Effects of Landscape Heterogeneity and Disperser Movement on Seed Dispersal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study System and Species
2.2. Model Design
2.3. Modelling Landscape
2.4. Simulation Experiments
2.5. Analyses
2.5.1. Seed Dispersal Effectiveness
2.5.2. Seed Dispersal Kernels
2.5.3. Population Size, Nearest Neighbor Distance and Connectivity
3. Results
3.1. Seed Dispersal Kernels
3.2. Seed Disperser Effectiveness
3.2.1. SDEQ and Proportion Dispersed
3.2.2. SDED
3.2.3. SDE
3.3. Route and Seed Dispersal Distances
3.4. Seed Deposition
3.5. Population Size, Nearest Neighbor Distance, and Connectivity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nathan, R.; Muller-Landau, H.C. Spatial Patterns of Seed Dispersal, Their Determinants and Consequences for Recruitment. Trends Ecol. Evol. 2000, 15, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Levin, S.A.; Muller-Landau, H.C.; Nathan, R.; Chave, J. The Ecology and Evolution of Seed Dispersal: A Theoretical Perspective. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 575–604. [Google Scholar] [CrossRef]
- Traveset, A.; Heleno, R.; Nogales, M. The Ecology of Seed Dispersal. In Seeds: The Ecology of Regeneration in Plant Communities; Gallagher, R., Ed.; CAB International: Wallingford, UK, 2014; pp. 62–93. ISBN 978 1 78064 183 6. [Google Scholar]
- Thomson, F.J.; Moles, A.T.; Auld, T.D.; Ramp, D.; Ren, S.; Kingsford, R.T. Chasing the Unknown: Predicting Seed Dispersal Mechanisms from Plant Traits. J. Ecol. 2010, 98, 1310–1318. [Google Scholar] [CrossRef]
- Carlo, T.A. Interspecific Neighbors Change Seed Dispersal Pattern of an Avian-Dispersed Plant. Ecology 2005, 86, 2440–2449. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Fiedler, W.; Safi, K.; Waldenström, J.; Wikelski, M.; van Toor, M.L. A Comprehensive Model for the Quantitative Estimation of Seed Dispersal by Migratory Mallards. Front. Ecol. Evol. 2019, 7, 40. [Google Scholar] [CrossRef]
- Rey, P.J.; Alcántara, J.M. Recruitment Dynamics of a Fleshy-Fruited Plant (Olea europaea): Connecting Patterns of Seed Dispersal to Seedling Establishment. J. Ecol. 2000, 88, 622–633. [Google Scholar] [CrossRef]
- Saracco, J.F.; Collazo, J.A.; Groom, M.J.; Carlo, T.A. Crop Size and Fruit Neighborhood Effects on Bird Visitation to Fruiting Schefflera morototoni Trees in Puerto Rico. Biotropica 2005, 37, 81–87. [Google Scholar] [CrossRef]
- Falcón, W.; Moll, D.; Hansen, D.M. Frugivory and Seed Dispersal by Chelonians: A Review and Synthesis. Biol. Rev. 2020, 95, 142–166. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.M.; Morán López, T. Mechanistic Models of Seed Dispersal by Animals. Oikos 2022, 2022, e08328. [Google Scholar] [CrossRef]
- Carlo, T.A.; Morales, J.M. Generalist Birds Promote Tropical Forest Regeneration and Increase Plant Diversity via Rare-Biased Seed Dispersal. Ecology 2016, 97, 1819–1831. [Google Scholar] [CrossRef]
- Levey, D.J.; Silva, W.R.; Galetti, M. (Eds.) Seed Dispersal and Frugivory: Ecology, Evolution and Conservation; CABI Publishing: New York, NY, USA, 2002; ISBN 0-85199-525-X. [Google Scholar]
- Bullock, J.M.; Mallada González, L.; Tamme, R.; Götzenberger, L.; White, S.M.; Pärtel, M.; Hooftman, D.A.P. A Synthesis of Empirical Plant Dispersal Kernels. J. Ecol. 2017, 105, 6–19. [Google Scholar] [CrossRef]
- Law, R.; Murrell, D.J.; Dieckmann, U. Population Growth in Space and Time: Spatial Logistic Equations. Ecology 2003, 84, 252–262. [Google Scholar] [CrossRef]
- Bustamante, R.O.; Canals, M. Dispersal Quality in Plants: How to Measure Efficiency and Effectiveness of a Seed Disperser. Oikos 1995, 73, 133. [Google Scholar] [CrossRef]
- Schupp, E.W.; Jordano, P.; Gómez, J.M. Seed Dispersal Effectiveness Revisited: A Conceptual Review. New Phytol. 2010, 188, 333–353. [Google Scholar] [CrossRef] [PubMed]
- Schupp, E.W. Quantity, Quality and the Effectiveness of Seed Dispersal by Animals. In Frugivory and Seed Dispersal: Ecological and Evolutionary Aspects. Vegetatio 1993, 107/108, 15–29. [Google Scholar] [CrossRef]
- Jordano, P. What Is Long-Distance Dispersal? And a Taxonomy of Dispersal Events. J. Ecol. 2017, 105, 75–84. [Google Scholar] [CrossRef]
- Bohrer, G.; Nathan, R.; Volis, S. Effects of Long-Distance Dispersal for Metapopulation Survival and Genetic Structure at Ecological Time and Spatial Scales. J. Ecol. 2005, 93, 1029–1040. [Google Scholar] [CrossRef]
- Morán-López, T.; Carlo, T.A.; Morales, J.M. The Role of Frugivory in Plant Diversity Maintenance—A Simulation Approach. Ecography 2018, 41, 24–31. [Google Scholar] [CrossRef]
- Carlo, T.A.; Morales, J.M. Inequalities in Fruit-Removal and Seed Dispersal: Consequences of Bird Behaviour, Neighbourhood Density and Landscape Aggregation. J. Ecol. 2008, 96, 609–618. [Google Scholar] [CrossRef]
- Pegman, A.P.M.; Perry, G.L.W.; Clout, M.N. Exploring the Interaction of Avian Frugivory and Plant Spatial Heterogeneity and Its Effect on Seed Dispersal Kernels Using a Simulation Model. Ecography 2017, 40, 1098–1109. [Google Scholar] [CrossRef]
- Skeate, S.T. Interactions between Birds and Fruits in a Northern Florida Hammock Community. Ecology 1987, 68, 297–309. [Google Scholar] [CrossRef]
- Morales, J.M.; García, D.; Martínez, D.; Rodriguez-Pérez, J.; Herrera, J.M. Frugivore Behavioural Details Matter for Seed Dispersal: A Multi-Species Model for Cantabrian Thrushes and Trees. PLoS ONE 2013, 8, e65216. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Alvarez, H.; Ríos-Casanova, L.; Peco, B. Are Large Frugivorous Birds Better Seed Dispersers than Medium- and Small-Sized Ones? Effect of Body Mass on Seed Dispersal Effectiveness. Ecol. Evol. 2020, 10, 6136–6143. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, N.; Böhning-Gaese, K.; Laube, I.; Schleuning, M. Short Seed-Dispersal Distances and Low Seedling Recruitment in Farmland Populations of Bird-Dispersed Cherry Trees. J. Ecol. 2012, 100, 1349–1358. [Google Scholar] [CrossRef]
- González-Castro, A.; Calviño-Cancela, M.; Nogales, M. Comparing Seed Dispersal Effectiveness by Frugivores at the Community Level. Ecology 2015, 96, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Côrtes, M.C.; Uriarte, M. Integrating Frugivory and Animal Movement: A Review of the Evidence and Implications for Scaling Seed Dispersal. Biol. Rev. 2013, 88, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Nield, A.P.; Nathan, R.; Enright, N.J.; Ladd, P.G.; Perry, G.L.W. The Spatial Complexity of Seed Movement: Animal-Generated Seed Dispersal Patterns in Fragmented Landscapes Revealed by Animal Movement Models. J. Ecol. 2020, 108, 687–701. [Google Scholar] [CrossRef]
- Will, H.; Tackenberg, O. A Mechanistic Simulation Model of Seed Dispersal by Animals. J. Ecol. 2008, 96, 1011–1022. [Google Scholar] [CrossRef]
- Abraham, A.J.; Prys-Jones, T.O.; De Cuyper, A.; Ridenour, C.; Hempson, G.P.; Hocking, T.; Clauss, M.; Doughty, C.E. Improved Estimation of Gut Passage Time Considerably Affects Trait-Based Dispersal Models. Funct. Ecol. 2020, 35, 860–869. [Google Scholar] [CrossRef]
- Jones, L.R.; Duke-Sylvester, S.M.; Leberg, P.L.; Johnson, D.M. Closing the Gaps for Animal Seed Dispersal: Separating the Effects of Habitat Loss on Dispersal Distances and Seed Aggregation. Ecol. Evol. 2017, 7, 5410–5425. [Google Scholar] [CrossRef]
- Cousens, R.D.; Hill, J.; French, K.; Bishop, I.D. Towards Better Prediction of Seed Dispersal by Animals. Funct. Ecol. 2010, 24, 1163–1170. [Google Scholar] [CrossRef]
- Fedriani, J.M.; Wiegand, T.; Ayllón, D.; Palomares, F.; Suárez-Esteban, A.; Grimm, V. Assisting Seed Dispersers to Restore Oldfields: An Individual-Based Model of the Interactions among Badgers, Foxes and Iberian Pear Trees. J. Appl. Ecol. 2018, 55, 600–611. [Google Scholar] [CrossRef]
- Wotton, D.M.; Kelly, D. Do Larger Frugivores Move Seeds Further? Body Size, Seed Dispersal Distance, and a Case Study of a Large, Sedentary Pigeon. J. Biogeogr. 2012, 39, 1973–1983. [Google Scholar] [CrossRef]
- Westcott, D.A.; Graham, D.L. Patterns of Movement and Seed Dispersal of a Tropical Frugivore. Oecologia 2000, 122, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Wenny, D.G. Advantages of Seed Dispersal: A Re-Evaluation of Directed Dispersal. Evol. Ecol. Res. 2001, 3, 51–74. [Google Scholar]
- Nams, V.O. Using Animal Movement Paths to Measure Response to Spatial Scale. Oecologia 2005, 143, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Thierry, H.; Rose, E.; Rogers, H. Landscape Configuration and Frugivore Identity Affect Seed Rain during Restoration. Oikos 2022, 2022, e08323. [Google Scholar] [CrossRef]
- Fedriani, J.M.; Ayllón, D.; Wiegand, T.; Grimm, V. Intertwined Effects of Defaunation, Increased Tree Mortality and Density Compensation on Seed Dispersal. Ecography 2020, 43, 1352–1363. [Google Scholar] [CrossRef]
- Joseph, M.B.; Preston, D.L.; Johnson, P.T.J. Integrating Occupancy Models and Structural Equation Models to Understand Species Occurrence. Ecology 2016, 97, 765–775. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.; Royle, J.; Pollock, K.; Bailey, L.; Hines, J. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier: New York, NY, USA, 2006. [Google Scholar]
- Gould, M.J.; Gould, W.R.; Cain, J.W.; Roemer, G.W. Validating the Performance of Occupancy Models for Estimating Habitat Use and Predicting the Distribution of Highly-Mobile Species: A Case Study Using the American Black Bear. Biol. Conserv. 2019, 234, 28–36. [Google Scholar] [CrossRef]
- Meyer, N.F.V.; Moreno, R.; Reyna-Hurtado, R.; Signer, J.; Balkenhol, N. Towards the Restoration of the Mesoamerican Biological Corridor for Large Mammals in Panama: Comparing Multi-Species Occupancy to Movement Models. Mov. Ecol. 2020, 8, 3. [Google Scholar] [CrossRef]
- Zeller, K.A.; McGarigal, K.; Whiteley, A.R. Estimating Landscape Resistance to Movement: A Review. Landsc. Ecol. 2012, 27, 777–797. [Google Scholar] [CrossRef]
- Evans, B.S.; Kilpatrick, A.M.; Hurlbert, A.H.; Marra, P.P. Dispersal in the Urban Matrix: Assessing the Influence of Landscape Permeability on the Settlement Patterns of Breeding Songbirds. Front. Ecol. Evol. 2017, 5, 63. [Google Scholar] [CrossRef]
- Vasudev, D.; Goswami, V.R.; Oli, M.K. Detecting Dispersal: A Spatial Dynamic Occupancy Model to Reliably Quantify Connectivity across Heterogeneous Conservation Landscapes. Biol. Conserv. 2021, 253, 108874. [Google Scholar] [CrossRef]
- Betts, M.G.; Rodenhouse, N.L.; Scott Sillett, T.; Doran, P.J.; Holmes, R.T. Dynamic Occupancy Models Reveal Within-Breeding Season Movement up a Habitat Quality Gradient by a Migratory Songbird. Ecography 2008, 31, 592–600. [Google Scholar] [CrossRef]
- Schupp, E.W.; Zwolak, R.; Jones, L.R.; Snell, R.S.; Beckman, N.G.; Aslan, C.; Cavazos, B.R.; Effiom, E.; Fricke, E.C.; Montaño-Centellas, F.; et al. Intrinsic and Extrinsic Drivers of Intraspecific Variation in Seed Dispersal Are Diverse and Pervasive. AoB PLANTS 2019, 11, plz067. [Google Scholar] [CrossRef]
- Wall, W.A.; Hohmann, M.G.; Walker, A.S.; Gray, J.B. Sex Ratios and Population Persistence in the Rare Shrub Lindera subcoriacea Wofford. Plant Ecol. 2013, 214, 1105–1114. [Google Scholar] [CrossRef]
- Wall, W.A.; Walker, A.S.; Gray, J.B.; Hohmann, M.G. Fire Effects on the Vital Rates and Stochastic Population Growth Rate of the Rare Shrub Lindera subcoriacea Wofford. Plant Ecol. 2021, 222, 119–131. [Google Scholar] [CrossRef]
- Herrera, J.M.; Morales, J.M.; García, D. Differential Effects of Fruit Availability and Habitat Cover for Frugivore-Mediated Seed Dispersal in a Heterogeneous Landscape. J. Ecol. 2011, 99, 1100–1107. [Google Scholar] [CrossRef]
- Bhakti, T.; Pena, J.C.; Niebuhr, B.B.; Sampaio, J.; Goulart, F.F.; de Azevedo, C.S.; Ribeiro, M.C.; Antonini, Y. Combining Land Cover, Animal Behavior, and Master Plan Regulations to Assess Landscape Permeability for Birds. Landsc. Urban Plan. 2021, 214, 104171. [Google Scholar] [CrossRef]
- Treep, J.; de Jager, M.; Bartumeus, F.; Soons, M.B. Seed Dispersal as a Search Strategy: Dynamic and Fragmented Landscapes Select for Multi-Scale Movement Strategies in Plants. Mov. Ecol. 2021, 9, 4. [Google Scholar] [CrossRef]
- Hanski, I. Habitat Connectivity, Habitat Continuity, and Metapopulations in Dynamic Landscapes. Oikos 1999, 87, 209. [Google Scholar] [CrossRef]
- Griffith, G.; Omernik, J.; Comstock, J.; Schafale, M.; NcNab, W.; Lenat, D.; MacPherson, T.; Glover, J.; Shelburne, V. Ecoregions of North Carolina and South Carolina (U.S. Geological Survey Map); U.S. Geological Survey: Reston, VA, USA, 2002. [Google Scholar]
- Sorrie, B.A.; Gray, J.B.; Crutchfield, P.J. The Vascular Flora of the Longleaf Pine Ecosystem of Fort Bragg and Weymouth Woods, North Carolina. Castanea 2006, 71, 129–161. [Google Scholar] [CrossRef]
- Just, M.G.; Hohmann, M.G.; Hoffmann, W.A. Where Fire Stops: Vegetation Structure and Microclimate Influence Fire Spread along an Ecotonal Gradient. Plant Ecol. 2016, 217, 631–644. [Google Scholar] [CrossRef]
- Hohmann, M.G.; Wall, W.A.; Just, M.G.; Huskins, S.D. Multiple Intrinsic and Extrinsic Drivers Influence the Quantity and Quality Components of Seed Dispersal Effectiveness in the Rare Shrub Lindera subcoriacea. PLoS ONE 2023, 18, e0283810. [Google Scholar] [CrossRef]
- Wall, W.; Just, M.; Huskins, S.; Hohmann, M. Enhancing Rare Plant Population Predictions through Demographic Modeling of Seed Predation, Dispersal, and Habitat Suitability. Plant Ecol. 2023, 225, 63–74. [Google Scholar] [CrossRef]
- NatureServe Lindera subcoriacea. NatureServe Network Biodiversity Location Data Accessed through NatureServe Explorer [Web Application]. NatureServe, Arlington, Virginia. Available online: https://explorer.natureserve.org/Taxon/ELEMENT_GLOBAL.2.133279/Lindera_subcoriacea (accessed on 7 February 2021).
- U.S. Fish and Wildlife Service Endangered and Threatened Wildlife and Plants. Partial 90-Day Finding on a Petition to List 404 Species in the Southeastern United States as Endangered or Threatened with Critical Habitat (FWS–R4–ES–2011–0049). Fed. Regist. 2011, 76, 59836–59862. [Google Scholar]
- Albert, S.; Kaschube, D.R.; Taylor, R. The 2016 Annual Report of the Monitoring Avian Productivity and Survivorship (MAPS) Program on Fort Bragg, North Carolina; The Institute for Bird Populations: Point Reyes Station, CA, USA, 2017; p. 22. [Google Scholar]
- Wall, W.; Hohmann, M.; Wilcox, M. Canopy Cover and Understory Heterogeneity Effects on Post-Breeding Avian Occupancy and Species Richness in a Longleaf Pine Ecosystem under Active Fire Management; ERDC-CERL: Champaign, IL, USA, 2024; To be submitted. [Google Scholar]
- Garcia, D.; Zamora, R.; Amico, G.C. Birds as Suppliers of Seed Dispersal in Temperate Ecosystems: Conservation Guidelines from Real-World Landscapes. Conserv. Biol. 2010, 24, 1070–1079. [Google Scholar] [CrossRef]
- Morales, J.M.; Carlo, T.A. The Effects of Plant Distribution and Frugivore Density on the Scale and Shape of Dispersal Kernels. Ecology 2006, 87, 1489–1496. [Google Scholar] [CrossRef]
- R Foundation for Statistical Computing. R Core Team R (4.1.0): A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bauduin, S.; McIntire, E.J.B.; Chubaty, A.M. NetLogoR: A Package to Build and Run Spatially Explicit Agent-Based Models in R. Ecography 2019, 42, 1841–1849. [Google Scholar] [CrossRef]
- Grimm, V.; Berger, U.; Bastiansen, F.; Eliassen, S.; Ginot, V.; Giske, J.; Goss-Custard, J.; Grand, T.; Heinz, S.K.; Huse, G.; et al. A Standard Protocol for Describing Individual-Based and Agent-Based Models. Ecol. Model. 2006, 198, 115–126. [Google Scholar] [CrossRef]
- Grimm, V.; Berger, U.; DeAngelis, D.L.; Polhill, J.G.; Giske, J.; Railsback, S.F. The ODD Protocol: A Review and First Update. Ecol. Model. 2010, 221, 2760–2768. [Google Scholar] [CrossRef]
- Smith, A.D.; McWilliams, S.R. Fruit Removal Rate Depends on Neighborhood Fruit Density, Frugivore Abundance, and Spatial Context. Oecologia 2014, 174, 931–942. [Google Scholar] [CrossRef]
- Robbins, C.S.S. Effect of Time of Day on Bird Activity. Stud. Avian Biol. 1981, 6, 275–286. [Google Scholar]
- Allen, J.C.; Krieger, S.M.; Walters, J.R.; Collazo, J.A. Associations of Breeding Birds with Fire-Influenced and Riparian-Upland Gradients in a Longleaf Pine Ecosystem. Auk 2006, 123, 1110–1128. [Google Scholar] [CrossRef]
- Alerstam, T.; Rosén, M.; Bäckman, J.; Ericson, P.G.P.; Hellgren, O. Flight Speeds among Bird Species: Allometric and Phylogenetic Effects. PLoS Biol. 2007, 5, 1656–1662. [Google Scholar] [CrossRef]
- Hampe, A. Fruit Tracking, Frugivore Satiation, and Their Consequences for Seed Dispersal. Oecologia 2008, 156, 137–145. [Google Scholar] [CrossRef]
- Krebs, C.J. Some Historical Thoughts on the Functional Responses of Predators to Prey Density. Front. Ecol. Evol. 2022, 10, 1052289. [Google Scholar] [CrossRef]
- Real, L.A. Ecological Determinants of Functional Response. Ecology 1979, 60, 481–485. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Kawakami, K.; Masaki, T. Allometric Scaling of Seed Retention Time in Seed Dispersers and Its Application to Estimation of Seed Dispersal Potentials of Theropod Dinosaurs. Oikos 2019, 128, 836–844. [Google Scholar] [CrossRef]
- Dunning, J., Jr. CRC Handbook of Avian Body Masses; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-4200-6444-5. [Google Scholar]
- Holling, C.S. Cross-Scale Morphology, Geometry, and Dynamics of Ecosystems. Ecol. Monogr. 1992, 62, 447–502. [Google Scholar] [CrossRef]
- Thibault, K.M.; White, E.P.; Hurlbert, A.H.; Ernest, S.K.M. Multimodality in the Individual Size Distributions of Bird Communities. Glob. Ecol. Biogeogr. 2011, 20, 145–153. [Google Scholar] [CrossRef]
- Hohmann, M.G.; Wall, W.A. A Species Distribution Modeling Informed Conservation Assessment of Bog Spicebush. ERDC/CERL TR-16-21; U.S. Army Engineer Research and Development Center: Champaign, IL, USA, 2016. [Google Scholar]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2013; ISBN 978-0-387-95457-8. [Google Scholar]
- Austerlitz, F.; Dick, C.W.; Dutech, C.; Klein, E.K.; Oddou-Muratorio, S.; Smouse, P.E.; Sork, V.L. Using Genetic Markers to Estimate the Pollen Dispersal Curve. Mol. Ecol. 2004, 13, 937–954. [Google Scholar] [CrossRef] [PubMed]
- Nathan, R.; Klein, E.K.; Robledo-Arnuncio, J.; Revilla, E. Dispersal Kernels: Review. In Dispersal Ecology and Evolution; Bullock, J., Clobert, J., Baguette, M., Benton, T., Eds.; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Gilbert, P.; Varadhan, R. The numDeriv Package, R Package Version 2016.8-1.1 2019; Available online: https://cran.r-project.org/web/packages/numDeriv/index.html (accessed on 5 April 2024).
- Pannell, J.R.; Barrett, S.C.H. Baker’s Law Revisited: Reproductive Assurance in a Metapopulation. Evolution 1998, 52, 657–668. [Google Scholar] [CrossRef] [PubMed]
- NatureServe Element Occurrence. Available online: https://help.natureserve.org/biotics/content/record_management/Element_Occurrence/EO_Element_Occurrence.htm (accessed on 26 May 2022).
- North Carolina Natural Heritage Program. Natural Heritage Data Explorer [Web Application]; Lindera subcoriacea; NCDNCR: Raleigh, NC, USA, 2022; Available online: www.ncnhp.org (accessed on 4 April 2022).
- Strimas-Mackey, M.; Brodie, J. Metacapa: Metapopulation Capacity-Based Conservation Prioritization; R Package Version 0.1.0; 2018. Available online: https://strimas.com/metacapa/ (accessed on 5 April 2024).
- Russo, S.E.; Portnoy, S.; Augspurger, C.K. Incorporating Animal Behavior into Seed Dispersal Models: Implications for Seed Shadows. Ecology 2006, 87, 3160–3174. [Google Scholar] [CrossRef] [PubMed]
- King, A.W.; With, K.A. Dispersal Success on Fractal Landscapes: A Consequence of Lacunarity Thresholds. Landsc. Ecol. 1999, 14, 73–82. [Google Scholar]
- Skelsey, P.; With, K.A.; Garrett, K.A. Why Dispersal Should Be Maximized at Intermediate Scales of Heterogeneity. Theor. Ecol. 2013, 6, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Pinder, J.E.; Wiener, J.G.; Smith, M.H. The Weibull Distribution: A New Method of Summarizing Survivorship Data. Ecology 1978, 59, 175–179. [Google Scholar] [CrossRef]
- Clark, J.S.; Macklin, E.; Wood, L. Stages and Spatial Scales of Recruitment Limitation in Southern Appalachian Forests. Ecol. Monogr. 1998, 68, 213–235. [Google Scholar] [CrossRef]
- Cook-Patton, S.C.; LaForgia, M.; Parker, J.D. Positive Interactions between Herbivores and Plant Diversity Shape Forest Regeneration. Proc. R. Soc. B Biol. Sci. 2014, 281, 20140261. [Google Scholar] [CrossRef]
- Lamanna, J.A.; Walton, M.L.; Turner, B.L.; Myers, J.A. Negative Density Dependence Is Stronger in Resource-Rich Environments and Diversifies Communities When Stronger for Common but Not Rare Species. Ecol. Lett. 2016, 19, 657–667. [Google Scholar] [CrossRef]
- Trakhtenbrot, A.; Nathan, R.; Perry, G.; Richardson, D.M. The Importance of Long-Distance Dispersal in Biodiversity Conservation. Divers. Distrib. 2005, 11, 173–181. [Google Scholar] [CrossRef]
- Zhu, J.; Hrušková, K.; Pánková, H.; Münzbergová, Z. Quantifying Patch-Specific Seed Dispersal and Local Population Dynamics to Estimate Population Spread of an Endangered Plant Species. Ecol. Evol. 2021, 11, 14070–14078. [Google Scholar] [CrossRef]
- Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A.; Nathan, R. Long-Distance Seed Dispersal. Annu. Plant Rev. 2009, 38, 204–237. [Google Scholar] [CrossRef]
- Nathan, R.; Schurr, F.M.; Spiegel, O.; Steinitz, O.; Trakhtenbrot, A.; Tsoar, A. Mechanisms of Long-Distance Seed Dispersal. Trends Ecol. Evol. 2008, 23, 638–647. [Google Scholar] [CrossRef]
- Baker, H.G. Self-Compatibility and Establishment After “Long-Distance” Dispersal. Evolution 1955, 9, 347. [Google Scholar] [CrossRef]
- Baker, H.G. Support for Baker’s Law-As a Rule. Evolution 1967, 21, 853. [Google Scholar] [CrossRef]
- Stebbins, G.L. Self Fertilization and Population Variability in the Higher Plants. Am. Nat. 1957, 91, 337–354. [Google Scholar] [CrossRef]
- Heilbuth, J.C.; Ilves, K.L.; Otto, S.P. The Consequences of Dioecy for Seed Dispersal: Modeling the Seed-Shadow Handicap. Evolution 2001, 55, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Vamosi, J.C.; Zhang, Y.; Wilson, W.G. Animal Dispersal Dynamics Promoting Dioecy over Hermaphroditism. Am. Nat. 2007, 170, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, T.S.; Walck, J.L.; Hidayati, S.N. Seed Ecology of Lindera melissifolia (Lauraceae) as It Relates to Rarity of the Species. J. Torrey Bot. Soc. 2011, 138, 298–307. [Google Scholar] [CrossRef]
- Cipollini, M.L.; Whigham, D.; O’Neill, J. Seed Size, Sexual Dimorphism, and Sex Ratio in Lindera benzoin L. (Lauraceae). J. Torrey Bot. Soc. 2013, 140, 300–312. [Google Scholar] [CrossRef]
- Di Musciano, M.; Di Cecco, V.; Bartolucci, F.; Conti, F.; Frattaroli, A.R.; Di Martino, L. Dispersal Ability of Threatened Species Affects Future Distributions. Plant Ecol. 2020, 221, 265–281. [Google Scholar] [CrossRef]
- Cain, M.L.; Milligan, B.G.; Strand, A.E. Long-Distance Seed Dispersal in Plant Populations. Am. J. Bot. 2000, 87, 1217–1227. [Google Scholar] [CrossRef]
- White, D.; Stiles, E. Fruit Harvesting by American Robins: Influence of Fruit Size. Wilson Bull. 1991, 103, 690–692. [Google Scholar]
- Stiles, E.W.; White, D.W. Seed Deposition Patterns: Influence of Season, Nutrients, and Vegetation Structure. In Frugivores and Seed Dispersal; Estrada, A., Fleming, T.H., Eds.; Springer: Dordrecht, The Netherlands, 1986; pp. 45–54. ISBN 978-94-009-4812-9. [Google Scholar]
- Barot, S.; Gignoux, J. How Do Sessile Dioecious Species Cope with Their Males? Theor. Popul. Biol. 2004, 66, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.J.; Bourg, N.A.; Howe, R.; Mcshea, W.J.; Johnson, D.J.; Bourg, N.A.; Howe, R.; Mcshea, W.J.; Wolf, A.; Clay, K. Conspecific Negative Density-Dependent Mortality and the Structure of Temperate Forests. Ecology 2017, 95, 2493–2503. [Google Scholar] [CrossRef]
- Lashley, M.; Chitwood, M.; Harper, C.; DePerno, C.; Moorman, C. Variability in Fire Prescriptions to Promote Wildlife Foods in the Longleaf Pine Ecosystem. Fire Ecol. 2015, 11, 62–79. [Google Scholar] [CrossRef]
- Nelson, A.S.; Whitehead, S.R. Fruit Secondary Metabolites Shape Seed Dispersal Effectiveness. Trends Ecol. Evol. 2021, 36, 1113–1123. [Google Scholar] [CrossRef]
- Kleyheeg, E.; van Leeuwen, C.H.A.; Morison, M.A.; Nolet, B.A.; Soons, M.B. Bird-Mediated Seed Dispersal: Reduced Digestive Efficiency in Active Birds Modulates the Dispersal Capacity of Plant Seeds. Oikos 2015, 124, 899–907. [Google Scholar] [CrossRef]
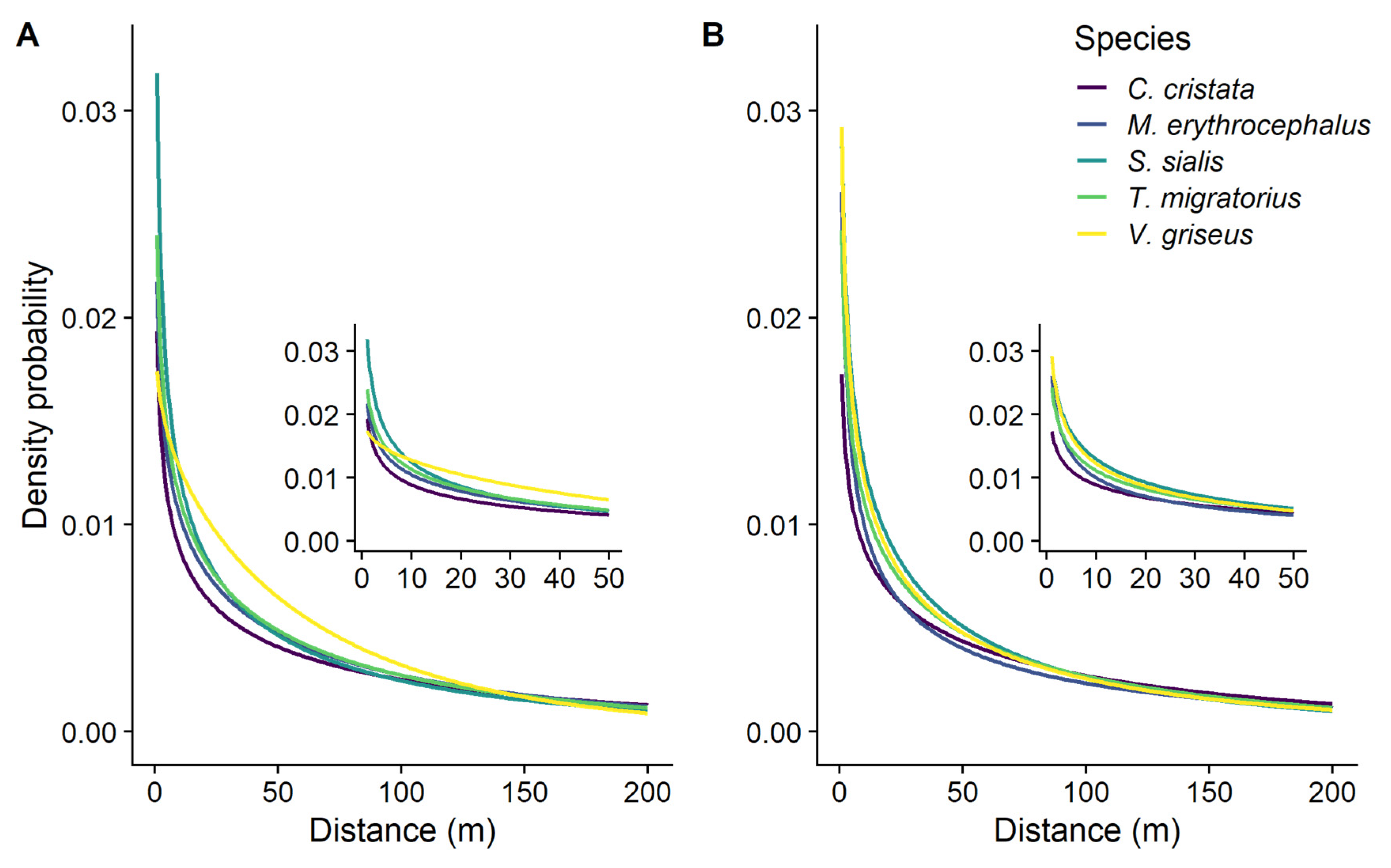
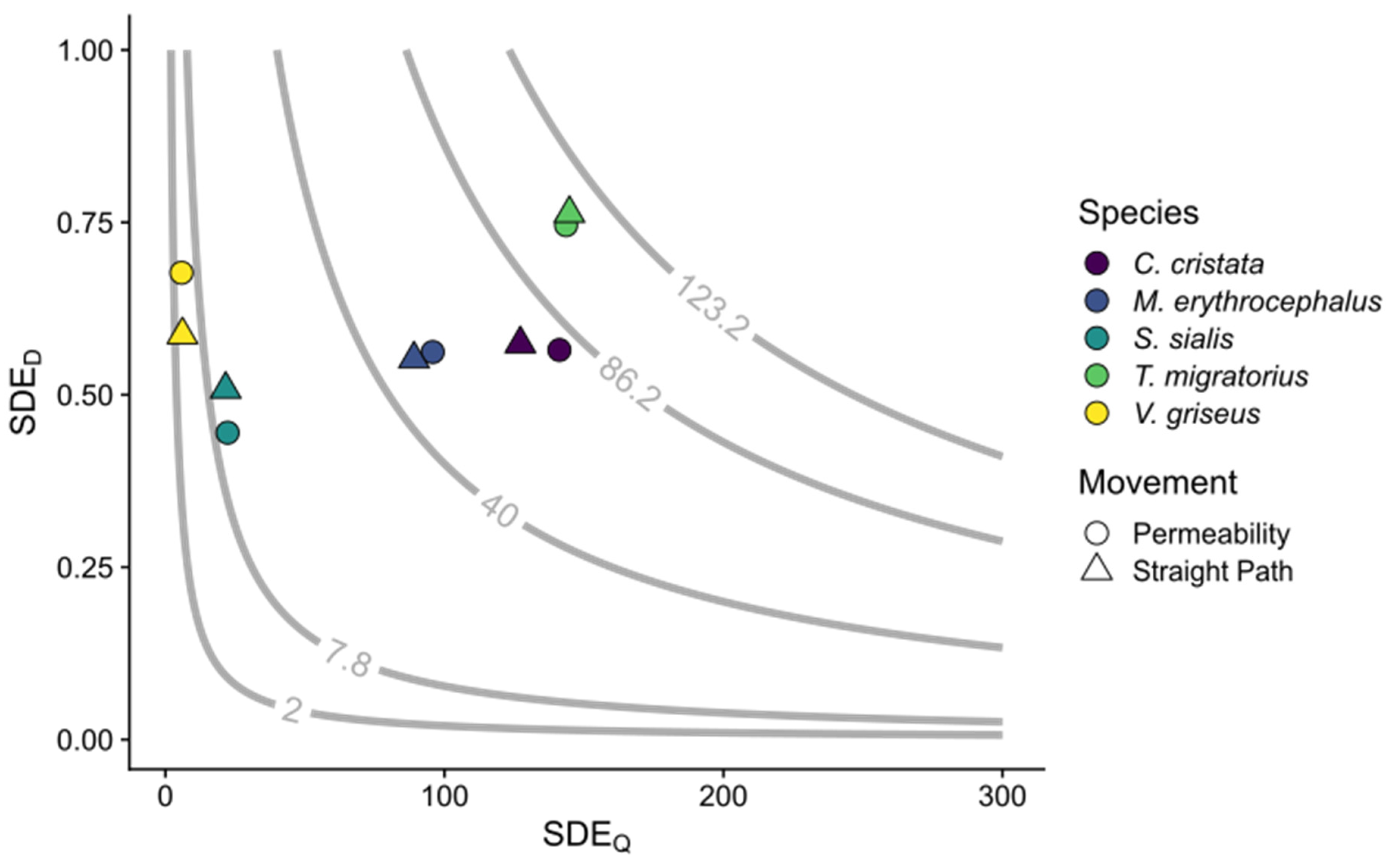
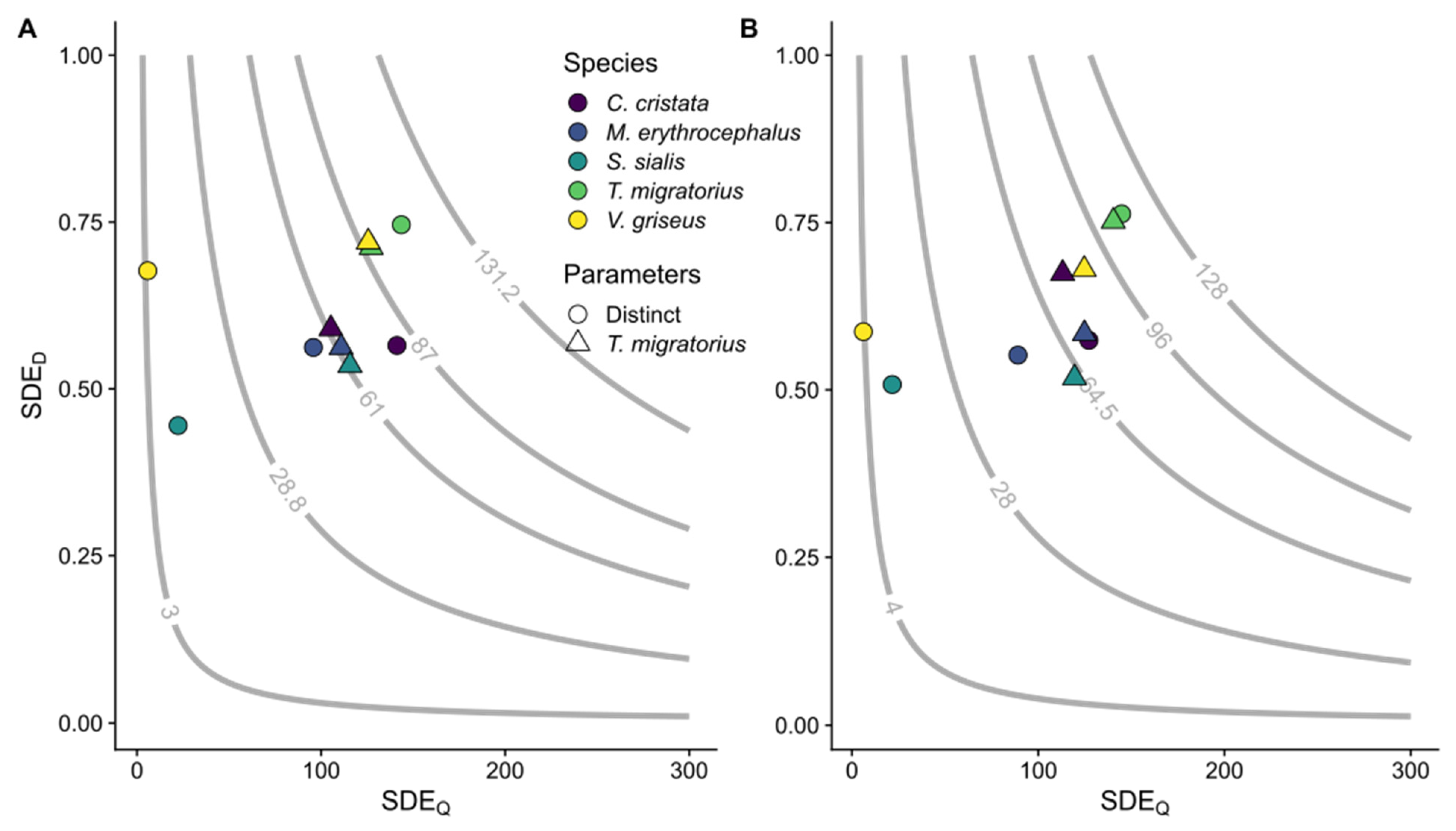
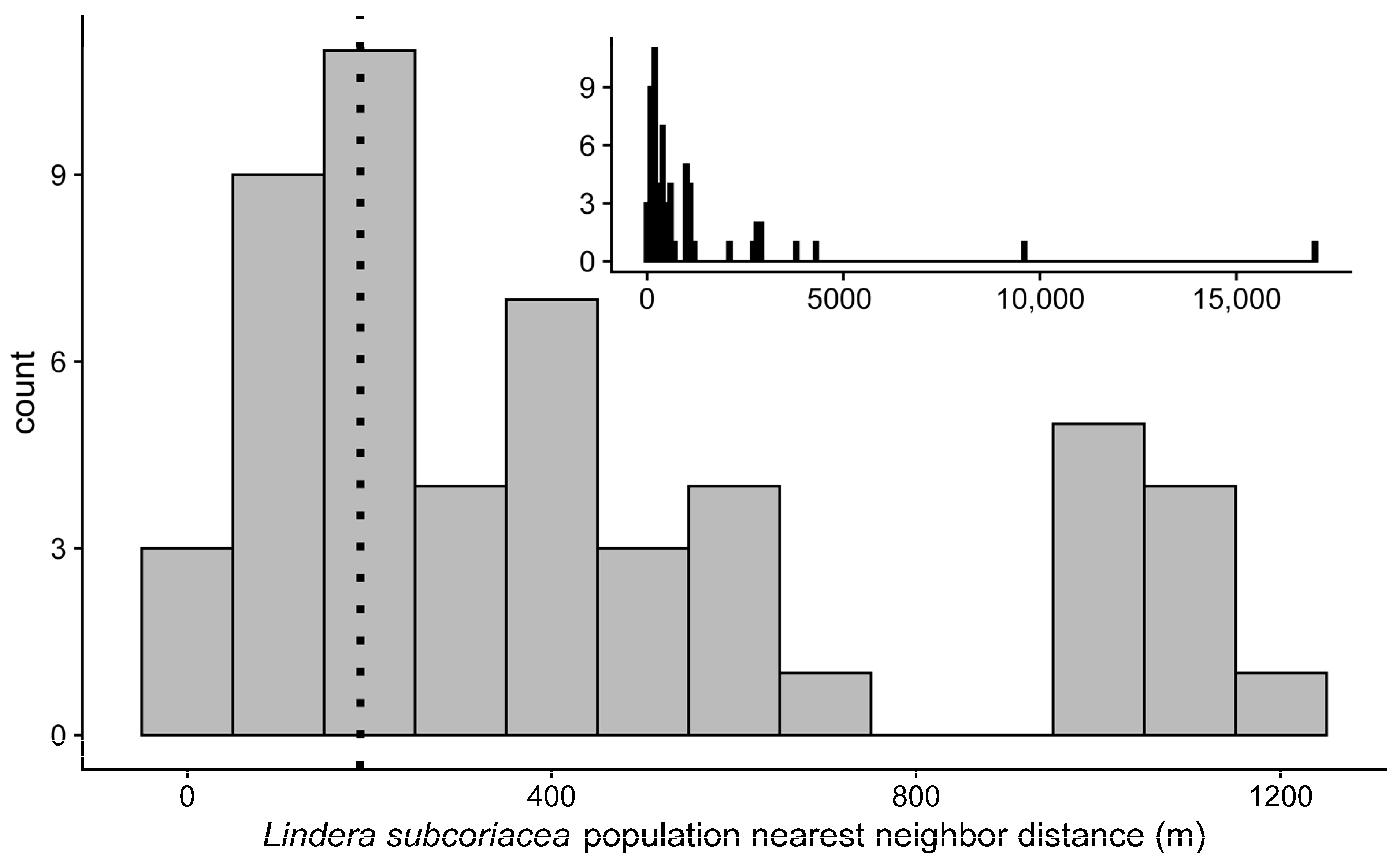
| Species | Body Mass (g) | Maximum Fruit | Gut Capacity | SRT (SD) (min) | Flight Speed (m/min) |
|---|---|---|---|---|---|
| C. cristata | 88.0 | 10 | 15 | 35.8 (17.92) | 695.6 |
| M. erythrocephalus | 69.5 | 8 | 12 | 28.51 (14.27) | 674.5 |
| S. sialis | 27.5 | 4 | 6 | 11.96 (5.99) | 597.9 |
| T. migratorius | 78.5 | 9 | 14 | 32.05 (16.04) | 685.3 |
| V. griseus | 11.4 | 3 | 4 | 5.62 (2.81) | 533.3 |
| Movement | Species | Scale (m) | Shape | Kurtosis |
|---|---|---|---|---|
| Permeability | C. cristata | 152.0 (10.35) | 0.71 (0.02) | 19.3 (2.1) |
| M. erythrocephalus | 111.4 (8.08) | 0.74 (0.02) | 16.5 (1.95) | |
| S. sialis | 86.4 (10.1) | 0.67 (0.03) | 23.8 (4.2) | |
| T. migratorius | 98.1 (6.47) | 0.74 (0.02) | 16.7 (1.77) | |
| V. griseus | 71.1 (9.07) | 0.93 (0.07) | 7.7 (1.88) | |
| Straight path | C. cristata | 141.6 (9.25) | 0.76 (0.02) | 15.3 (1.61) |
| M. erythrocephalus | 134.6 (11.21) | 0.65 (0.02) | 27.8 (3.56) | |
| S. sialis | 78.9 (8.77) | 0.73 (0.03) | 17.8 (3.09) | |
| T. migratorius | 102.1 (6.77) | 0.73 (0.02) | 17.8 (1.89) | |
| V. griseus | 88.6 (14.94) | 0.70 (0.05) | 20.8 (5.26) |
| Permeability | Straight Path | Permeability | Straight Path | |||
|---|---|---|---|---|---|---|
| Species | SDEQ | SDEQ | U | % Disp. | % Disp. | U |
| C. cristata | 135.4 (8.36) a | 122.4 (6.93) ab | 519 | 33.73 (1.57) a | 32.09 (1.49) a | 492 NS |
| M. erythrocephalus | 93.3 (4.91) b | 87.1 (4.24) a | 520.5 | 23.65 (1.16) b | 23.17 (1.08) b | 475 NS |
| S. sialis | 22.1 (1.29) c | 21.4 (1.72) c | 471 | 5.56 (0.27) c | 5.59 (0.42) c | 447.5 NS |
| T. migratorius | 141.4 (5.95) a | 143 (7.05) b | 469.5 | 35.59 (1.25) a | 37.58 (1.47) a | 403 NS |
| V. griseus | 5.7 (0.57) d | 6 (0.55) d | 416.5 | 1.46 (0.16) d | 1.57 (0.14) d | 402 NS |
| Permeability | Straight Path | ||
|---|---|---|---|
| Species | SDED | SDED | U |
| C. cristata | 0.57 (0.03) ab | 0.57 (0.04) a | 431 NS |
| M. erythrocephalus | 0.56 (0.03) ab | 0.55 (0.03) a | 471 NS |
| S. sialis | 0.44 (0.03) a | 0.51 (0.04) a | 339.5 NS |
| T. migratorius | 0.75 (0.02) c | 0.76 (0.02) b | 403 NS |
| V. griseus | 0.68 (0.06) bc | 0.59 (0.07) a | 512 NS |
| Permeability | Straight Path | ||
|---|---|---|---|
| Species | SDE | SDE | U |
| C. cristata | 76.6 (6.06) ab | 73 (6.55) ab | 474.5 NS |
| M. erythrocephalus | 53.7 (4.21) a | 49.8 (4.29) a | 511 NS |
| S. sialis | 9.7 (0.75) c | 10.8 (1.19) c | 430 NS |
| T. migratorius | 104.2 (4.5) b | 107 (4.79) b | 435 NS |
| V. griseus | 3.8 (0.49) c | 3.7 (0.46) c | 443.5 NS |
| Permeability | Straight Path | Permeability | Straight Path | |||
|---|---|---|---|---|---|---|
| Species | Route Dist. (m) | Route Dist. (m) | U | Disp. Dist. (m) | Disp. Dist. (m) | U |
| C. cristata | 320.9 (51.8) a | 270.9 (40.9) ab | 508 | 242.3 (29.97) a | 217.8 (28.66) ab | 509 NS |
| M. erythrocephalus | 230.2 (41.08) a | 302.2 (40.77) a | 333 | 200.5 (34.93) ab | 288.1 (39.42) a | 299 * |
| S. sialis | 278.9 (60.45) a | 233.1 (77.15) cd | 558 | 161.7 (34.15) bc | 135.8 (33.13) cd | 494 NS |
| T. migratorius | 272.6 (43.73) a | 179.3 (34.14) bc | 563 | 165.8 (18.47) ab | 159.7 (25.72) bc | 499 NS |
| V. griseus | 92.4 (17.82) b | 158.3 (62.08) d | 439.5 | 74.4 (14.61) c | 127.5 (43.08) d | 410.5 NS |
| Species | % Not Disp. | % Not Disp. | U | % Flight | % Flight | U |
| C. cristata | 84.3 (0.62) a | 85.8 (0.58) a | 330 | 1.28 (0.51) a | 1.28 (0.48) a | 406 NS |
| M. erythrocephalus | 88.8 (0.49) b | 88.8 (0.53) a | 427 | 0.82 (0.34) a | 0.84 (0.38) a | 460 NS |
| S. sialis | 95.3 (0.25) c | 95.6 (0.35) b | 412 | 3.16 (0.8) a | 3.11 (1.38) a | 504.5 NS |
| T. migratorius | 86.6 (0.53) ab | 86.9 (0.54) a | 421 | 1.13 (0.38) a | 0.4 (0.22) a | 538.5 NS |
| V. griseus | 98.3 (0.16) d | 98.4 (0.14) b | 444.5 | 3.5 (1.99) a | 0.88 (0.66) a | 468 NS |
| Not Suitable | Suitable | Total | ||||
|---|---|---|---|---|---|---|
| Dispersal/Movement | n | Distance (m) | n | Distance (m) | n | Distance (m) |
| Flight | ||||||
| Permeability | 117 | 1735.4 (164.5) | 35 | 1070.0 (85.4) | 152 | 1582.1 (130.0) |
| Straight Path | 94 | 1594.9 (144.6) | 1 | 4626.2 | 95 | 1626.8 (146.6) |
| Perched | ||||||
| Permeability | 4386 | 218.6 (9.9) | 7403 | 155.6 (7.8) | 11,789 | 179.0 (6.1) |
| Straight Path | 3974 | 302.6 (19.1) | 7329 | 136.1 (6.9) | 11,303 | 194.6 (8.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Just, M.G.; Wall, W.A.; Huskins, S.D.; Hohmann, M.G. Effects of Landscape Heterogeneity and Disperser Movement on Seed Dispersal. Ecologies 2024, 5, 198-217. https://doi.org/10.3390/ecologies5020013
Just MG, Wall WA, Huskins SD, Hohmann MG. Effects of Landscape Heterogeneity and Disperser Movement on Seed Dispersal. Ecologies. 2024; 5(2):198-217. https://doi.org/10.3390/ecologies5020013
Chicago/Turabian StyleJust, Michael G., Wade A. Wall, Stacy D. Huskins, and Matthew G. Hohmann. 2024. "Effects of Landscape Heterogeneity and Disperser Movement on Seed Dispersal" Ecologies 5, no. 2: 198-217. https://doi.org/10.3390/ecologies5020013






