Comparing Energetics and Physiological Trait Patterns of North American Birds to Support Ecological Risk Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source, Selection Criteria, and Guild Classification
2.2. DEB Model, Parameters, and Traits
2.3. Multidimensional Scaling (MDS)
2.4. Software
3. Results and Discussion
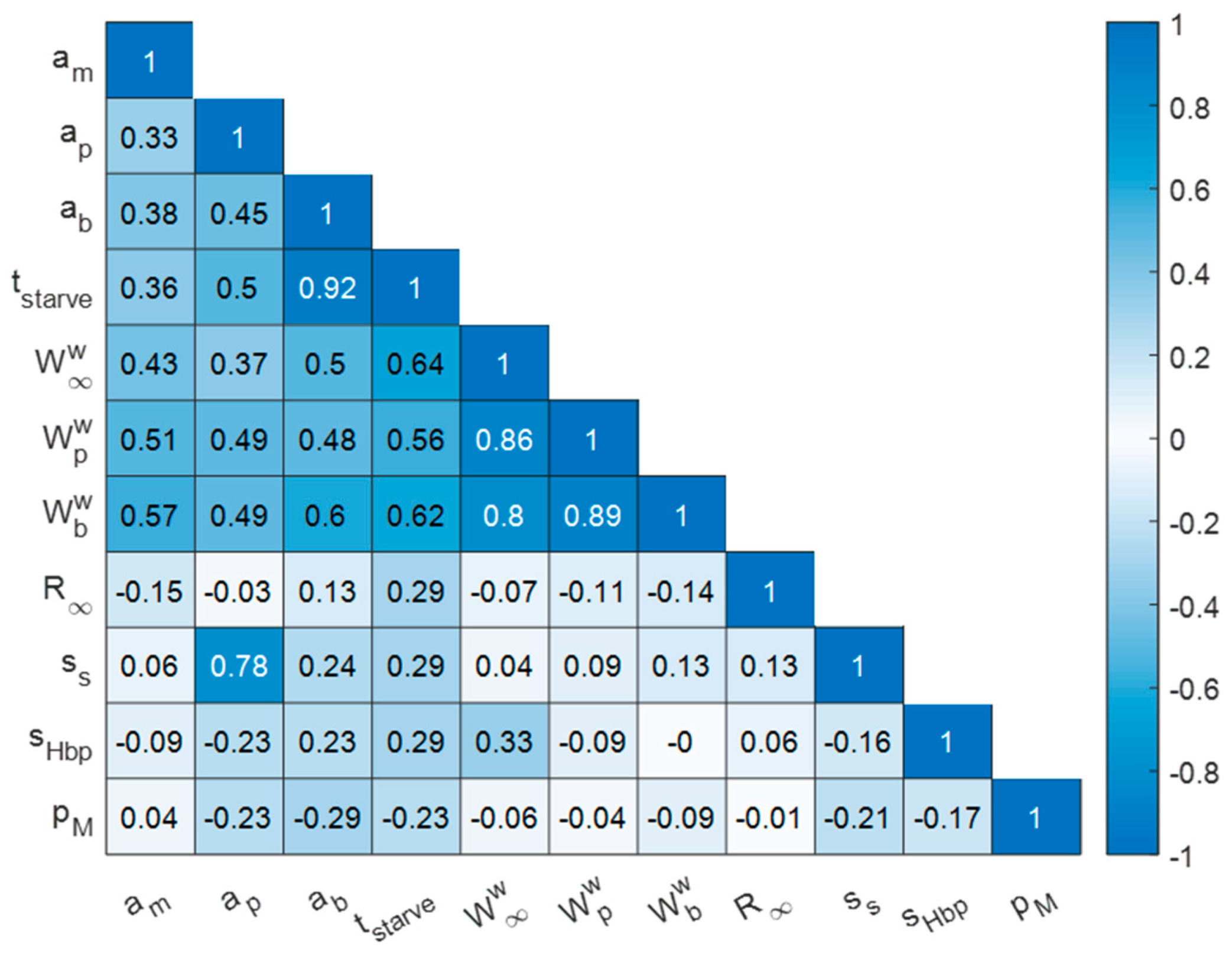
3.1. Quality of AmP Entries
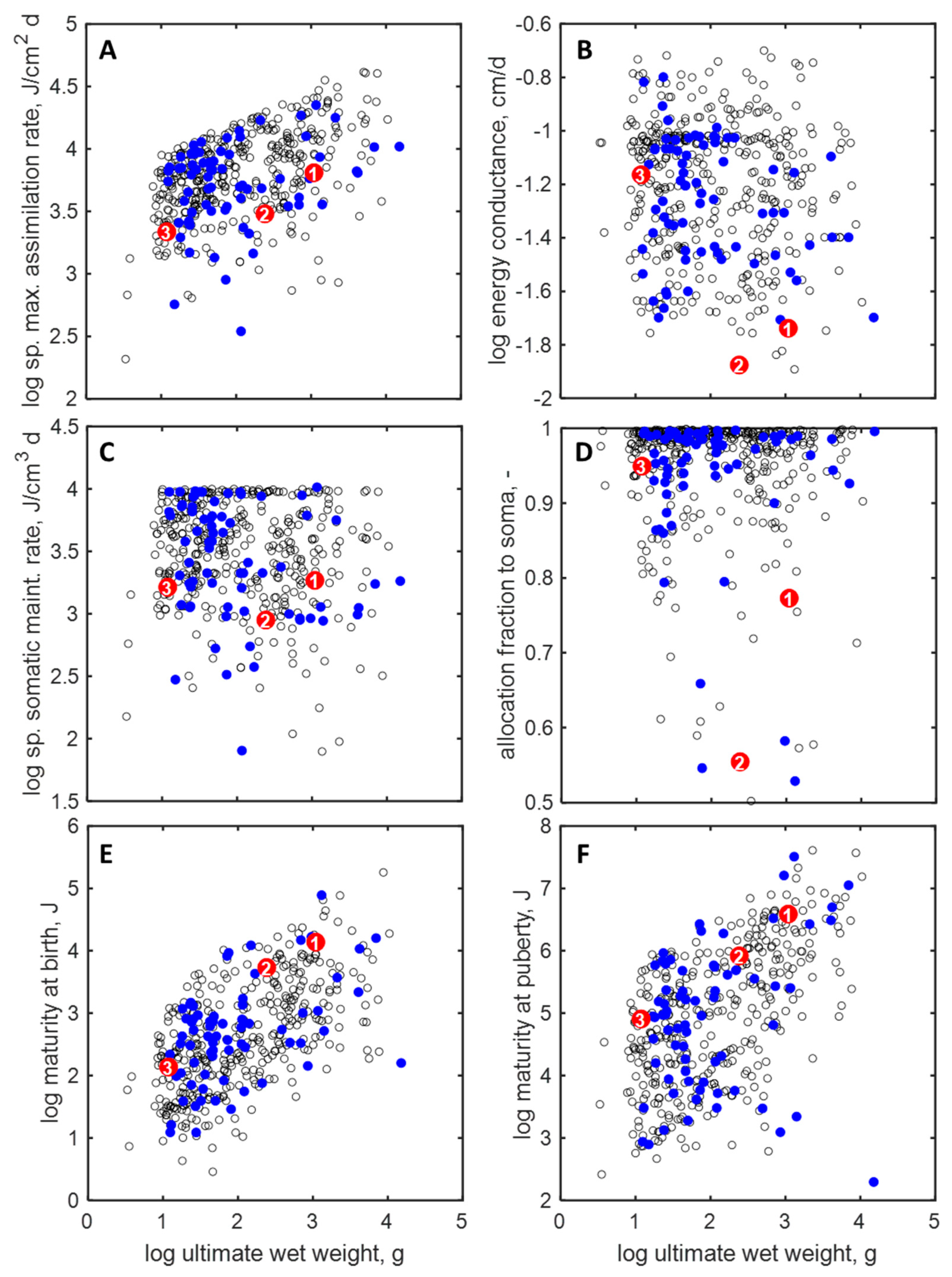
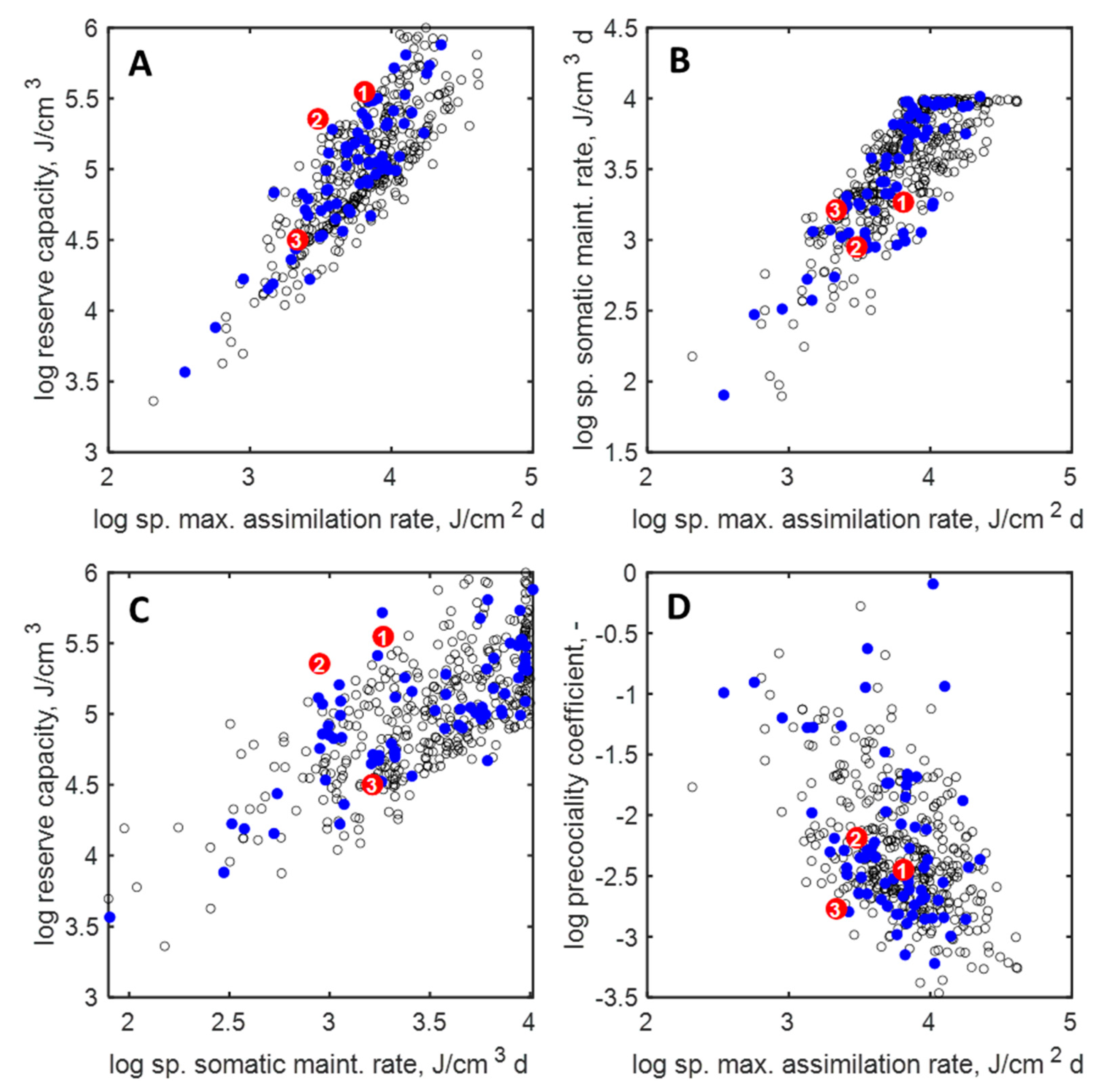
3.2. Parameters and Traits of Terrestrial Holarctic Birds
3.3. Parameters and Traits of Species of ERA Interest and Laboratory Species
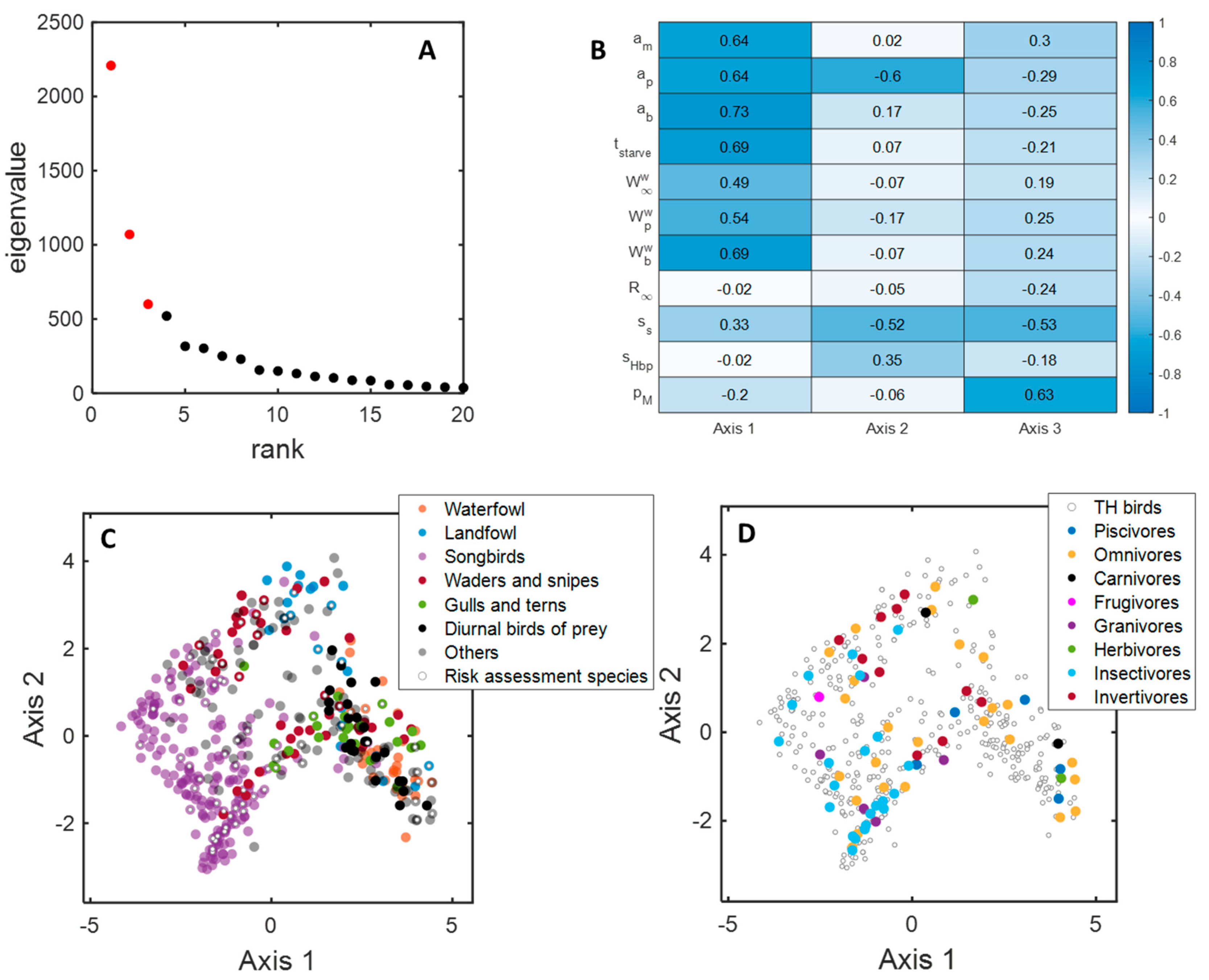
3.4. MDS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD. Test No. 206: Avian Reproduction Test; Organisation for Economic Co-Operation and Development: Paris, France, 1984; Available online: https://www.oecd-ilibrary.org/environment/test-no-206-avian-reproduction-test_9789264070028-en (accessed on 14 September 2022).
- US EPA. Ecological Effects Test Guidelines OCSPP 850.2300: Avian Reproduction Test; Guideline EPA-HQ-OPPT-2009-0154-0012; US EPA: Washington, DC, USA, 2012; pp. 1–27. [Google Scholar]
- EFSA; Aagaard, A.; Berny, P.; Chaton, P.-F.; Antia, A.L.; McVey, E.; Arena, M.; Fait, G.; Ippolito, A.; Linguadoca, A.; et al. Risk assessment for Birds and Mammals. EFSA J. 2023, 21, e07790. [Google Scholar] [CrossRef]
- Galic, N.; Forbes, V.E.; Grimm, V.; Schmolke, A.; Vaugeois, M.; Brain, R.A. Ecological risk assessment when species-specific data are scarce: How trait-based approaches and modeling can help. BioScience, in press.
- Moore, A.P.; Galic, N.; Brain, R.A.; Hornbach, D.J.; Forbes, V.E. Validation of freshwater mussel life-history strategies: A database and multivariate analysis of freshwater mussel life-history traits. Aquat. Conserv. 2021, 31, 3386–3402. [Google Scholar] [CrossRef]
- Schmolke, A.; Galic, N.; Feken, M.; Thompson, H.; Sgolastra, F.; Pitts-Singer, T.; Elston, C.; Pamminger, T.; Hinarejos, S. Assessment of the Vulnerability to Pesticide Exposures Across Bee Species. Environ Toxic Chem. 2021, 40, 2640–2651. [Google Scholar] [CrossRef] [PubMed]
- Etterson, M.A.; Bennett, R.S.; Kershner, E.L.; Walk, J.W. Markov chain estimation of avian seasonal fecundity. Ecol. Appl. 2009, 19, 622–630. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Markov Chain Nest Productivity Model. 2017. Available online: https://www.epa.gov/chemical-research/markov-chain-nest-productivity-model (accessed on 1 June 2023).
- Nisbet, R.M.; Muller, E.B.; Lika, K.; Kooijman, S.A.L.M. From molecules to ecosystems through dynamic energy budget models. J. Anim. Ecol. 2000, 69, 913–926. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M. Dynamic Energy Budget Theory for Metabolic Organisation; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Jusup, M.; Sousa, T.; Domingos, T.; Labinac, V.; Marn, N.; Wang, Z.; Klanjšček, T. Physics of metabolic organization. Phys. Life Rev. 2017, 20, 1–39. [Google Scholar] [CrossRef]
- Jager, T.; Goussen, B.; Gergs, A. Using the standard DEB animal model for toxicokinetic-toxicodynamic analysis. Ecol. Model. 2023, 475, 110187. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M. Energy budgets can explain body size relations. J. Theor. Biol. 1986, 121, 269–282. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M.; Lika, K.; Augustine, S.; Marn, N. Multidimensional scaling for animal traits in the context of dynamic energy budget theory. Conserv. Physiol. 2021, 9, coab086. [Google Scholar] [CrossRef]
- Add My Pet Portal. Available online: http://www.bio.vu.nl/thb/deb/deblab/add_my_pet/ (accessed on 24 October 2022).
- Lika, K.; Augustine, S.; Kooijman, S.A.L.M. The comparative energetics of the ray-finned fish in an evolutionary context. Conserv. Physiol. 2022, 10, coac039. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M.; Augustine, S. The comparative energetics of the carnivorans and pangolins. Conserv. Physiol. 2022, 10, coac052. [Google Scholar] [CrossRef]
- Marn, N.; Kooijman, S.A.L.M. The comparative energetics of the turtles and crocodiles. Ecol. Evol. 2022, 12, e8996. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M.; Augustine, S. The comparative energetics of the cephalopods: They neither grow nor reproduce fast. J. Sea Res. 2022, 184, 102205. [Google Scholar] [CrossRef]
- Kooijman, S.A.L.M. Waste to hurry: Dynamic energy budgets explain the need of wasting to fully exploit blooming resources. Oikos 2013, 122, 348–357. [Google Scholar] [CrossRef]
- Lika, K.; Augustine, S.; Pecquerie, L.; Kooijman, S.A.L.M. The bijection from data to parameter space with the standard DEB model quantifies the supply–demand spectrum. J. Theor. Biol. 2014, 354, 35–47. [Google Scholar] [CrossRef]
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. Birds of the World. Available online: https://birdsoftheworld.org/bow/home (accessed on 24 October 2022).
- Bennett, R.S.; Etterson, M.A. Estimating Pesticide Effects on Fecundity Rates of Wild Birds Using Current Laboratory Reproduction Tests. Hum. Ecol. Risk Assess. Int. J. 2006, 12, 762–781. [Google Scholar] [CrossRef]
- Mineau, P. A Review and Analysis of Study Endpoints Relevant to the Assessment of “Long Term” Pesticide Toxicity in Avian and Mammalian Wildlife. Ecotoxicology 2005, 14, 775–799. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.S.; Etterson, M.A. Incorporating Results of Avian Toxicity Tests into a Model of Annual Reproductive Success. Integr. Environ. Assess. Manag. 2007, 3, 498. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P.R.; Dobkin, D.S.; Wheye, D. The Birder’s Handbook: A Field Guide to the Natural History of North American Birds: Including All Species That Regularly Breed North of Mexico; Simon & Schuster: New York, NY, USA, 1988; ISBN 978-0-671-62133-9. [Google Scholar]
- Lika, K.; Kearney, M.R.; Freitas, V.; van der Veer, H.W.; van der Meer, J.; Wijsman, J.W.M.; Pecquerie, L.; Kooijman, S.A.L.M. The “covariation method” for estimating the parameters of the standard Dynamic Energy Budget model I: Philosophy and approach. J. Sea Res. 2011, 66, 270–277. [Google Scholar] [CrossRef]
- Marques, G.M.; Augustine, S.; Lika, K.; Pecquerie, L.; Domingos, T.; Kooijman, S.A.L.M. The AmP project: Comparing species on the basis of dynamic energy budget parameters. PLoS Comput. Biol. 2018, 14, e1006100. [Google Scholar] [CrossRef]
- Add My Pet Parameter Estimation. Available online: https://debportal.debtheory.org/docs/AmPestimation.html#Introduction (accessed on 12 June 2024).
- AmPTool. 2023. Available online: https://github.com/addmy-pet/AmPtool (accessed on 31 January 2023).
- Ducatez, S.; Field, D.J. Disentangling the avian altricial-precocial spectrum: Quantitative assessment of developmental mode, phylogenetic signal, and dimensionality. Evolution 2021, 75, 2717–2735. [Google Scholar] [CrossRef] [PubMed]

| Primary DEB parameters 1 | ||
| J | Maturity threshold of birth | |
| J | Maturity threshold of puberty | |
| J cm−2 | Surface-area-specific maximum assimilation rate (assimilation capacity) | |
| J cm−3 | Volume-specific somatic maintenance rate | |
| cm day−1 | Energy conductance (measures how fast ingested food is made available for production, maintenance and maturation) | |
| - | Allocation fraction to soma (fraction of mobilized energy reserve allocated to growth and somatic maintenance) | |
| Secondary parameters and traits 2 | ||
| day | Age at birth (counted from time of egg fertilization) | |
| day | Maximum age | |
| day | Age at puberty 3 | |
| J cm−3 | Reserve capacity | |
| day−1 | Von Bertalanffy growth rate | |
| # day−1 | Maximum reproduction rate | |
| - | Precociality coefficient (ratio of maturity thresholds of birth and puberty) | |
| - | Supply stress (measures maintenance requirements relative to assimilation capacity) | |
| day | Maximum starvation time (time to deplete energy reserve in well-fed, full grown bird if only used for somatic maintenance demands) | |
| g | Wet weight at birth | |
| g | Wet weight at puberty | |
| g | Ultimate wet weight (weight of a full-grown, well-fed adult) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muller, E.B.; Romoli, C.; Goussen, B.; Maul, J.D.; Brain, R.; Galic, N. Comparing Energetics and Physiological Trait Patterns of North American Birds to Support Ecological Risk Assessment. Ecologies 2024, 5, 354-367. https://doi.org/10.3390/ecologies5030022
Muller EB, Romoli C, Goussen B, Maul JD, Brain R, Galic N. Comparing Energetics and Physiological Trait Patterns of North American Birds to Support Ecological Risk Assessment. Ecologies. 2024; 5(3):354-367. https://doi.org/10.3390/ecologies5030022
Chicago/Turabian StyleMuller, Erik B., Carlo Romoli, Benoit Goussen, Jonathan D. Maul, Richard Brain, and Nika Galic. 2024. "Comparing Energetics and Physiological Trait Patterns of North American Birds to Support Ecological Risk Assessment" Ecologies 5, no. 3: 354-367. https://doi.org/10.3390/ecologies5030022
APA StyleMuller, E. B., Romoli, C., Goussen, B., Maul, J. D., Brain, R., & Galic, N. (2024). Comparing Energetics and Physiological Trait Patterns of North American Birds to Support Ecological Risk Assessment. Ecologies, 5(3), 354-367. https://doi.org/10.3390/ecologies5030022






