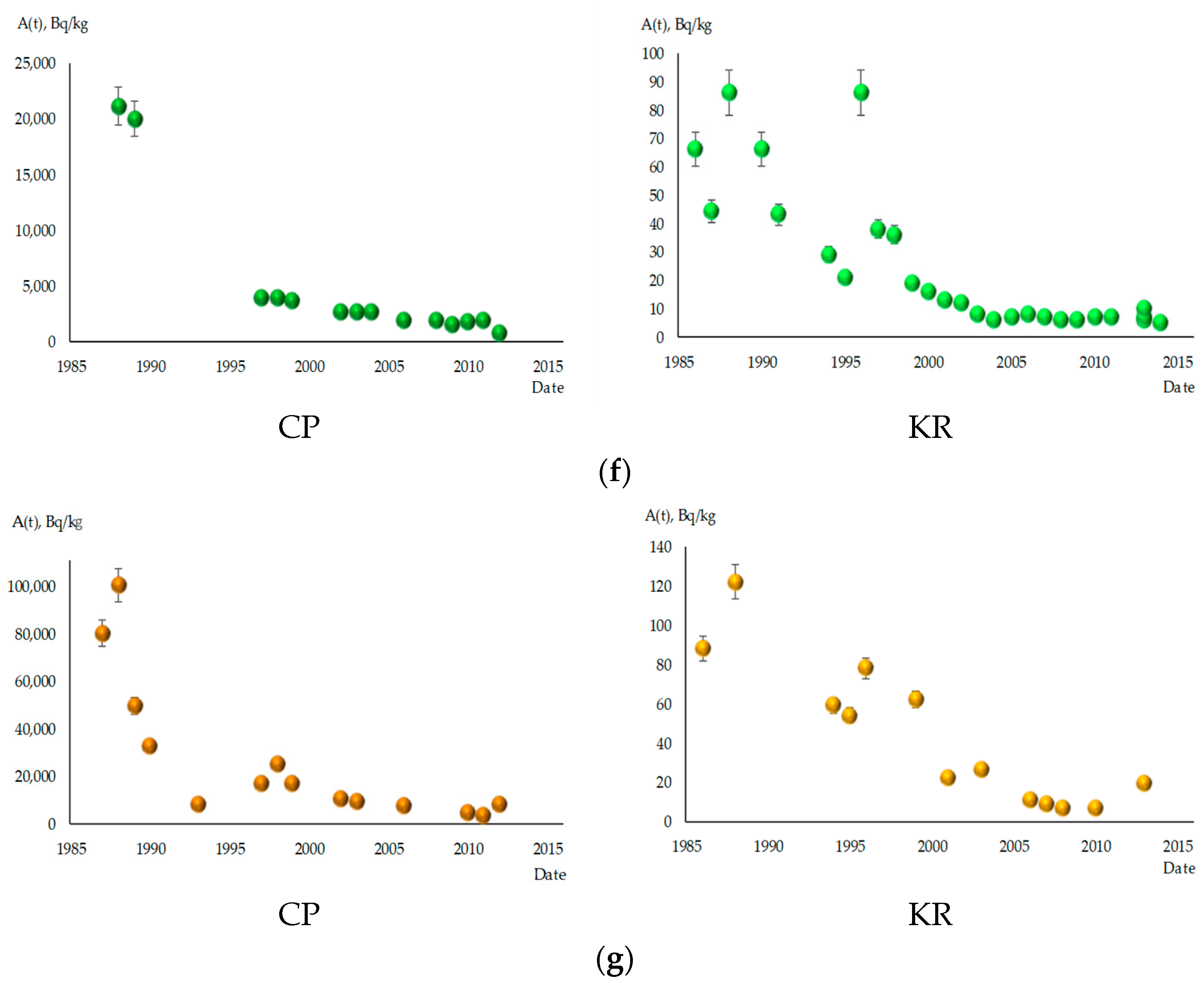
3.1. Dynamics of 137Cs Content in Fish from the Cooling Pond of ChNPP and Kaniv Reservoir
3.2. Calculation of Teco of 137Cs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Atomic Energy Agency. Environmental Consequences of the Chernobyl Accident and Their Remediation: Twenty Years of Experience; STI/PUB/1239; Report of the Chernobyl Forum Expert Group “Environment”; Radiological Assessment Reports Series; International Atomic Energy Agency: Vienna, Austria, 2006; ISBN 92-0-114705-8. [Google Scholar]
- Voitsekhovich, O.V.; Kanivets, V.V.; Laptev, G.V. The Current State of Radioactive Contamination of Water Objects in the Zone of Influence of the Accident. In Radioecology of Water Objects in the Zone of Influence of the Accident at the Chernobyl Nuclear Power Plant; Chernobyltekhinform: Kyiv, Ukraine, 1997; pp. 60–96. (In Ukrainian) [Google Scholar]
- Kuzmenko, M.I. Radioecological Problems of Water Bodies of Ukraine. Hydrobiol. J. 1998, 34, 95–119. (In Ukrainian) [Google Scholar] [CrossRef]
- Kononovich, A.L.; Oskolkov, B.Y.; Korotkov, V.T. Radiation State of the Cooling-Pond of the Chernobyl Nuclear Power Plant and Its Radioecological Status. In Proceedings of the 4th International Scientific and Technical Conference “Results of 8 Years of Work to Eliminate the Consequences of the Chernobyl Accident”. 160–164. (In Ukrainian)
- Zarubin, O.L. Dynamics of the Content of Cesium-137 in Some Fish Species of the Cooling-pond of the Chernobyl Nuclear Power Plant. In Proceedings of the Scientific Conference of the Institute for Nuclear Research, Kyiv, Ukraine, 22–26 January 1996; pp. 286.2–290.2. (In Ukrainian). [Google Scholar]
- Zarubin, O.L.; Vishnevsky, I.N.; Trishin, V.V.; Zalissky, A.A.; Laktionov, V.A. Optimization of the Selection of Biological Objects in Radioecological Monitoring of Freshwater Reservoirs. In Proceedings of the International Conference BIORAD-2001 “Biological Effects of Low Doses of Ionizing Radiation and Radioactive Contamination Environment”, Syktyvkar, Russia, 20–24 March 2001; pp. 129–130. [Google Scholar]
- Zarubin, O.L.; Laktionov, V.A.; Lukashev, D.V.; Zalissky, A.A.; Golovach, A.I. Application of Biological Objects in Radiation Monitoring of Water Bodies. In Proceedings of the International Conference “Fifteen Years of the Chernobyl Disaster. Experience of Overcoming”, Kyiv, Ukraine, 18–20 April 2001. (In Ukrainian). [Google Scholar]
- Zarubin, O.L. Optimization of the Choice of Biotic Objects for a Comprehensive Assessment of Radionuclide Pollution of Water Bodies. In Proceedings of the Russian Scientific Conference “Medico-Biological Problems of Anti-Radiation and Anti-Chemical Protection”, Saint-Petersburg, Russia, 20–21 May 2004; pp. 453–454. (In Russian). [Google Scholar]
- Polikarpov, G.G. Radioecology of Marine Plants and Animals. In Modern Problems of Radiobiology; Atomizdat: Moscow, Russia, 1971; pp. 354–367. (In Russian) [Google Scholar]
- Polikarpov, G.G. Radioecology of Marine Organisms; Atomizdat: Moscow, Russia, 1964. (In Russian) [Google Scholar]
- Ilyin, D.I.; Moskalev, Y.I. On the Distribution, Excretion and Accumulation Coefficients of Strontium-90, Cesium-137 and Phosphorus-32 in Fish. In Distribution, Biological Action and Migration of Radioactive Isotopes; Medgiz: Moscow, Russia, 1961. (In Russian) [Google Scholar]
- Kanevsky, Y.P. Dynamics of K, Rb, and Cs Metabolism in Anadromous Cyclostomes during the Freshwater (pre-spawning) Period of Life. In Radioecology of Aquatic Organisms; Zinatne: Riga, Latvia, 1973; p. 129. (In Russian) [Google Scholar]
- Fleishman, D.G. Accumulation of Artificial Radionuclides by Freshwater Fish. In Modern Problems of Radiobiology; Atomizdat: Moscow, Russia, 1971; p. 395. (In Russian) [Google Scholar]
- Gustafson, P.F. Comments on Radionuclides in Aquatic Ecosystems. In Radioecological Concentration Processes; Pergamon Press: Oxford, UK, 1967; p. 853. [Google Scholar]
- Häsänen, E. Biological Half–Time of Caesium-137 in Three Species of Fresh-Water Fish: Perch, Roach and Rainbow Trout. In Radioecological Concentration Processes; Pergamon Press: Oxford, UK, 1967; p. 921. [Google Scholar]
- King, S.F. Uptake and Transfer of Caesium-137 by Chlamydomonads, Daphnie and Bluegill Fingerlings. Ecology 1964, 45, 852–859. [Google Scholar] [CrossRef]
- Zarubin, O.L.; Zarubina, N.E.; Zalissky, A.A.; Malyuk, I.A.; Kostyuk, V.A. Dynamics of Specific Activity of 137Cs in Fish of Different Types of Food in the Cooling-Pond of the Chernobyl Nuclear Power Plant. Hydrobiol. J. 2014, 50, 107–119. (In Ukrainian) [Google Scholar] [CrossRef]
- Kryshev, I.I.; Ryabov, I.N. About the Efficiency of Trophic Levels in the Accumulation of Cs-137 in Fish of the Chernobyl NPP’ Cooling Pond. In Proceedings of the First International Conference “Biological and Radioecological Aspects of the Consequences of the Chernobyl NPP accident”, Zeleny Mys, Ukraine, 10–18 September 1990. (In Russian). [Google Scholar]
- Zarubin, O.L.; Kostyuk, V.A.; Zalissky, A.A.; Babenko, V.V.; Litvinskaya, T.A.; Malyuk, I.A.; Laktionov, V.A.; Moshna, B.A. Dynamics of Distribution of 137Cs in Organs and Tissues of Fish from Different Ecological Groups of the Cooling Pond. Hydrobiol. J. 2012, 48, 109–115. (In Ukrainian) [Google Scholar] [CrossRef]
- Zarubin, O.L.; Zarubina, N.E. Radionuclide Contamination of the Kaniv Reservoir and Coastal Terrestrial Ecosystems. In Scientific notes of Ternopil National Pedagogical University named after Volodymyr Hnatyuk. Series: Biology. Special Issue “Hydroecology”. 2010; Volume 2, 201–203. (In Ukrainian) [Google Scholar]
- Zarubin, O.L.; Kanivets, V.V. The Content of Radionuclides in the Water of the Kanevsky Reservoir after the 1986 Chernobyl NPP Accident. Collect. Sci. Work Inst. Nucl. Res. 2005, 3, 110–130. (In Ukrainian) [Google Scholar]
- GN 6.6.1.1-130-2006; Permissible Levels of Radionuclides 137Cs and 90Sr in Food and Drinking Water. State Hygienic Standards; Approved. Order of the Ministry of Health of Ukraine from 19.08.97 No. 255. Ministry of Health of Ukraine: Kyiv, Ukraine, 2006. (In Ukrainian)
- Arai, T. Radioactive Cesium Accumulation in Freshwater Fishes after the Fukushima Nuclear Accident. SpringerPlus 2014, 3, 479. [Google Scholar] [CrossRef] [PubMed]
- Ishii, N.; Furota, T.; Kagami, M.; Tagami, K.; Uchida, S. Inequality in the Distribution of 137Cs Contamination within Freshwater Fish Bodies and its Affecting Factors. Nat. Sci. Rep. 2021, 11, 5769. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Tomiya, A.; Enomoto, M.; Sato, T.; Morishita, D.; Izumi, S.; Niizeki, K.; Suzuki, S.; Morita, T.; Kawata, G. Radiological Impact of the Nuclear Power Plant Accident on Freshwater Fish in Fukushima: An Overview of Monitoring Results. J. Environ. Radioact. 2016, 151, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Tateda, Y.; Tsumune, D.; Tsubono, T.; Aono, T.; Kanda, J.; Ishimaru, T. Radiocesium Biokinetics in Olive Flounder Inhabiting the Fukushima Accident-Affected Pacific Coastal Waters of Eastern Japan. J. Environ. Radioact. 2015, 147, 130–141. [Google Scholar] [CrossRef]
- Tateda, Y.; Tsumune, D.; Misumi, K.; Aono, T.; Kanda, J.; Ishimaru, T. Biokinetics of Radiocesium Depuration in Marine Fish Inhabiting the Vicinity of the Fukushima Dai-ichi Nuclear Power Plant. J. Environ. Radioact. 2017, 166, 67–73. [Google Scholar] [CrossRef]
- Wada, T.; Fujita, T.; Nemoto, Y.; Shimamura, S.; Mizuno, T.; Sohtome, T.; Kamiyama, K.; Narita, K.; Watanabe, M.; Hatta, N.; et al. Effects of the Nuclear Disaster on Marine Products in Fukushima: An Update after Five Years. J. Environ. Radioact. 2016, 164, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Pouila, S.; Oberhänslia, F.; Swarzenskia, P.W.; Bustamanteb, P.; Metian, M. The Role of Salinity in the Trophic Transfer of 137Cs in Euryhaline Fish. J. Environ. Radioact. 2018, 189, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Sohtome, T.; Wada, T.; Mizuno, T.; Nemoto, Y.; Igarashi, S.; Nishimune, A.; Aono, A.; Ito, Y.; Kanda, J.; Ishimaru, T. Radiological impact of TEPCO’s Fukushima Dai-ichi Nuclear Power Plant Accident on Invertebrates in the Coastal Benthic Food Web. J. Environ. Radioact. 2014, 138, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Brittain, J.; Bergström, U.; Håkanson, L.; Heling, R.; Monte, L.; Suolanen, V. Estimation of Ecological Half-lives of Caesium-137 in Lakes Contaminated by Chernobyl Fallout. In Proceedings of the International Symposium on Environmental Impact of Radioactive Releases, Vienna, Austria, 8–12 May 1995; IAEA-SM-339/58. pp. 291–298. [Google Scholar]
- Zibold, G.; Klemt, E. Ecological Half-Times of 137Cs and 90Sr in Forest and Freshwater Ecosystems. Radioprotection 2005, 40, S497–S502. [Google Scholar] [CrossRef]
- Paller, M.H.; Jannik, G.T.; Baker, R.A. Effective Half-Life of Caesium-137 in Various Environmental Media at the Savannah River Site. J. Environ. Radioact. 2014, 131, 81–88. [Google Scholar] [CrossRef]
- Available online: https://uk.wikipedia.org/wiki/KanivReservoir (accessed on 5 July 2023).
- Kryshev, I.I.; Ryabov, I.N.; Chumak, V.K.; Zarubin, O.L.; Blynova, L.D.; Nikityn, A.I. Radioecological Processes in the Cooling Reservoir of the Chernobyl NPP. In Radioecological Consequences of the Chernobyl Accident; Nuclear Society of the USSR: Moscow, Russia, 1991; pp. 54–70. (In Russian) [Google Scholar]
- Dzepo, S.P.; Skalsky, A.S.; Bugai, A.D. Results of Monitoring and Special Studies of Radioactive Contamination of Groundwater in the Exclusion Zone. In Radioecology of Water Objects in the Zone of Influence of the Accident at the Chernobyl Nuclear Power Plant; Chernobyltechinform: Kyiv, Ukraine, 1997; pp. 166–196. (In Ukrainian) [Google Scholar]
- Freyhof, J.; Kottelat, M. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland; Berlin, Germany, 2007; ISBN 978-2-8399-0298-4. [Google Scholar]
- Bé, M.-M.; Chisté, V.; Dulieu, C.; Browne, E.; Baglin, C.; Chechev, V.; Kuzmenko, N.; Helmer, R.; Kondev, F.; MacMahon, D.; et al. Table of Radionuclides, Cs-137; Monographie BIPM-5; Bureau International des Poids et Mesures: Sèvres, France, 2006; Volume 3, ISBN 92-822-2218-7. [Google Scholar]
- Zarubina, N.E.; Semak, V.; Burdo, O.S.; Ponomarenko, L.P. Ecological Half-Life of 137Cs in Fungi. Ecologies 2023, 4, 11–19. [Google Scholar] [CrossRef]
- Zarubin, O.L. 137Сs in Fish of Kaniv Reservoir after 20 Years after Accident on ChNPP. Nucl. Phys. At. Energy 2006, 2, 106–114. (In Ukrainian) [Google Scholar]
- Zarubin, O.L.; Zarubina, N.E.; Kostyuk, V.A.; Malyuk, I.A.; Osadchaya, N.N.; Kanivets, V.V.; Zalissky, A.A. On the dynamics of the specific activity of 137Cs in fish from the Kaniv reservoir and the cooling pond of the Chornobyl NPP. In Proceedings of the IX International Scientific and Practical Conference “Ecological Safety: Problems and Ways of Excellence”, Alushta, Ukraine, 9–13 September 2013; V. 1. Rider: Kharkiv, Ukraine, 2013; pp. 115–119. [Google Scholar]
- Luckey, T.D. Radiation Hormesis: The Good, the Bad, and the Ugly. Dose-Response 2006, 4, 169–190. [Google Scholar] [CrossRef]
- Grodzinsky, D.M.; Shilina, Y.V.; Мikhyeyev, O.N.; Guscha, M.I. Radiation Hormesis—Retrospectivity and Modernity. Saf. Probl. Nucl. Pow. Plan. Chorn. 2005, 3, 17–30. (In Ukrainian) [Google Scholar]
- Moffett, J.R. Miasmas, Germs, Homeopathy and Hormesis: Commentary on the Relationship Between Homeopathy and Hormesis. Hum. Exp. Toxicol. 2010, 29, 539–543. [Google Scholar] [CrossRef]
- Tang, S.; Liang, J.; Xiang, C.; Xiao, Y.; Wang, X.; Wu, J.; Li, G.; Cheke, R.A. A General Model of Hormesis in Biological Systems and Its Application to Pest Management. J. R. Soc. Interface 2019, 16, 20190468. [Google Scholar] [CrossRef] [PubMed]
- Grodzinsky, D.M.; Kolomiets, K.D.; Kutlahmedov, Y.O. Anthropogenic Radionuclide Anomaly and Plants; Grodzinsky, D.M., Ed.; Lybid: Kyiv, Ukraine, 1991. (In Ukrainian) [Google Scholar]
- Luckey, T.D. A Rosetta Stone for Ionizing Radiation. Radiat. Prot. Manag. 1994, 11, 73–79. [Google Scholar]
- Ostrovskaya, S.S.; Krizhanovsky, D.G.; Trushenko, O.S.; Shevchenko, I.F.; Gerasimchuk, P.G.; Konovalova, O.S. Influence of Ionizing Radiation and Heavy Metals on Organisms with the Impact of Modeling Effects and Radiation Hormesis. Bull. Probl. Biol. Med. 2022, 4, 84–91. [Google Scholar] [CrossRef]
- Hudkov, I.M. Basics of General and Agricultural Radiobiology; Publishing House of the Ukrainian Academy of Sciences: Kyiv, Ukraine, 1991; ISBN 7987-0005-4. (In Ukrainian) [Google Scholar]
- Grodzinsky, D.M. Radiobiology, 2nd ed.; Lybid: Kyiv, Ukraine, 2000; ISBN 966-06-0143-3. (In Ukrainian) [Google Scholar]

| Binomial Nomenclature | English Name | Ukrainian Name | Sampling Duration Range | |
|---|---|---|---|---|
| CP | KR | |||
| Abramis brama (L.) | Bream | Лящ | 1987–2011 | 1986–2014 |
| Blicca bjoerkna (L.) | Silver bream | Густера | 1986–2012 | 1986–2014 |
| Aspius aspius (L.) | Asp | Білизна | 1988–2009 | 1986–2012 |
| Perca fluviatilis (L.) | Perch | Окунь | 1986–2012 | 1986–2014 |
| Sander lucioperca (L.) | Pikeperch | Судак | 1986–2011 | 1986–2013 |
| Rutilus rutilus (L.) | Roach | Плoтва | 1988–2012 | 1986–2014 |
| Silurus glanis (L.) | European Сatfish | Сoм | 1987–2012 | 1986–2013 |
| Fish Specie | CP | KR | ||||
|---|---|---|---|---|---|---|
| Teco (years) | β (years−1) | Teff (years) | Teco (years) | β (years−1) | Teff (years) | |
| A. brama | 5.84 | 0.17 | 4.89 | 9.11 | 0.11 | 6.99 |
| B. bjoerkna | 6.67 | 0.15 | 5.46 | 8.56 | 0.12 | 6.66 |
| A. aspius | 6.94 | 0.14 | 5.64 | 7.12 | 0.14 | 5.76 |
| P. fluviatilis | 5.58 | 0.18 | 4.71 | 9.92 | 0.10 | 7.46 |
| S. lucioperca | 6.15 | 0.16 | 5.11 | 8.40 | 0.12 | 6.56 |
| R. rutilus | 11.94 | 0.08 | 8.54 | 8.72 | 0.11 | 6.76 |
| S. glanis | 11.50 | 0.09 | 8.32 | 7.06 | 0.14 | 5.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarubina, N.; Semak, V.; Burdo, O.S.; Ponomarenko, L.P. Ecological Half-Life of 137Cs in Fish. Ecologies 2023, 4, 463-477. https://doi.org/10.3390/ecologies4030030
Zarubina N, Semak V, Burdo OS, Ponomarenko LP. Ecological Half-Life of 137Cs in Fish. Ecologies. 2023; 4(3):463-477. https://doi.org/10.3390/ecologies4030030
Chicago/Turabian StyleZarubina, Nataliia, Vladislav Semak, Oleg S. Burdo, and Liliia P. Ponomarenko. 2023. "Ecological Half-Life of 137Cs in Fish" Ecologies 4, no. 3: 463-477. https://doi.org/10.3390/ecologies4030030
APA StyleZarubina, N., Semak, V., Burdo, O. S., & Ponomarenko, L. P. (2023). Ecological Half-Life of 137Cs in Fish. Ecologies, 4(3), 463-477. https://doi.org/10.3390/ecologies4030030







