Exploring mtDNA Databases to Evaluate the Population Structure and Genetic Diversity of Tursiops truncatus in the Atlantic Ocean: Implications for the Conservation of a Small, Offshore Population
Abstract
:1. Introduction
2. Materials and Methods
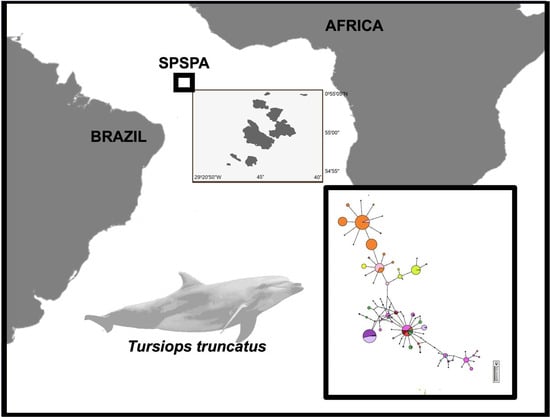
2.1. Sample Collection
2.2. Genetic Diversity and Differentiation
2.3. Demographic Equilibrium and Population Expansion
3. Results
3.1. Genetic Diversity
3.2. Population Structure
3.3. Tests of Demographic Equilibrium and Population Expansion
4. Discussion
4.1. Insights into Offshore Populations of Bottlenose Dolphins in the Atlantic Ocean
4.2. Implications for the Conservation of the SPSPA Population
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frankham, R.; Ballou, J.D.; Ralls, K.; Eldridge, M.; Dudash, M.R.; Fenster, C.B.; Lacy, R.C.; Sunnucks, P. Genetic Management of Fragmented Animal and Plant Populations; Oxford University Press: Oxford, UK, 2017; ISBN 987-0-19-878340. [Google Scholar]
- Simmonds, M.P.; Isaac, S.J. The impacts of climate change on marine mammals: Early signs of significant problems. Oryx 2007, 41, 19–26. [Google Scholar] [CrossRef]
- Bowen, W.D. Role of marine mammals in aquatic ecosystems. Mar. Ecol. Prog. Ser. 1997, 158, 267–274. [Google Scholar] [CrossRef]
- Roman, J.; Estes, J.A.; Morissette, L.; Smith, C.; Costa, D.; McCarthy, J.; Nation, J.; Nicol, S.; Pershing, A.; Smetacek, V. Whales as marine ecosystem engineers. Front. Ecol. Environ. 2014, 12, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.S.; Scott, M.D. Bottlenose dolphin-Tursiops truncates (Montagu, 1821). In Handbook of Marine Mammals; Ridgway, S.H., Harrison, S.R., Eds.; Academic Press: San Diego, CA, USA, 1999; Volume 6, pp. 137–182. ISBN 978-0125885065. [Google Scholar]
- Reynolds, J.E.; Wells, R.S.; Eide, S.D. The Bottlenose Dolphin: Biology and Conservation; University Press of Florida: Gainesville, FL, USA, 2000; ISBN 0-813-01775-0. [Google Scholar]
- Hoelzel, A.R.; Potter, C.W.; Best, P.B. Genetic Differentiation between Parapatric ‘Nearshore’ and ‘Offshore’ Populations of the Bottlenose Dolphin. Proc. R. Soc. Lond. B 1998, 265, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Segura, I.; Rocha-Olivares, A.; Flores-Ramírez, S.; Rojas-Bracho, L. Conservation Implications of the Genetic and Ecological Distinction of Tursiops truncatus Ecotypes in the Gulf of California. Biol. Conserv. 2006, 133, 336–346. [Google Scholar] [CrossRef]
- Tezanos-Pinto, G.; Baker, C.S.; Russell, K.; Martien, K.; Baird, R.W.; Hutt, A.; Stone, G.; Mignucci-Giannoni, A.A.; Caballero, S.; Endo, T.; et al. A worldwide perspective on the population structure and genetic diversity of bottlenose dolphins (Tursiops truncatus) in New Zealand. J. Hered. 2009, 100, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.; Viricel, A.; Lucas, T.; Peltier, H.; Alfonsi, E.; Berrow, S.; Brownlow, A.; Covelo, P.; Dabin, W.; Deaville, R.; et al. Habitat-Driven Population Structure of Bottlenose Dolphins, Tursiops truncatus, in the North-East Atlantic. Mol. Ecol. 2014, 23, 857–874. [Google Scholar] [CrossRef] [PubMed]
- Fruet, P.F.; Secchi, E.R.; DiTullio, J.C.; Simões-Lopes, P.C.; Daura-Jorge, F.; Costa, A.P.B.; Vermeulen, E.; Flores, P.A.C.; Genoves, R.C.; Laporta, P.; et al. Genetic divergence between two phenotypically distinct bottlenose dolphin ecotypes suggests separate evolutionary trajectories. Ecol. Evol. 2017, 7, 9131–9143. [Google Scholar] [CrossRef] [PubMed]
- Querouil, S.; Silva, M.A.; Freitas, L.; Prieto, R.; Magalhães, S.; Dinis, A.; Alves, F.; Matos, J.A.; Mendonça, D.; Hammond, P.S.; et al. High gene flow in oceanic bottlenose dolphins (Tursiops truncatus) of the North Atlantic. Conserv. Genet. 2007, 8, 1405. [Google Scholar] [CrossRef]
- Nykänen, M.; Louis, M.; Dillane, E.; Alfonsi, E.; Berrow, S.; O’Brien, J.; Brownlow, A.; Covelo, P.; Dabin, W.; Deaville, R.; et al. Fine-scale population structure and connectivity of bottlenose dolphins, Tursiops truncatus, in European waters and implications for conservation. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 197–211. [Google Scholar] [CrossRef]
- Louis, M.; Galimberti, M.; Archer, F.; Berrow, S.; Brownlow, A.; Fallon, R.; Nykänen, M.; O’Brien, J.; Roberston, M.K.; Rosel, P.E.; et al. Selection on ancestral genetic variation fuels repeated ecotype formation in bottlenose dolphins. Sci. Adv. 2021, 7, eabg1245. [Google Scholar] [CrossRef] [PubMed]
- Castilho, C.S.; Pedone, F.V.; Bertuol, F.; Fruet, P.; Genoves, R.; Di Tullio, J.; Caon, G.; Hoffmann, L.S.; Freitas, T.R.O. Insights about the genetic diversity and population structure of an offshore group of common bottlenose dolphins (Tursiops truncatus) in the Mid-Atlantic. Genet. Mol. Res. 2015, 14, 3387–3399. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.P.B.; Mcfee, W.; Wilcox, L.A.; Archer, F.I.; Rosel, P.E. The common bottlenose dolphin (Tursiops truncatus) ecotypes of the western North Atlantic revisited: An integrative taxonomic investigation supports the presence of distinct species. Zool. J. Linn. Soc. 2022, 196, 1608–1636. [Google Scholar] [CrossRef]
- Nykänen, M.; Kaschner, K.; Dabin, W.; Brownlow, A.; Davison, N.J.; Deaville, R.; Garilao, C.; Kesner-Reyes, K.; Gilbert, M.T.P.; Penrose, R.; et al. Postglacial Colonization of Northern Coastal Habitat by Bottlenose Dolphins: A Marine Leading-Edge Expansion? J. Hered. 2019, 110, 662–674. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Islas-Villanueva, V.; Tezanos-Pinto, G.; Duchene, S.; Delgado-Estrella, A.; Sanchez-Okrucky, R.; Mignucci-Giannoni, A.A. Phylogeography, genetic diversity and population structure of common bottlenose dolphins in the Wider Caribbean inferred from analyses of mitochondrial DNA control region sequences and microsatellite loci: Conservation and management implications. Anim. Conserv. 2012, 15, 95–112. [Google Scholar] [CrossRef]
- Silva, A.M.; Prieto, R.; Magalhães, S.; Seabra, M.I.; Santos, R.S.; Hammond, P.S. Ranging patterns of bottlenose dolphins living in oceanic waters: Implications for population structure. Mar. Biol. 2008, 156, 179–192. [Google Scholar] [CrossRef]
- Costa, A.P.B.; Fruet, P.F.; Secchi, E.R.; Daura-Jorge, F.G.; Simões-Lopes, P.C.; DiTullio, J.C.; Rosel, P.E. Ecological divergence and speciation in common bottlenose dolphins in the western South Atlantic. J. Evol. Biol. 2021, 34, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.P.B.; Rosel, P.E.; Daura-Jorge, F.G.; Simões-Lopes, P.C. Offshore and coastal common bottlenose dolphins of the western South Atlantic face-to-face: What the skull and the spine can tell us. Mar. Mamm. Sci. 2016, 32, 1433–1457. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Fraga, L.D.; Ott, P.H.; Siciliano, S.; Lopes, F.; Almeida, R.; Wickert, J.C.; Milmann, L.; Danilewicz, D.; Emin-Lima, N.R.; et al. Population structure, phylogeography, and genetic diversity of the common bottlenose dolphin in the tropical and subtropical southwestern Atlantic Ocean. J. Mammal. 2019, 100, 564–577. [Google Scholar] [CrossRef]
- Pratt, E.A.L.; Beheregaray, L.B.; Fruet, P.; Tezanos-Pinto, G.; Bilgmann, K.; Zanardo, N.; Diaz-Aguirre, F.; Secchi, E.R.; Freitas, T.R.O.; Möller, L. Genomic Divergence and the Evolution of Ecotypes in Bottlenose Dolphins (Genus Tursiops). Genome Biol. Evol. 2023, 15, evad199. [Google Scholar] [CrossRef] [PubMed]
- Campos, T.F.C.; Bezerra, F.H.R.; Srivastava, N.K.; Vieira, M.M.; Vita-Finzi, C. Holocene tectonic uplift of the St Peter and St Paul Rocks (Equatorial Atlantic) consistent with emplacement by extrusion. Mar. Geol. 2010, 271, 177–186. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Ott, P.H.; Moreno, I.B.; Tavares, M.; Siciliano, S.; Bonatto, S.L. Effective population size of an offshore population of bottlenose dolphins, Tursiops truncatus, from the São Pedro and São Paulo Archipelago, Brazil. Lat. Am. J. Aquat. Mamm. 2016, 11, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Milmann, L.C.; Danilewicz, D.; Baumgarten, J.; Ott, P.H. Temporal-spatial distribution of an island-based offshore population of common bottlenose dolphins (Tursiops truncatus) in the equatorial Atlantic. Mar. Mammal. Sci. 2016, 33, 496–519. [Google Scholar] [CrossRef]
- Hoffmann, L.S.; Freitas, T.R. Osgolfinhos do Arquipélago de São Pedroe São Paulo. In Arquipélago de São Pedro e São Paulo: 20 anos de Pesquisa; Oliveira, J.E.L., Viana, D.L., Souza, M.A.C., Eds.; Via Design Publicações: Recife, Brasil, 2018; pp. 184–197. ISBN 978-85-93906-01-5. [Google Scholar]
- Rosel, P.E.; Hancock-Hanser, B.L.; Archer, F.I.; Robertson, K.M.; Martien, K.K.; Leslie, M.S.; Berta, A.; Cipriano, F.; Viricel, A.; Viaud-Martinez, K.A.; et al. Examining metrics and magnitudes of molecular genetic differentiation used to delimit cetacean subspecies based on mitochondrial DNA control region sequences. Mar. Mammal. Sci. 2017, 33, 76–100. [Google Scholar] [CrossRef]
- Natoli, A.; Peddemors, V.M.; Hoelzel, A.R. Population structure and speciation in the genus Tursiops based on microsatellite and mitochondrial DNA analyses. J. Evol. Biol. 2004, 17, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Parsons, K.M.; Durban, J.W.; Claridge, D.E.; Herzing, D.L.; Balcomb, K.C.; Noble, L.R. Population genetic structure of coastal bottlenose dolphins (Tursiops truncatus) in the northern Bahamas. Mar. Mammal. Sci. 2006, 22, 276–298. [Google Scholar] [CrossRef]
- Fernández, R.; Santos, M.B.; Pierce, G.J.; Llavona, A.; López, A.; Silva, M.A.; Ferreira, M.; Carrillo, M.; Cermeño, P.; Lens, S.; et al. Fine-scale genetic structure of bottlenose dolphins, Tursiops truncatus, in Atlantic coastal waters of the Iberian Peninsula. Hydrobiologia 2011, 670, 111–125. [Google Scholar] [CrossRef]
- Richards, V.P.; Greig, T.W.; Fair, P.A.; McCulloch, S.D.; Politz, C.; Natoli, A.; Driscoll, C.A.; Hoelzel, A.R.; David, V.; Bossart, G.D.; et al. Patterns of Population Structure for Inshore Bottlenose Dolphins along the Eastern United States. J. Hered. 2013, 104, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Barrera, D.C.; May-Collado, L.J.; Tezanos-Pinto, G.; Islas-Villanueva, V.; Correa-Cárdenas, C.A.; Caballero, S. High genetic structure and low mitochondrial diversity in bottlenose dolphins of the Archipelago of Bocas del Toro, Panama: A population at risk? PLoS ONE 2017, 12, e0189370. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, N.L.; Rosel, P.E.; Mullin, K.D.; Schwacke, L.H.; Garrison, L.P.; Balmer, B.C.; Barry, K.; Martinez, A.; Quigley, B.M.; Sinclair, C.; et al. Assessing common bottlenose dolphin (Tursiops truncatus) population structure in Mississippi Sound and coastal waters of the north central Gulf of Mexico. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 2951–2966. [Google Scholar] [CrossRef]
- Mirimin, L.; Miller, R.; Dillane, E.; Berrow, S.D.; Ingram, S.; Cross, T.F.; Rogan, E. Fine-scale population genetic structuring of bottlenose dolphins in Irish coastal waters. Anim. Conserv. 2011, 14, 342–353. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H. An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2015, 1, 47–50. [Google Scholar]
- Meirmans, P.G. Using the AMOVA framework to estimate a standardized genetic differentiation measure. Evolution 2006, 60, 2399–2402. [Google Scholar] [CrossRef] [PubMed]
- Meirmans, P.G. GENODIVE version 3.0: Easy-to-use software for the analysis of genetic data of diploids and polyploids. Mol. Ecol. Resour. 2020, 20, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Excofier, L.; Smouse, P.E.; Quattro, J.M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: Application to human mitochondrial DNA restriction data. Genetics 1992, 131, 479–491. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 2 December 2023).
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987; ISBN 9780231886710. [Google Scholar]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Archer, F.I.; Adams, P.E.; Schneiders, B.B. Stratag: An r package for manipulating, summarizing and analysing population genetic data. Mol. Ecol. Resour. 2016, 17, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.X. Statistical Tests of Neutrality of Mutations Against Population Growth, Hitchhiking and Background Selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Tajima, F. Statistical Method for Testing the Neutral Mutation Hypothesis by DNA Polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Rosel, P.E.; Taylor, B.L.; Hancock-Hanser, B.L.; Morin, P.A.; Archer, F.I.; Lang, A.R.; Mesnick, S.L.; Pease, V.L.; Perrin, W.F.; Robertson, K.M.; et al. A review of molecular genetic markers and analytical approaches that have been used for delimiting marine mammal subspecies and species. Mar. Mamm. Sci. 2017, 33, 56–75. [Google Scholar] [CrossRef]
- Rubinoff, D.; Holland, B.S. Between two extremes: Mitochondrial DNA is neither the panacea nor the nemesis of phylogenetic and taxonomic inference. Syst. Biol. 2005, 54, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Zink, R.M.; Barrowclough, G.F. Mitochondrial DNA under siege in avian phylogeography. Mol. Ecol. 2008, 17, 2107–2121. [Google Scholar] [CrossRef] [PubMed]
- Martien, K.K.; Leslie, M.S.; Taylor, B.L.; Morin, P.A.; Archer, F.I.; Hancock-Hanser, B.L.; Rosel, P.E.; Vollmer, N.L.; Viricul, A.; Cipriano, F. Analytical approaches to subspecies delimitation with genetic data. Mar. Mamm. Sci. 2017, 33, 27–55. [Google Scholar] [CrossRef]
- Marko, P.B.; Hart, M.W. Retrospective coalscent methods and there construction of metapopulation histories in the sea. Evol. Ecol. 2011, 26, 291–315. [Google Scholar] [CrossRef]
- Natoli, A.; Birkun, A.; Aguilar, A.; Lopez, A.; Hoelzel, A.R. Habitat structure and the dispersal of male and female bottlenose dolphins (Tursiops truncatus). Proc. Royal Soc. B 2005, 272, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.E.; Shreves, K.; Pilot, M.; Andrews, K.R.; Moore, D.M.; Kishida, T.; Möller, L.; Natoli, A.; Gaspari, S.; McGowen, M.; et al. Phylogenomics of the genus Tursiops and closely related Delphininae reveals extensive reticulation among lineages and provides inference about eco-evolutionary drivers. Mol. Phylogenet. Evol. 2020, 146, 106756. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.; Nielsen, D.; Vilstrup, J.; Moreno-Mayar, V.; Gilbert, T.; Gray, H.; Natoli, A.; Moller, L.; Hoelzel, A.R. Recent Diversification of a Marine Genus (Tursiops spp.) Tracks Habitat Preference and Environmental Change. Syst. Biol. 2013, 62, 865–877. [Google Scholar] [CrossRef]
- Amaral, A.R.; Beheregaray, L.B.; Bilgmann, K.; Boutov, D.; Freitas, L.; Robertson, K.M.; Sequeira, M.; Stockin, K.A.; Coelho, M.M.; Moeller, L.M. Seascape Genetics of a Globally Distributed, Highly Mobile Marine Mammal: The Short-Beaked Common Dolphin (Genus Delphinus). PLoS ONE 2012, 7, e31482. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.R.; Smith, B.D.; Mansur, R.M.; Brownell, R.L., Jr.; Rosenbaum, H.C. Oceanographic drivers of population differentiation in Indo-Pacific bottlenose (Tursiops aduncus) and humpback (Sousa spp.) dolphins of the northern Bay of Bengal. Conserv. Genet. 2017, 18, 371–381. [Google Scholar] [CrossRef]
- Stevick, P.T.; McConnell, B.J.; Hammond, P.S. Patterns of movement. In Marine Mammal Biology: An Evolutionary Approach; Hoelzel, A.R., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 185–216. ISBN 978-0-632-05232-5. [Google Scholar]
- Rosel, P.E.; Hansen, L.; Hohn, A.A. Restricted dispersal in a continuously distributed marine species: Common bottlenose dolphins Tursiops truncatus in coastal waters of the western North Atlantic. Mol. Ecol. 2009, 18, 5030–5045. [Google Scholar] [CrossRef] [PubMed]
- Connor, R.C.; Wells, R.S.; Mann, J.; Read, A.J. The bottlenose dolphin, social relationship in a fission-fusion society. In Cetacean Societies, Field Studies of Dolphins and Whales; Mann, J., Connor, R.C., Tyack, P.L., Whitehead, H., Eds.; The University of Chicago Press: Chicago, IL, USA, 2000; pp. 91–126. ISBN 0-226-50341-0. [Google Scholar]
- McGuire, T.L.; Henningsen, T. Movement Patterns and Site Fidelity of River Dolphins (Inia geoffrensis and Sotalia fluviatilis) in the Peruvian Amazon as Determined by Photo-Identification. Aquat. Mamm. 2007, 33, 359–367. [Google Scholar] [CrossRef]
- Parra, G.J.; Corkeron, P.J.; Marsh, H. Population sizes, site fidelity and residence patterns of Australian snubfin and Indo-Pacific humpback dolphins: Implications for conservation. Biol. Conserv. 2006, 129, 167–180. [Google Scholar] [CrossRef]
- Zanardo, N.; Parra, G.; Moller, L.M. Site fidelity, residency, and abundance of bottlenose dolphins (Tursiop ssp.) in Adelaide’s coastal waters, South Australia. Mar. Mammal. Sci. 2016, 32, 1381–1401. [Google Scholar] [CrossRef]
- Hunt, T.N.; Bejder, L.; Allen, S.J.; Rankin, R.W.; Hanf, D.M.; Parra, G.J. Demographic characteristics of Australian humpback dolphins reveal important habitat toward the southwestern limit of their range. Endanger. Species Res. 2017, 32, 71–88. [Google Scholar] [CrossRef]
- Gowans, S.; Würsig, B.; Karczmarski, L. The Social Structure and Strategies of Delphinids: Predictions Based on an Ecological Framework. Adv. Mar. Biol. 2007, 53, 195–294. [Google Scholar] [CrossRef] [PubMed]
- Hoelzel, A.R.; Goldsworthy, S.D.; Fleischer, R.C. Population genetics. In Marine Mammal Biology: An Evolutionary Approach; Hoelzel, A.R., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 325–352. ISBN 978-0-632-05232-5. [Google Scholar]
- Sellas, A.B.; Wells, R.S.; Rosel, P.E. Mitochondrial and nuclear DNA analyses reveal fine scale geographic structure in bottlenose dolphins (Tursiops truncatus) in the Gulf of Mexico. Conserv. Genet. 2005, 6, 715–728. [Google Scholar] [CrossRef]
- Wiszniewski, J.; Beheregaray, L.B.; Allen, S.J.; Möller, L.M. Environmental and social influences on the genetic structure of bottlenose dolphins (Tursiops aduncus) in Southeastern Australia. Conserv. Genet. 2010, 11, 1405–1419. [Google Scholar] [CrossRef]
- Kopps, A.M.; Ackermann, C.Y.; Sherwin, W.B.; Allen, S.J.; Bejder, L.; Krützen, M. Cultural transmission of tool use combined with habitat specializations leads to fine-scale genetic structure in bottlenose dolphins. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133245. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Bryant, K.; Kraus, R.; Loneragan, N.; Kopps, A.; Brown, A.; Gerber, L.; Krützen, M. Genetic isolation between coastal and fishery-impacted, offshore bottlenose dolphin (Tursiops spp.) populations. Mol. Ecol. 2016, 25, 2735–2753. [Google Scholar] [CrossRef]
- Genovês, R.C.; Fruet, P.F.; Di Tullio, J.C.; Hoffmann, L.S. Tamanho Mínimo da População de Golfinhos-nariz-de-garrafa Tursiops truncatus Associado ao Arquipélago de São Pedro e São Paulo, Brasil. In Proceedings of the I Encontro Sul Americano de Pesquisa e Conservação de Tursiops truncatus: Integrando Conhecimento Sobre a Espécie no Atlântico Sul Ocidental DT61, Rio Grande, Brazil, 1–23 May 2010; pp. 1–4. [Google Scholar]
- Ott, P.H.; Tavares, M.; Moreno, I.B.; Oliveira, L.R. Os Cetáceos do Arquipélago de São Pedro e São Paulo. In Ilhas Oceânicas Brasileiras: Da Pesquisa ao Manejo; Mohr, L.V., Castro, J.W.A., Costa, P.M.S., Alves, R.J., Eds.; Ministério do Meio Ambiente: Brasília, Brasil, 2009; pp. 283–300. ISBN 978-85-7738-076-3. [Google Scholar]
- Queiroz, J.D.G.R.; Bezerra, N.A.; Macena, B.; Hazin, F. Back from the dead? Not really. The tale of the Galapagos shark (Carcharhinus galapagensis) in a remote Brazilian archipelago. Biol. Conserv. 2021, 256, 109097. [Google Scholar] [CrossRef]
- Lailson-Brito, J.; Manhaes, B.; Santos, N.E.B.; Azevedo, A.; Bisi, T.L.; Hoffmann, L.S.; Freitas, T.R.O. Bottlenose Dolphin, Tursiops truncatus, as a Sentinel of the Halogenated Trophic Flow in Remote Ocean Waters Adjacent to the São Pedro and São Paulo Archipelago, Brazil. In Proceedings of the XII Congresso de La Sociedad Latinoamericana de Especialistas em Mamiferos Acuaticos-RT 18, Lima, Peru, 5 November 2018; p. 185. [Google Scholar]
- Butchart, S.H.; Walpole, M.; Collen, B.; van Strien, A.; Scharlemann, J.P.W.; Almond, R.E.A.; Baillie, J.E.M.; Bomhard, B.; Brown, C.; Bruno, J.; et al. Global biodiversity: Indicators of recent declines. Science 2010, 328, 1164–1168. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.L.; Eikeset, A.M.; McCauley, D.J.; Payne, J.L.; Sunday, J.M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 2019, 569, 108–111. [Google Scholar] [CrossRef] [PubMed]
- National Marine Fisheries Service. Fisheries of the United States. U.S. Department of Commerce, NOAA Current Fishery Statistics No. 2020, 2022. Available online: https://www.fisheries.noaa.gov/national/sustainable-fisheries/fisheries-united-states (accessed on 2 December 2023).
- Stat, M.; Huggett, M.J.; Bernasconi, R.; Di Battista, J.D.; Berry, T.E.; Newman, S.J.; Harvey, E.S.; Bunce, M. Ecosystem biomonitoring with eDNA: Metabarcoding across the tree of life in a tropical marine environment. Sci. Rep. 2017, 7, 12240. [Google Scholar] [CrossRef]
- Berry, T.E.; Saunders, B.J.; Coghlan, M.L.; Stat, M.; Jarman, S.; Richardson, A.J.; Davies, C.H.; Berry, O.; Harvey, E.S.; Bunce, M. Marine environmental DNA biomonitoring reveals seasonal patterns in biodiversity and identifies ecosystem responses to anomalous climatic events. PLoS Genet. 2019, 15, e1007943. [Google Scholar] [CrossRef] [PubMed]




| Groups | Location | N | Ecotype | Publication |
|---|---|---|---|---|
| Saint Peter Saint Paul Archipelago (SPSPA) | Saint Peter Saint Paul Archipelago, Brazil | 19 | offshore | de Oliveira et al., 2019 [22] |
| Caribbean Offshore (CAO) | Golfo de Morrosquillo, Córdoba Province, Colombia | 3 | offshore | Caballero et al., 2012 [18] |
| Ciénaga, Magdalena Province, Colombia | 1 | Caballero et al., 2012 [18] | ||
| Gandoca-Manzanillo, Costa Rica | 2 | Barragan-Barrera et al., 2017 [33] | ||
| Bahía de Buenavista, Cuba | 5 | Caballero et al., 2012 [18] | ||
| Between la Ceiba and Bahia de Trujillo, Honduras | 4 | Caballero et al., 2012 [18] | ||
| Puerto Rico | 20 | Caballero et al., 2012 [18] | ||
| 35 | ||||
| Gulf of Mexico Offshore (GMO) | Gulf of Mexico, USA | 28 | offshore | Vollmer et al., 2021 [34] |
| Holbox, Mexico | 5 | Caballero et al., 2012 [18] | ||
| Isla Mujeres, Mexico | 1 | Caballero et al., 2012 [18] | ||
| 34 | ||||
| North Atlantic Offshore (NAO) | The Azores, Portugal | 84 | offshore | Querouil et al., 2007 [12] |
| Madeira, Portugal | 18 | Querouil et al., 2007 [12] | ||
| Canarias, Spain | 4 | Fernandéz et al., 2011 [31] | ||
| Pelagic Atlantic, North-East Atlantic Ocean | 101 | Louis et al., 2014 [10] | ||
| 207 | ||||
| Caribbean Inshore (CAI) | East Abaco, Bahamas | 29 | inshore | Parsons et al., 2006 [30] |
| South Abaco, Bahamas | 21 | Parsons et al., 2006 [30] | ||
| White Sand Ridge, Bahamas | 5 | Parsons et al., 2006 [30] | ||
| Bahía de Buenavista, Cuba | 60 | Caballero et al., 2012 [18] | ||
| 115 | ||||
| Gulf of Mexico Inshore (GMI) | Gulf of Mexico, USA | 525 | inshore | Vollmer et al., 2021 [34] |
| Celestun, Mexico | 1 | Caballero et al., 2012 [18] | ||
| Holbox, Mexico | 4 | Caballero et al., 2012 [18] | ||
| Laguna Alvarado, Mexico | 2 | Caballero et al., 2012 [18] | ||
| Laguna Terminos, Mexico | 2 | Caballero et al., 2012 [18] | ||
| Matamoros, Mexico | 4 | Caballero et al., 2012 [18] | ||
| Paraiso, Mexico | 16 | Caballero et al., 2012 [18] | ||
| Tampico, Mexico | 5 | Caballero et al., 2012 [18] | ||
| 559 | ||||
| Namibia (NAM) | Namíbia | 12 | * | Natoli et al., 2004 [29] |
| Northeast of Brazil (NBR) | Bahia, Brazil | 6 | * | de Oliveira et al., 2019 [22] |
| Ceará, Brazil | 3 | de Oliveira et al., 2019 [22] | ||
| Pará, Brazil | 1 | de Oliveira et al., 2019 [22] | ||
| Rio Grande do Norte, Brazil | 4 | de Oliveira et al., 2019 [22] | ||
| 14 | ||||
| Northeast Atlantic—Coastal North (NEAn) | Shannon Estuary, Ireland | 44 | inshore | Mirimin et al., 2010 [35] |
| Connemara–Mayo, Ireland | 12 | Mirimin et al., 2010 [35] | ||
| Cork Harbor, Ireland | 4 | Mirimin et al., 2010 [35] | ||
| Coastal North, North-East Atlantic | 76 | Louis et al., 2014 [10] | ||
| 136 | ||||
| Northeast Atlantic—Coastal South (NEAs) | Mauritania | 1 | inshore | Natoli et al., 2004 [29] |
| Coastal South, North-East Atlantic | 115 | Louis et al., 2014 [10] | ||
| 116 | ||||
| Panama (PAN) | Bocas del Toro, Panamá | 25 | inshore | Barragan-Barrera et al., 2017 [33] |
| USA Inshore (USA) | Charleston Harbor, USA | 35 | inshore | Richards et al., 2013 [32] |
| Indian River, USA | 97 | Richards et al., 2013 [32] | ||
| 132 | ||||
| South of Brazil (SBR) | Campos and Santos Basins, Brazil | 44 | * | de Oliveira et al., 2019 [22] |
| Northern coast of Rio Grande do Sul, Brazil | 29 | de Oliveira et al., 2019 [22] | ||
| 73 | ||||
| USA Inshore (USA) | Charleston Harbor, USA | 35 | inshore | Richards et al., 2013 [32] |
| Indian River, USA | 97 | Richards et al., 2013 [32] | ||
| 132 |
| Source of Variation | df | % Var | F | p | |
|---|---|---|---|---|---|
| All groups | Among individual | 1464 | 0.674 | 1 | 0.001 |
| Among population | 12 | 0.326 | 0.326 | 0.001 | |
| Inshore groups | Among individual | 1105 | 0.605 | 1 | 0.001 |
| Among population | 6 | 0.395 | 0.395 | 0.001 | |
| Offshore groups | Among individual | 291 | 0.793 | 1 | 0.001 |
| Among population | 3 | 0.207 | 0.207 | 0.001 |
| Groups | n | S | H | h ± SD | π ± SD | Tajima’s D | Fu’s FS |
|---|---|---|---|---|---|---|---|
| Saint Peter Saint Paul Archipelago (SPSPA) | 19 | 1 | 2 | 0.105 ± 0.092 | 0.00044 ± 0.00039 | −1.16480 | −0.838 |
| Caribbean Offshore (CAO) | 35 | 11 | 7 | 0.605 ± 0.070 | 0.00735 ± 0.00174 | −1.26300 | −0.626 |
| Gulf of Mexico Offshore (GMO) | 34 | 5 | 7 | 0.838 ± 0.029 | 0.00592 ± 0.00058 | 0.40461 | −1.363 |
| North Atlantic Offshore (NAO) | 207 | 26 | 40 | 0.937 ± 0.007 | 0.02245 ± 0.00057 | 0.34314 | −13.050 |
| Caribbean Inshore (CAI) | 115 | 19 | 11 | 0.574 ± 0.048 | 0.01008 ± 0.00139 | −0.92802 | −0.456 |
| Gulf of Mexico Inshore (GMI) | 559 | 17 | 20 | 0.805 ± 0.007 | 0.00730 ± n.d. | −0.81701 | −6.136 |
| Namibia (NAM) | 12 | 6 | 3 | 0.545 ± 0.144 | 0.00989 ± 0.00283 | 0.72327 | 2.792 |
| Northeast of Brazil (NBR) | 14 | 17 | 9 | 0.912 ± 0.059 | 0.02293 ± 0.00349 | −0.02084 | −1.222 |
| Northeast Atlantic—Coastal North (NEAn) | 136 | 8 | 3 | 0.443 ± 0.040 | 0.01126 ± 0.00110 | 1.95996 | 9.058 |
| Northeast Atlantic—Coastal South (NEAs) | 116 | 7 | 3 | 0.204 ± 0.047 | 0.00159 ± 0.00061 | −1.63199 | 0.224 |
| Panama (PAN) | 25 | 0 | 1 | - | - | - | - |
| South of Brazil (SBR) | 73 | 25 | 17 | 0.888 ± 0.016 | 0.01901 ± 0.00161 | −0.59038 | −2.055 |
| USA (USA) | 132 | 4 | 5 | 0.367 ± 0.048 | 0.00189 ± 0.00028 | −0.74014 | −1.589 |
| Total | 1477 | 43 | 74 | 0.909 ± n.d. | 0.02230 ± n.d. | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandre, B.G.; Cruz, M.M.; do Amaral, K.B.; Hoffmann, L.S.; de Freitas, T.R.O.; Zanini, R. Exploring mtDNA Databases to Evaluate the Population Structure and Genetic Diversity of Tursiops truncatus in the Atlantic Ocean: Implications for the Conservation of a Small, Offshore Population. Ecologies 2024, 5, 170-187. https://doi.org/10.3390/ecologies5020011
Alexandre BG, Cruz MM, do Amaral KB, Hoffmann LS, de Freitas TRO, Zanini R. Exploring mtDNA Databases to Evaluate the Population Structure and Genetic Diversity of Tursiops truncatus in the Atlantic Ocean: Implications for the Conservation of a Small, Offshore Population. Ecologies. 2024; 5(2):170-187. https://doi.org/10.3390/ecologies5020011
Chicago/Turabian StyleAlexandre, Brenda Godoy, Marcelo Merten Cruz, Karina Bohrer do Amaral, Lilian Sander Hoffmann, Thales Renato Ochotorena de Freitas, and Rebeca Zanini. 2024. "Exploring mtDNA Databases to Evaluate the Population Structure and Genetic Diversity of Tursiops truncatus in the Atlantic Ocean: Implications for the Conservation of a Small, Offshore Population" Ecologies 5, no. 2: 170-187. https://doi.org/10.3390/ecologies5020011
APA StyleAlexandre, B. G., Cruz, M. M., do Amaral, K. B., Hoffmann, L. S., de Freitas, T. R. O., & Zanini, R. (2024). Exploring mtDNA Databases to Evaluate the Population Structure and Genetic Diversity of Tursiops truncatus in the Atlantic Ocean: Implications for the Conservation of a Small, Offshore Population. Ecologies, 5(2), 170-187. https://doi.org/10.3390/ecologies5020011