Implications of Climate-Induced Localised Extinction of Bull Kelp (Durvillaea amatheiae) for Holdfast Macrofauna
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
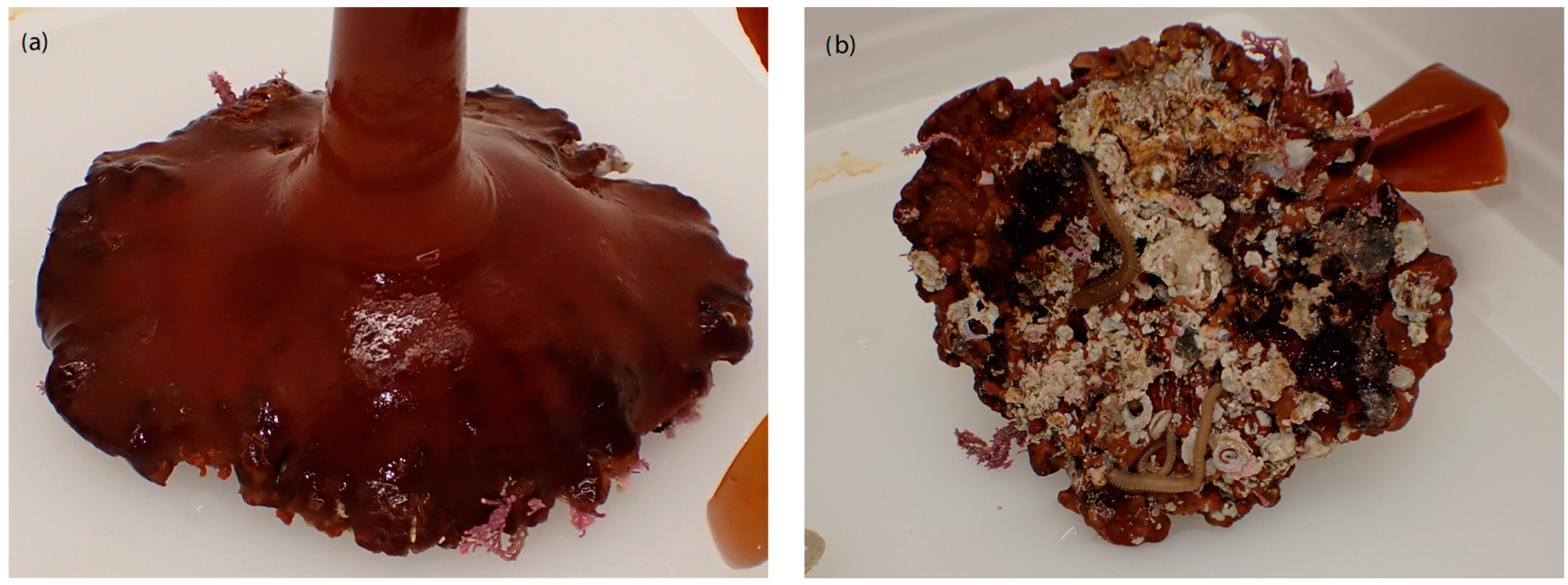
2.2. Holdfast Sampling
2.3. Holdfast Geometry
2.4. Macrofaunal Sampling
2.5. Comparison with Ecklonia Radiata Holdfast Fauna
2.6. Statistical Analysis
3. Results
3.1. Holdfast Geometry and Habitat Description
3.2. Macrofaunal Assemblages
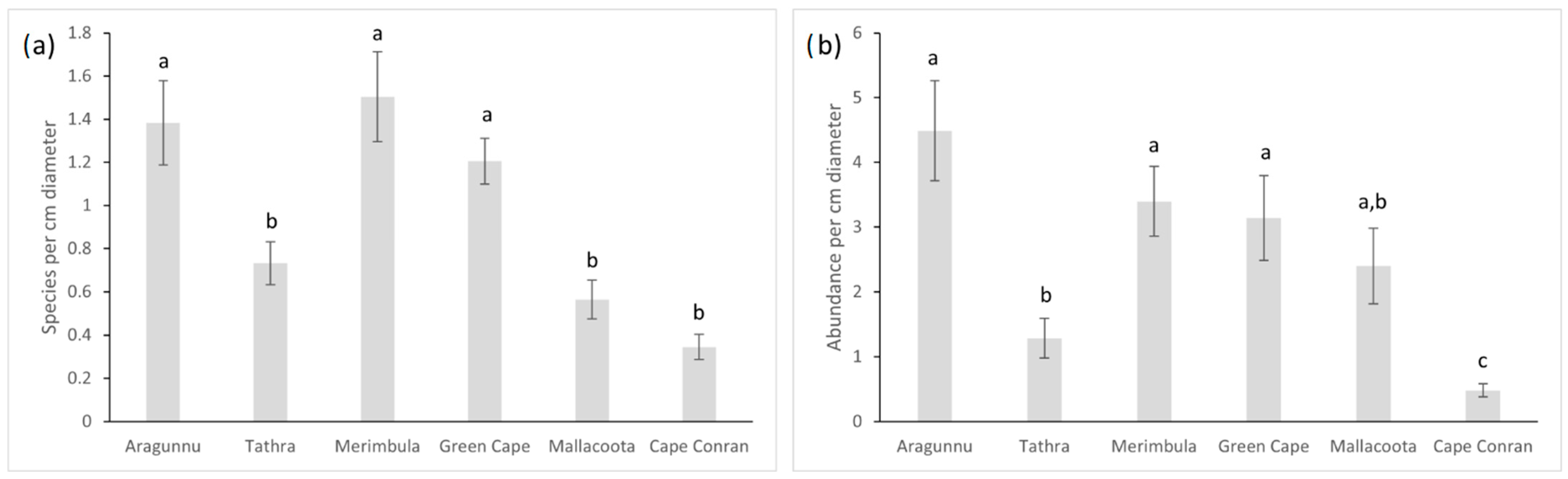
3.3. Macrofaunal Species Richness and Abundance
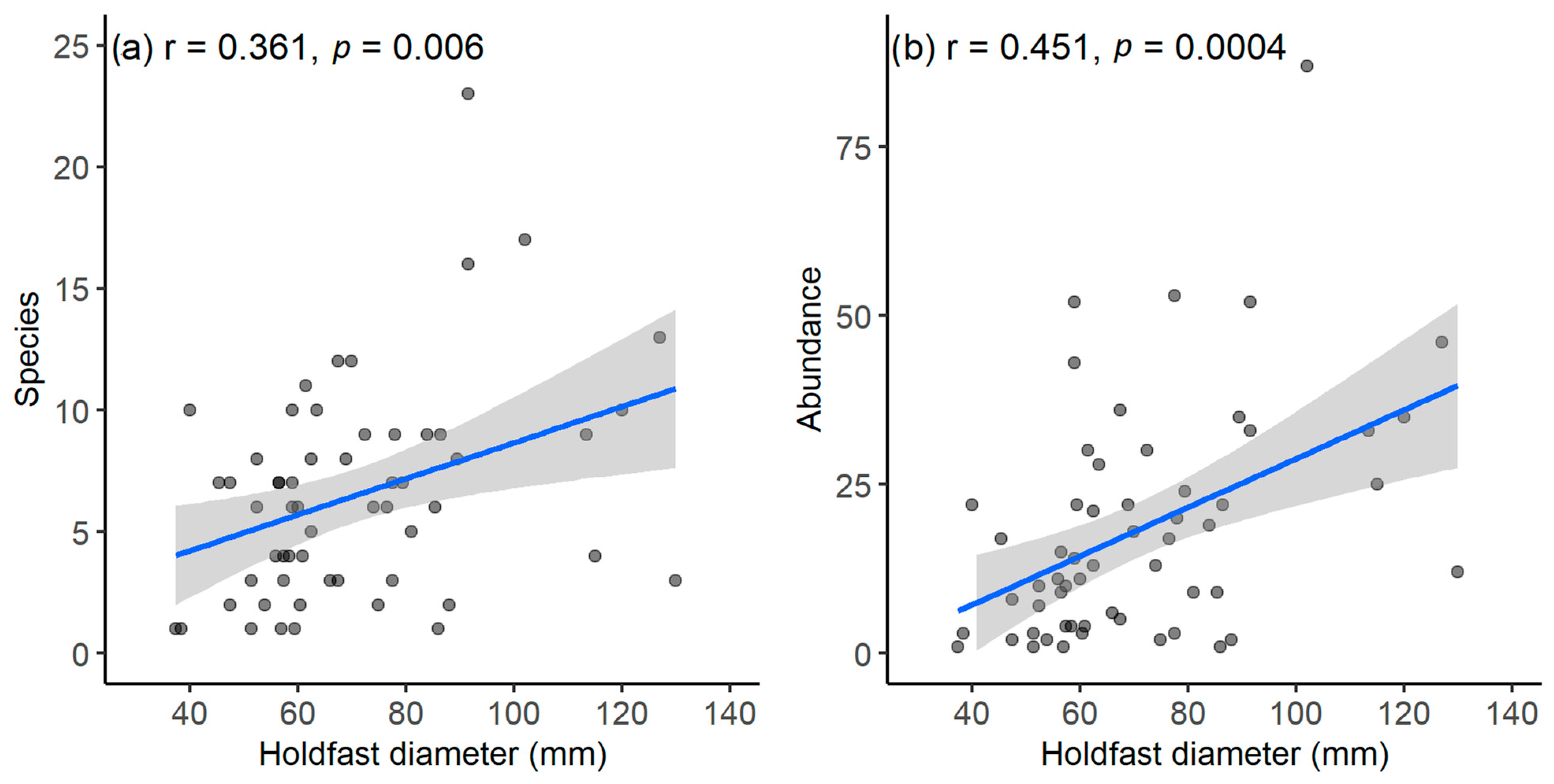
3.4. Factors Associated with Variations in Macrofaunal Assemblage Structures
4. Discussion
4.1. Comparison of D. amatheiae and Co-Occurring E. radiata Holdfast Macrofauna
4.2. Comparison of D. amatheiae Holdfast Macrofauna to Other Kelp Species
4.3. Variations in D. amatheiae Holdfast Macrofauna among Locations
4.4. Influence of D. amatheiae Holdfast Size on Macrofaunal Assemblages
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Phylum | Class | Family | Species | Count |
|---|---|---|---|---|
| Annelida | Polychaeta | Chrysopetalidae | Chrysopetalidae sp. | 2 |
| Polychaeta | Cirratulidae | Cirriformia capensis | 11 | |
| Polychaeta | Cirratulidae | Cirriformia filigera | 4 | |
| Polychaeta | Dorvilleidae | Dorvilleidae sp. | 1 | |
| Polychaeta | Eunicidae | Marphysa sp. 1 | 12 | |
| Polychaeta | Eunicidae | Marphysa sp. 2 | 8 | |
| Polychaeta | Lumbrineridae | Lumbrinereis sp. 1 | 1 | |
| Polychaeta | Lumbrineridae | Lumbrinereis sp. 2 | 44 | |
| Polychaeta | Nereidae | Ceratonereis | 70 | |
| Polychaeta | Nereidae | Nereididae sp. 2 | 21 | |
| Polychaeta | Nereidae | Nereididae sp. 3 | 2 | |
| Polychaeta | Nereidae | Nereididae sp. 4 | 1 | |
| Polychaeta | Phyllodocidae | Eulalia sp. | 1 | |
| Polychaeta | Phyllodocidae | Phyllodocidae sp. | 3 | |
| Polychaeta | Sabellariidae | Idanthyrsus pennatus | 2 | |
| Polychaeta | Scalibregmatidae | Hyboscolex sp. | 9 | |
| Polychaeta | Serpulidae | Galeolaria caespitosa | 7 | |
| Polychaeta | Sphaerodorididae | Sphaerodorum sp. | 1 | |
| Polychaeta | Spionidae | Spionidae sp. | 9 | |
| Polychaeta | Syllidae | Sf. Syllinae | 3 | |
| Polychaeta | Syllidae | Syllis gracilis | 80 | |
| Polychaeta | Syllidae | Syllis sp. | 18 | |
| Polychaeta | Syllidae | Syllis variegata | 2 | |
| Sipuncula | Sipuncula | Sipuncula | 4 | |
| Cnidaria | Actiniaria | Actiniidae | Actiniidae sp. | 2 |
| Crustacea | Amphipoda | Amphilochidae | Amphilochidae sp. 1 | 2 |
| Amphipoda | Amphilochidae | Amphilochidae sp. 2 | 2 | |
| Amphipoda | Aoridae | Aora hebes | 20 | |
| Amphipoda | Eophliantidae | Eophliantidae sp. | 3 | |
| Amphipoda | Hyalidae | Hyale sp. | 25 | |
| Amphipoda | Ischyroceridae | Ischyroceros sp. | 25 | |
| Amphipoda | Leucothoidae | Leucothoe sp. | 2 | |
| Amphipoda | Maeridae | Maera mastersi | 1 | |
| Cirripedia | Cirripedia | Balanus amphitrite | 7 | |
| Isopoda | Anthuridae | Anthuridae sp. | 1 | |
| Isopoda | Janiridae | Janiridae sp. | 5 | |
| Isopoda | Sphaeromatidae | Sphaeromatidae | 22 | |
| Tanaid | Tanaidae | Tanaidae sp. | 19 | |
| Tanaidacea | Apseudidae | Apseudidae sp. | 1 | |
| Foraminifera | Foraminifera | Foraminifera | Foraminifera sp. | 1 |
| Insecta | Insect | Diptera | Diptera sp. | 2 |
| Mollusca | Bivalvia | Hiatellidae | Hiatella australis | 15 |
| Bivalvia | Lasaeidae | Lasaea australis | 413 | |
| Bivalvia | Mytilidae | Mytilidae spp. | 96 | |
| Chitonida | Acanthochitonidae | Acanthochitona sp. | 1 | |
| Chitonida | Chitonidae | Chitonidae sp. | 1 | |
| Gastropod | Fissurellidae | Emarginula sp. | 1 | |
| Gastropoda | Patellidae | Scutellastra peronii | 3 | |
| Nematoda | Nematoda | Nematoda | Nematoda spp. | 25 |
| Nemertea | Nemerteans | Nemertea | Nemertea spp. | 28 |
| Platyhelminthes | Platyhelminthes | Platyhelminthes | Platyhelminth sp. | 1 |
Appendix B
| Location | Sample | Diameter (mm) | Height (mm) | Volume (mL) | Species | Abundance | Abundance Per mL |
|---|---|---|---|---|---|---|---|
| Aragunnu | A1 | 59.0 | 15 | 20 | 6 | 14 | 0.70 |
| Aragunnu | A2 | 91.5 | 25 | 90 | 16 | 33 | 0.37 |
| Aragunnu | A3 | 59.0 | 20 | 22 | 7 | 43 | 1.95 |
| Aragunnu | A4 | 67.5 | 15 | 32 | 12 | 36 | 1.13 |
| Aragunnu | A5 | 37.5 | 10 | 3 | 1 | 1 | 0.33 |
| Aragunnu | A6 | 59.0 | 15 | 17 | 10 | 52 | 3.06 |
| Aragunnu | A7 | 63.5 | 22 | 23 | 10 | 28 | 1.22 |
| Aragunnu | A8 | 89.5 | 22 | 49 | 8 | 35 | 0.71 |
| Aragunnu | A9 | 91.5 | 22 | 68 | 23 | 52 | 0.76 |
| Aragunnu | A10 | 69.0 | 20 | 22 | 8 | 22 | 1.00 |
| Cape Conran | CC1 | 57.0 | 12 | 12 | 1 | 1 | 0.08 |
| Cape Conran | CC2 | 88.0 | 20 | 48 | 2 | 2 | 0.04 |
| Cape Conran | CC3 | 57.5 | 15 | 15 | 3 | 4 | 0.27 |
| Cape Conran | CC4 | 47.5 | 11 | 10 | 2 | 2 | 0.20 |
| Cape Conran | CC5 | 38.5 | 10 | 10 | 1 | 3 | 0.30 |
| Cape Conran | CC6 | 51.5 | 14 | 10 | 1 | 1 | 0.10 |
| Cape Conran | CC7 | 86.0 | 22 | 70 | 1 | 1 | 0.01 |
| Cape Conran | CC8 | 85.5 | 17 | 42 | 6 | 9 | 0.21 |
| Cape Conran | CC9 | 67.5 | 10 | 15 | 3 | 5 | 0.33 |
| Cape Conran | CC10 | 77.5 | 25 | 45 | 3 | 3 | 0.07 |
| Green Cape | GC1 | 102.0 | 32 | 80 | 17 | 87 | 1.09 |
| Green Cape | GC2 | 127.0 | 35 | 160 | 13 | 46 | 0.29 |
| Green Cape | GC3 | 56.5 | 23 | 20 | 7 | 9 | 0.45 |
| Green Cape | GC4 | 86.5 | 23 | 79 | 9 | 22 | 0.28 |
| Green Cape | GC5 | 70.0 | 16 | 21 | 12 | 18 | 0.86 |
| Green Cape | GC6 | 74.0 | 22 | 32 | 6 | 13 | 0.41 |
| Green Cape | GC7 | 72.5 | 23 | 35 | 9 | 30 | 0.86 |
| Green Cape | GC8 | 113.5 | 32 | 108 | 9 | 33 | 0.31 |
| Green Cape | GC9 | 60.0 | 20 | 21 | 6 | 11 | 0.52 |
| Green Cape | GC10 | 52.5 | 20 | 15 | 8 | 10 | 0.67 |
| Mallacoota | MA1 | 130.0 | 25 | 135 | 3 | 12 | 0.09 |
| Mallacoota | MA2 | 115.0 | 28 | 120 | 4 | 25 | 0.21 |
| Mallacoota | MA3 | 120.0 | 25 | 98 | 10 | 35 | 0.36 |
| Mallacoota | MA4 | 77.5 | 15 | 58 | 7 | 53 | 0.91 |
| Mallacoota | MA5 | 62.5 | 15 | 21 | 5 | 13 | 0.62 |
| Mallacoota | MA6 | 75.0 | 23 | 39 | 2 | 2 | 0.05 |
| Mallacoota | MA7 | 81.0 | 15 | 32 | 5 | 9 | 0.28 |
| Mallacoota | MA8 | 59.5 | 12 | 16 | 1 | 22 | 1.38 |
| Mallacoota | MA9 | 76.5 | 15 | 25 | 6 | 17 | 0.68 |
| Mallacoota | MA10 | 57.5 | 15 | 13 | 4 | 10 | 0.77 |
| Merimbula | M1 | 61.5 | 17 | 30 | 11 | 30 | 1.00 |
| Merimbula | M2 | 62.5 | 16 | 19 | 8 | 21 | 1.11 |
| Merimbula | M3 | 56.5 | 18 | 18 | 7 | 15 | 0.83 |
| Merimbula | M4 | 40.0 | 16 | 10 | 10 | 22 | 2.20 |
| Merimbula | M5 | 56.0 | 16 | 20 | 4 | 11 | 0.55 |
| Merimbula | M6 | 45.5 | 12 | 11 | 7 | 17 | 1.55 |
| Merimbula | M7 | 47.5 | 14 | 9 | 7 | 8 | 0.89 |
| Tathra | T1 | 66.0 | 20 | 29 | 3 | 6 | 0.21 |
| Tathra | T2 | 60.5 | 20 | 20 | 2 | 3 | 0.15 |
| Tathra | T3 | 78.0 | 22 | 29 | 9 | 20 | 0.69 |
| Tathra | T4 | 79.5 | 22 | 36 | 7 | 24 | 0.67 |
| Tathra | T5 | 58.5 | 18 | 20 | 4 | 4 | 0.20 |
| Tathra | T6 | 54.0 | 20 | 20 | 2 | 2 | 0.10 |
| Tathra | T7 | 51.5 | 12 | 10 | 3 | 3 | 0.30 |
| Tathra | T8 | 84.0 | 25 | 50 | 9 | 19 | 0.38 |
| Tathra | T9 | 52.5 | 15 | 20 | 6 | 7 | 0.35 |
| Tathra | T10 | 61.0 | 17 | 19 | 4 | 4 | 0.21 |
Appendix C
| Variable | df | SS | MS | Pseudo-F | P (Perm) | Unique Perms |
|---|---|---|---|---|---|---|
| Location | 5 | 38131 | 7626.1 | 3.9326 | 0.001 | 998 |
| Location Pair | t | P (Perm) | Unique Perms |
|---|---|---|---|
| Mallacoota, GreenCape | 1.6992 | 0.002 | 995 |
| Mallacoota, CapeConran | 2.3745 | 0.001 | 978 |
| Mallacoota, Merimbula | 1.9139 | 0.001 | 973 |
| Mallacoota, Aragunnu | 1.9161 | 0.008 | 993 |
| Mallacoota, Tathra | 1.6125 | 0.022 | 996 |
| GreenCape, CapeConran | 2.2959 | 0.001 | 973 |
| GreenCape, Merimbula | 1.3795 | 0.019 | 975 |
| GreenCape, Aragunnu | 1.4188 | 0.019 | 991 |
| GreenCape, Tathra | 1.7763 | 0.005 | 992 |
| CapeConran, Merimbula | 1.7273 | 0.008 | 740 |
| CapeConran, Aragunnu | 2.6244 | 0.001 | 955 |
| CapeConran, Tathra | 2.5017 | 0.002 | 975 |
| Merimbula, Aragunnu | 1.6583 | 0.004 | 951 |
| Merimbula, Tathra | 2.081 | 0.001 | 973 |
| Aragunnu, Tathra | 2.2181 | 0.002 | 991 |
References
- Ceballos, G.; García, A.; Ehrlich, P.R. The Sixth Extinction Crisis: Loss of Animal Populations and Species. J. Cosmol. 2010, 8, 31. [Google Scholar]
- Régnier, C.; Achaz, G.; Lambert, A.; Cowie, R.H.; Bouchet, P.; Fontaine, B. Mass Extinction in Poorly Known Taxa. Proc. Natl. Acad. Sci. USA 2015, 112, 7761–7766. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.R. Modern Insect Extinctions, the Neglected Majority. Conserv. Biol. 2005, 19, 1030–1036. [Google Scholar] [CrossRef]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; De Siqueira, M.F.; Grainger, A.; Hannah, L. Extinction Risk from Climate Change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Brooks, T.M.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.B.; Rylands, A.B.; Konstant, W.R.; Flick, P.; Pilgrim, J.; Oldfield, S.; Magin, G. Habitat Loss and Extinction in the Hotspots of Biodiversity. Conserv. Biol. 2002, 16, 909–923. [Google Scholar] [CrossRef]
- Chen, I.-C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Sorte, C.J.B.; Williams, S.L.; Carlton, J.T. Marine Range Shifts and Species Introductions: Comparative Spread Rates and Community Impacts. Glob. Ecol. Biogeogr. 2010, 19, 303–316. [Google Scholar] [CrossRef]
- Booth, D.J.; Bond, N.; Macreadie, P. Detecting Range Shifts among Australian Fishes in Response to Climate Change. Mar. Freshw. Res. 2011, 62, 1027–1042. [Google Scholar] [CrossRef]
- Wernberg, T.; Bennett, S.; Babcock, R.C.; De Bettignies, T.; Cure, K.; Depczynski, M.; Dufois, F.; Fromont, J.; Fulton, C.J.; Hovey, R.K.; et al. Climate-Driven Regime Shift of a Temperate Marine Ecosystem. Science 2016, 353, 169–172. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L.; Booth, D.J.; Coleman, M.A.; Feary, D.A. The Tropicalization of Temperate Marine Ecosystems: Climate-Mediated Changes in Herbivory and Community Phase Shifts. Proc. R. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Martínez, B.; Radford, B.; Thomsen, M.S.; Connell, S.D.; Carreño, F.; Bradshaw, C.J.A.; Fordham, D.A.; Russell, B.D.; Gurgel, C.F.D.; Wernberg, T. Distribution Models Predict Large Contractions of Habitat-forming Seaweeds in Response to Ocean Warming. Divers. Distrib. 2018, 24, 1350–1366. [Google Scholar] [CrossRef]
- DCCEEW. Threatened Species Strategy Action Plan 2022–2032; DCCEEW: Canberra, Australia, 2022. [Google Scholar]
- Weber, X.A.; Edgar, G.J.; Banks, S.C.; Waters, J.M.; Fraser, C.I. A Morphological and Phylogenetic Investigation into Divergence among Sympatric Australian Southern Bull Kelps (Durvillaea Potatorum and D. Amatheiae Sp. Nov.). Mol. Phylogenet. Evol. 2017, 107, 630–643. [Google Scholar] [CrossRef] [PubMed]
- Glasby, T.M.; Gibson, P.T.; Cruz-Motta, J.J. Differences in Rocky Reef Habitats Related to Human Disturbances across a Latitudinal Gradient. Mar. Environ. Res. 2017, 129, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Mondardini, L.; Alestra, T.; Gerrity, S.; Tait, L.; South, P.M.; Lilley, S.A.; Schiel, D.R. Local Extinction of Bull Kelp (Durvillaea Spp.) Due to a Marine Heatwave. Front. Mar. Sci. 2019, 6, 84. [Google Scholar] [CrossRef]
- Velásquez, M.; Fraser, C.I.; Nelson, W.A.; Tala, F.; Macaya, E.C. Concise Review of the Genus Durvillaea Bory de Saint-Vincent, 1825. J. Appl. Phycol. 2020, 32, 3–21. [Google Scholar] [CrossRef]
- Smith, S.D.A.; Simpson, R.D. Spatial Variation in the Community Structure of Intertidal Habitats at Macquarie Island (Sub-Antarctic). Antarct. Sci. 2002, 14, 374–384. [Google Scholar] [CrossRef]
- Montie, S.; Thomsen, M.S. Long-term Community Shifts Driven by Local Extinction of an Iconic Foundation Species Following an Extreme Marine Heatwave. Ecol. Evol. 2023, 13, e10235. [Google Scholar] [CrossRef] [PubMed]
- Arranz, V.; Liggins, L.; Aguirre, J.D. Metabarcoding Hyperdiverse Kelp Holdfast Communities on Temperate Reefs: An Experimental Approach to Inform Future Studies. Environ. DNA 2022, 4, 492–509. [Google Scholar] [CrossRef]
- Smith, S.D.A.; Simpson, R.D.; Cairns, S.C. The Macrofaunal Community of Ecklonia Radiata Holdfasts: Description of the Faunal Assemblage and Variation Associated with Differences in Holdfast Volume. Aust. J. Ecol. 1996, 21, 81–95. [Google Scholar] [CrossRef]
- Carbajal, P.; Gamarra Salazar, A.; Moore, P.J.; Pérez-Matus, A. Different Kelp Species Support Unique Macroinvertebrate Assemblages, Suggesting the Potential Community-wide Impacts of Kelp Harvesting along the Humboldt Current System. Aquat. Conserv. 2022, 32, 14–27. [Google Scholar] [CrossRef]
- Montie, S.; Thomsen, M.S. Facilitation of Animals Is Stronger during Summer Marine Heatwaves and around Morphologically Complex Foundation Species. Ecol. Evol. 2023, 13, e10512. [Google Scholar] [CrossRef] [PubMed]
- Tuya, F.; Larsen, K.; Platt, V. Patterns of Abundance and Assemblage Structure of Epifauna Inhabiting Two Morphologically Different Kelp Holdfasts. Hydrobiologia 2011, 658, 373–382. [Google Scholar] [CrossRef]
- Smith, S.D.A.; Simpson, R.D. Effects of the “Nella Dan” Oil Spill on the Fauna of Durvillaea Antarctica Holdfasts. Mar. Ecol. Prog. Ser. 1995, 121, 73–89. [Google Scholar] [CrossRef]
- Smith, S.D.A. The Macrofaunal Community of Ecklonia Radiata Holdfasts: Variation Associated with Sediment Regime, Sponge Cover and Depth. Aust. J. Ecol. 1996, 21, 144–153. [Google Scholar] [CrossRef]
- Smith, S.D.A. Impact of Domestic Sewage Effluent versus Natural Background Variability: An Example from Jervis Bay, New South Wales. Mar. Freshw. Res. 1994, 45, 1045–1064. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2015. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Burnham, K.P.; Anderson, D.R.; Huyvaert, K.P. AIC Model Selection and Multimodel Inference in Behavioral Ecology: Some Background, Observations, and Comparisons. Behav. Ecol. Sociobiol. 2011, 65, 23–35. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A Review of Methods to Deal with It and a Simulation Study Evaluating Their Performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Davis, T.R.; Champion, C.; Coleman, M.A. Ecological Interactions Mediate Projected Loss of Kelp Biomass under Climate Change. Divers. Distrib. 2022, 28, 306–317. [Google Scholar] [CrossRef]
- Anderson, M.J.; Diebel, C.E.; Blom, W.M.; Landers, T.J. Consistency and Variation in Kelp Holdfast Assemblages: Spatial Patterns of Biodiversity for the Major Phyla at Different Taxonomic Resolutions. J. Exp. Mar. Biol. Ecol. 2005, 320, 35–56. [Google Scholar] [CrossRef]
- Ojeda, F.P.; Santelices, B. Invertebrate Communities in Holdfasts of the Kelp Macrocystic Pyrifera from Southern Chile. Mar. Ecol. Prog. Ser. Oldendorf 1984, 16, 65–73. [Google Scholar] [CrossRef]
- Teagle, H.; Moore, P.J.; Jenkins, H.; Smale, D.A. Spatial Variability in the Diversity and Structure of Faunal Assemblages Associated with Kelp Holdfasts (Laminaria Hyperborea) in the Northeast Atlantic. PLoS ONE 2018, 13, e0200411. [Google Scholar] [CrossRef] [PubMed]
- Novaczek, I. Stipe Growth Rings in Ecklonia Radiata (C. AG.) J. AG.(Laminariales). Br. Phycol. J. 1981, 16, 363–371. [Google Scholar] [CrossRef]
- Hill, R.; Bellgrove, A.; Macreadie, P.I.; Petrou, K.; Beardall, J.; Steven, A.; Ralph, P.J. Can Macroalgae Contribute to Blue Carbon? An A Ustralian Perspective. Limnol. Oceanogr. 2015, 60, 1689–1706. [Google Scholar] [CrossRef]
- Thurstan, R.H.; Brittain, Z.; Jones, D.S.; Cameron, E.; Dearnaley, J.; Bellgrove, A. Aboriginal Uses of Seaweeds in Temperate Australia: An Archival Assessment. J. Appl. Phycol. 2018, 30, 1821–1832. [Google Scholar] [CrossRef]
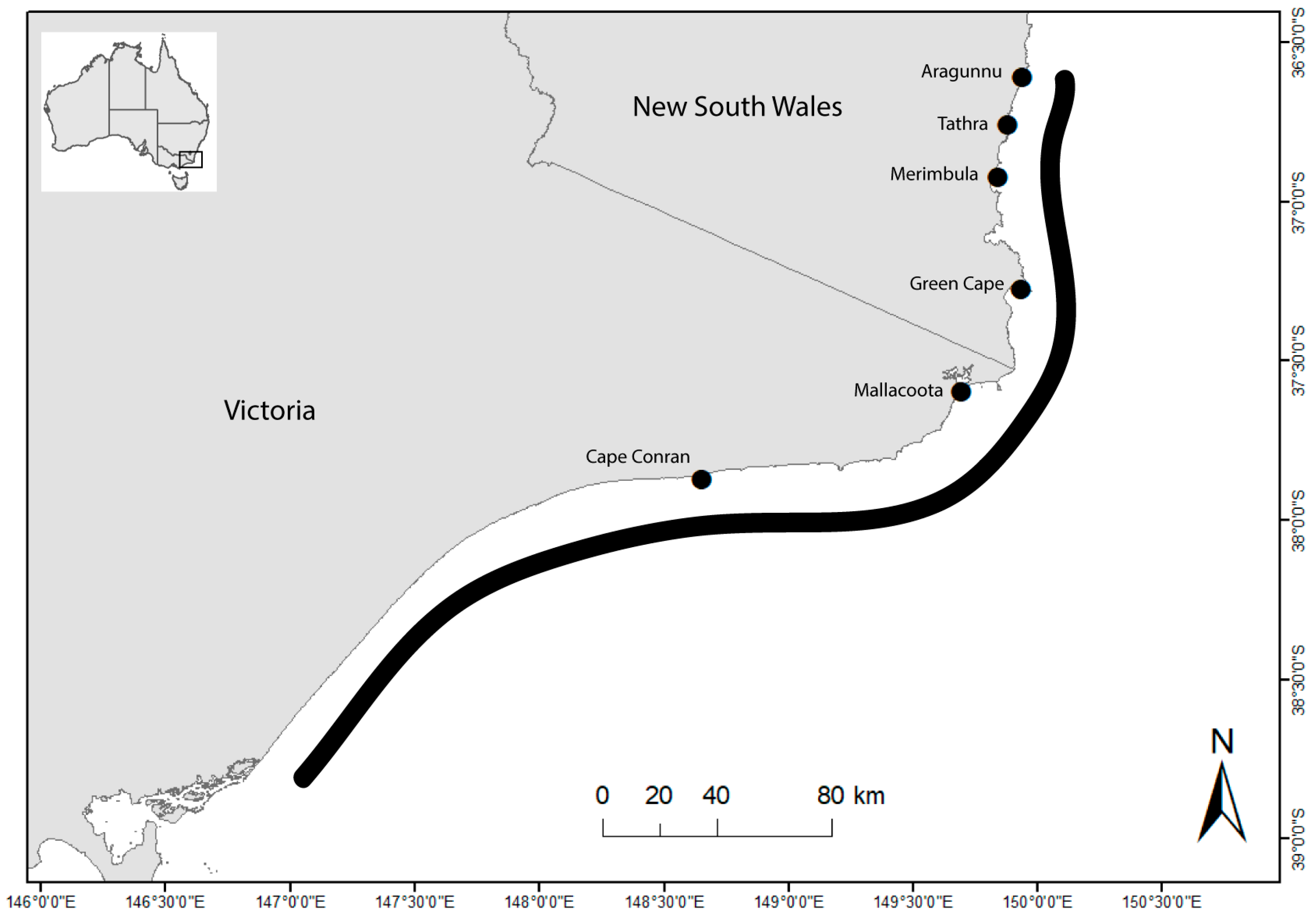

| Location | Latitude (° S) | Longitude (° E) | Samples | Mean Annual Sea Surface Temperature (°C) |
|---|---|---|---|---|
| Aragunnu | 36.5910 | 150.0435 | 10 | 18.38 |
| Tathra | 36.7379 | 149.9846 | 10 | 18.12 |
| Merimbula | 36.9005 | 149.9371 | 7 | 17.94 |
| Green Cape | 37.2552 | 150.0133 | 10 | 17.51 |
| Mallacoota | 37.5719 | 149.7648 | 10 | 16.98 |
| Cape Conran | 37.8125 | 148.7268 | 10 | 15.83 |
| Phylum | Aragunnu (10) | Tathra (10) | Merimbula (7) | Green Cape (10) | Mallacoota (10) | Cape Conran (10) | Total (57) |
|---|---|---|---|---|---|---|---|
| Annelida | 14 | 9 | 11 | 13 | 11 | 6 | 24 |
| Cnidaria | 0 | 1 | 0 | 1 | 0 | 0 | 1 |
| Crustacea | 9 | 3 | 7 | 10 | 3 | 1 | 14 |
| Foraminifera | 0 | 0 | 1 | 0 | 0 | 0 | 1 |
| Insecta | 1 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mollusca | 7 | 3 | 3 | 4 | 3 | 2 | 7 |
| Nematoda | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Nemertea | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Platyhelminthes | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Total | 33 | 18 | 24 | 31 | 19 | 11 | 51 |
| Species | Contribution to Differences among Assemblages % | Cumulative Contribution % |
|---|---|---|
| Lasaea australis | 15.9% | 15.9% |
| Mytilidae spp. | 7.8% | 23.7% |
| Syllis gracilis | 7.4% | 31.1% |
| Ceratonereis | 6.8% | 37.9% |
| Lumbrinereis sp. 2 | 4.9% | 42.7% |
| Nemertea spp. | 3.9% | 46.6% |
| Nereididae sp. 2 | 3.3% | 49.9% |
| Nematoda spp. | 2.9% | 52.8% |
| Model | Explanatory Variables | AIC | R2 | RSS |
|---|---|---|---|---|
| 1 | Latitude, holdfast diameter | 413.18 | 0.110 | 115,030 |
| 2 | Latitude | 413.73 | 0.066 | 120,690 |
| 3 | Latitude, sediment content | 414.05 | 0.095 | 116,940 |
| 4 | Latitude, diameter, sediment | 414.53 | 0.121 | 113,630 |
| 5 | Holdfast diameter | 415.36 | 0.037 | 124,470 |
| 6 | Sediment content | 415.51 | 0.034 | 124,820 |
| 7 | Diameter, sediment | 416.21 | 0.057 | 121,800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, T.R.; Nimbs, M.J.; Benson, A.; Smith, S.D.A. Implications of Climate-Induced Localised Extinction of Bull Kelp (Durvillaea amatheiae) for Holdfast Macrofauna. Ecologies 2024, 5, 218-232. https://doi.org/10.3390/ecologies5020014
Davis TR, Nimbs MJ, Benson A, Smith SDA. Implications of Climate-Induced Localised Extinction of Bull Kelp (Durvillaea amatheiae) for Holdfast Macrofauna. Ecologies. 2024; 5(2):218-232. https://doi.org/10.3390/ecologies5020014
Chicago/Turabian StyleDavis, Tom R., Matt J. Nimbs, Andrew Benson, and Steve D. A. Smith. 2024. "Implications of Climate-Induced Localised Extinction of Bull Kelp (Durvillaea amatheiae) for Holdfast Macrofauna" Ecologies 5, no. 2: 218-232. https://doi.org/10.3390/ecologies5020014
APA StyleDavis, T. R., Nimbs, M. J., Benson, A., & Smith, S. D. A. (2024). Implications of Climate-Induced Localised Extinction of Bull Kelp (Durvillaea amatheiae) for Holdfast Macrofauna. Ecologies, 5(2), 218-232. https://doi.org/10.3390/ecologies5020014







