Abstract
The Panchthar–Ilam–Taplejung Corridor in Eastern Nepal, managed through community forestry, is a crucial habitat for the Himalayan red panda, an endangered carnivore threatened by forest degradation and illegal trade. We deployed the altitude line intercept and ten-tree plotless methods to evaluate the distribution of Himalayan red pandas and the environmental factors affecting them within four community forests, namely Singhadevi, Chitre-Hile, Chhipchhipe, and Kalikhop-Dadehli, of the corridor. We established a total of 23 transects and 92 plots, identifying 41 plots with evidence of the Himalayan red panda’s presence. The sign occurrence revealed a clumped distribution of the species across all four community forests. The Himalayan red panda signs were observed between 2200 m and 2700 m above sea level (asl) and the majority of them were from habitats with a moderate slope within elevations of 2400 m to 2500 m asl. The primary sites for the defecation were large horizontal tree branches (78.12%), followed by forest ground (15.62%) and rocks (6.25%). The dominant tree species in their habitats included Lithocarpus pachyphylla (Importance value index, IVI = 45.05), Symplocus theifolia (IVI = 37.19), Symplocos pyrifolia (IVI = 20.99), Quercus lamellosa (IVI = 19.25), and Magnolia campbellii (IVI = 17.25). Among the thirteen environmental variables examined, proximity to water, distance to road, bamboo density, and Normalized Difference Vegetation Index were identified as the major factors influencing the Himalayan red panda’s distribution. This research provides crucial insights to develop site-specific habitat management plans for community forestry.
1. Introduction
Red pandas are the only remaining representatives of the family Ailuridae, order Carnivora, and have adapted to the herbivorous diet, primarily bamboo [1,2]. Of the two recently recognized species based on genomic analysis [3,4,5,6], the Himalayan red panda (Ailurus fulgens) is found west of the Yalu Zangbu River and distributed in the southern part of Tibet, India, Bhutan, and Nepal [5,7,8]. The Chinese red panda (Ailurus styani) occurs east of the Yalu Zangbu River and is distributed in China, Myanmar, and India [3,5]. Due to the loss of habitats and hunting for trade, the population of red pandas has plausibly declined by 50% over the last three generations and the decline is projected to continue [9,10].
Nepal holds a large portion of the distribution range of the Himalayan red panda, where they occur in fragmented populations with a low genetic diversity [5,7,11,12]. The species is distributed across 27 districts, including protected areas such as the Rara National Park (NP), Langtang NP, Sagarmatha NP, and Makalu Barun NP, Dhorpatan Hunting Reserve, Annapurna Conservation Area (CA), Manaslu CA, Gaurishankar CA, and Kanchenjunga CA [2,11,13]. It is listed as an endangered (EN) species in the IUCN Red List of Threatened Species and the National Red List of Nepal, and it is legally protected in Nepal by the National Park and Wildlife Conservation Act of 1973 [10,13,14].
Due to variations in climate and topography, the habitat of red panda includes different vegetation compositions, including evergreen forests, evergreen and deciduous mixed broad-leaved forests, deciduous forests, deciduous and coniferous mixed forests, and coniferous forests with associated bamboo thicket understories [11,15,16,17,18]. Several microhabitat characteristics create suitable habitat conditions for the red pandas, including a sparse understory, fallen logs, fruit-bearing shrubs, bamboo cover, proximity to water sources, and canopy cover [11,15,19,20,21,22,23,24]. They frequently defecate in tree branches and other substrates such as rocks, forest ground, fallen logs, and cut stumps [19,25,26]. Tall trees with hollow trunks, such as Rhododendron spp., are preferred to escape predators and bask in the winter. Hardwood species, such as Viburnum erubescens, Symplocos pyrifolia, Magnolia campbellii, Quercus lamellosa, Lindera pulcherrima, Lithocarpus elegans, Machilus edulis, Eurya acuminata, Litsea salicifolia, and Acer sp., are other preferred vegetations in the habitat of the Himalayan red panda.
The Himalayan red panda population in Nepal is declining due to habitat loss and degradation, poaching and illegal trade, effects of development activities, and illegal collection of herbal plants [11,13,25]. The Panchthar-Ilam-Teplejung (PIT) Corridor in Eastern Nepal is assumed to support 25% of the Himalayan red panda population of the country and plays a vital role in linking the protected areas of Nepal and India [27,28,29]; however, the habitat ecology of the species is still understudied [29,30,31,32,33]. Agriculture and resource harvesting practices, including livestock grazing, are the major drivers of deforestation and habitat degradation in the PIT Corridor [30,32]. Poaching is increasing in this region due to the habitat in the cross-border area [34]. Information on the status, ecology, and causes of population decline is limited, particularly outside the protected areas of Nepal [11,26,35]. Habitats of the Himalayan red panda outside the protected area are at a higher risk due to human-induced activities [24,26,36]. The Ilam district of Eastern Nepal holds one of the major habitats of the Himalayan red panda. Still, habitat-associated information at a fine scale is insufficiently documented from the community forests, which hinders the implementation of effective conservation measures by the community forestry user groups at the local scale [33]. Here, we assessed the distribution and environmental variables affecting the abundance of the Himalayan red panda in the community forests of the PIT Corridor. The findings provide information on the habitat characteristics of the Himalayan red panda, which plays a crucial role in creating community forest corridors to link habitats into the protected area system in Eastern Nepal. This information will contribute to policymakers in developing effective management plans and conservation strategies in Nepal.
2. Materials and Methods
2.1. Study Area
This study was conducted in Eastern Nepal’s PIT Corridor encompassing the Panchthar, Ilam, and Taplejung districts. The Ilam district is a major PIT community forest Corridor in Eastern Nepal that borders Panchthar in the northwest, Morang in the southwest, Jhapa in the southeast, and the Darjeeling district of India in the east (Figure 1). The study site is extended from the Suryodaya Municipality to Mai Jogmai Municipality (88°04′–88°58′ E, 26°54′–27°15′ N) of the Ilam District. An intensive study was conducted in four community forests, which included Singhadevi (88°5′ E, 27°00′ N; 2100–2400 m above sea level, asl), Chitre-Hile (88°04′ E, 26°59′ N; 2300–2500 m asl), Chhipchhipe (88°5′ E, 27°00′ N; 2000–2700 m asl), and the Kalikhop-Dadehli, first and second blocks (88°03’ E, 27°01′ N; 2000–2600 m asl). The study area covered 13.42 km2, ranging between 2000 m and 2700 m asl. The study area consists of lower temperate broad-leaved forests and upper temperate broad-leaved forests [26,33]. The forests of the Eastern Himalayas are dominated by deciduous conifer and broad-leaved forest (Quercus sp., Lauraceae sp.) in the lower part (2000 m and 2500 m asl) and a mixture of evergreen conifers (Tsuga sp., Taxus sp.) and deciduous broad-leaf species (Acer sp., Betula sp., and Magnolia sp.) in the upper part (2500 m and 3000 m asl). Bamboo (Arundinaria sp.) is an important understory species. The major faunas are the leopard (Panthera pardus), clouded leopard (Neofelis nebulosa), Himalayan black bear (Ursus thibetanus), leopard cat (Prionailurus bengalensis), Asiatic golden cat (Catopuma temminckii) [37], and Himalayan red panda [33]. Prominent bird species include the yellow-vented warbler (Phylloscopus cantator), rufous-throated wren babbler (Spelaeornis caudatus), spiny babbler (Turdoides nipalensis), hoary-throated barwing (Actinodura nipalensis), and Asian fairy bluebird (Irena puella) [38]. The mountain horned frog (Megophrys monticola), Sikkim high altitude toad (Scutiger sikimmensis) Assam torrent frog (Amolops formosus), Himalayan tree frog (Polypedates himalayensis) and Himalayan bubble nest frog (Raorchestes annandalii) are the amphibians distributed in the area.
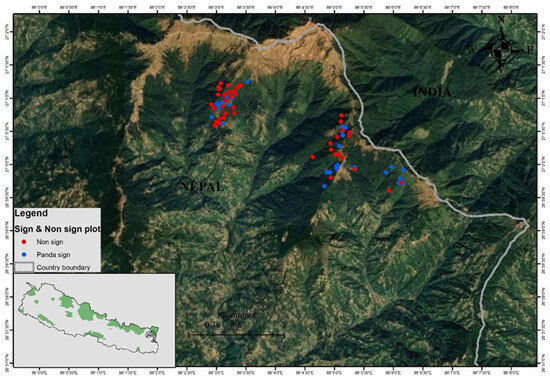
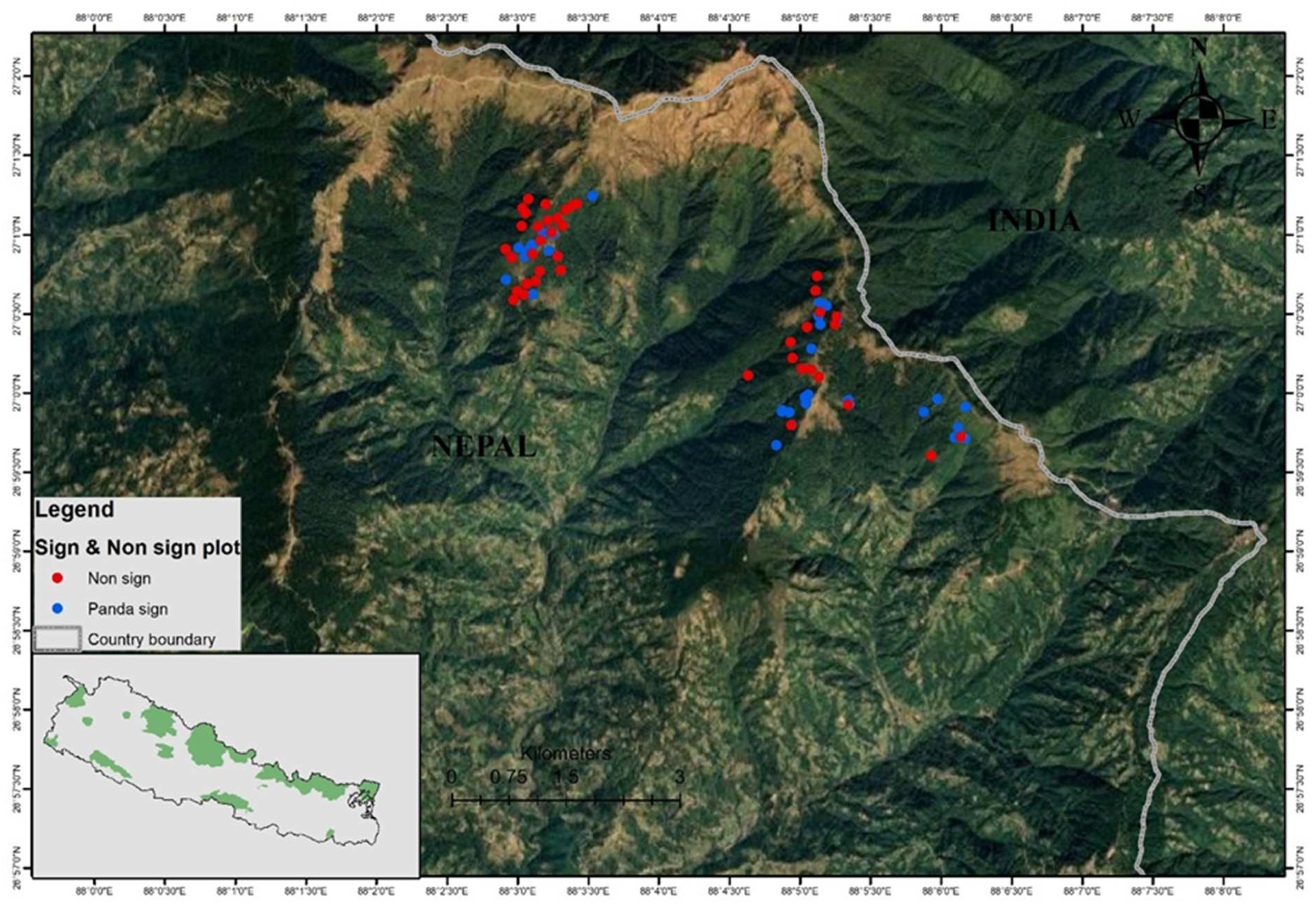
Figure 1.
Map showing the study area. Black dots indicate non-sign plots and blue dots represent occurrence records of Himalayan red pandas (sign plots) in the community forests.
2.2. Field Survey
The distribution and habitat composition of the Himalayan red pandas were determined using the altitudinal line intercept method. The survey was conducted between 2100 m and 2741 m asl. Due to topographic steepness and rocky cliff, the length of transects ranged between 0.5 km and 1.5 km with a width of 10 m. Twenty-three transects were established across the existing trails, four in Singhadevi, three in Chitre-Hile, six in Chhipchhipe, five in Kalikhop-Dadehli, first block, and five in Kalikhop-Dadehli, second block. Surveys were conducted along those transects in search of direct observation and indirect signs (fecal pellets) of the Himalayan red panda. Fecal pellets were used as indirect evidence because they are considered a valid indicator of presence in the area and are distinct from other species [11,15,22]. When evidence of the Himalayan red panda was encountered, a Garmin eTrex® 10 GPS was used to record the latitude and longitude of the location.
2.3. Ten-Tree Plotless Method
The ten-tree plotless method [39] was used to assess the habitat characteristics of the Himalayan red panda. The study plots were categorized into two types as follows: (i) sign plots where the Himalayan red panda signs were found, and (ii) no-sign plots where the Himalayan red panda signs were not observed. When a red panda sign was encountered in a transect, a sign plot was laid out using the observed substrate as the plot center. The plot area was determined by using the average distance from the center to the tenth and eleventh trees as the radius of the plot [39]. A no-sign plot/pseudoabsence plot was systematically established at 200 m intervals from the sign plot along the transect adopted from Williams [40]. The habitat parameters, including elevation, bamboo cover, bamboo density, bamboo height, bamboo cutting, canopy cover, distance to water source, effects of livestock grazing, shrub density, slope, and tree height at breast, were recorded in the field. The number of fecal pellet groups and the type of substrate used for defecation were also recorded in each plot.
Sampling for the vegetation composition was carried out using quadrats of different sizes. The quadrats used for shrub sampling were 3 m × 3 m and were established to measure the shrubs’ density, cover, and frequency (woody plants below 3 m in height) within each ten-tree plot. Similarly, 1 m × 1 m quadrates were established for herbs (plants up to 1 m in height) in the center and each of the four corners within the same plot to determine the density, frequency, species, height, and cover of bamboo. Additionally, based on the coverage of bamboo in a 1 m × 1 m plot, the bamboo cover in the plot was estimated by counting the number of culms. Apart from the understory covers of bamboo and shrub cover, we extracted the Normalized Difference Vegetation Index (NDVI) for vegetation indices as the proxy for canopy cover (https://land.copernicus.eu/en, accessed on 10 March 2024). Vegetation was identified using a reference book of flowering plants in Nepal [41].
2.4. Data Analysis
Sign plot locations were converted into a data layer and that was used to create a distribution map in ArcGIS 10.2. To determine the distribution pattern of the Himalayan red panda fecal pellet groups, variance to mean ratio [42] was used. A chi-squared goodness-of-fit test was performed to determine which habitat variables influence the Himalayan red panda distribution. The vegetation between sign plots and no-sign plots was studied by calculating Sorensen’s Index of Similarity. The relative abundance of trees was measured using the Shannon–Wiener Index (H) and species dominance using Simpson’s index of dominance. Student’s t-test was used to determine the significance of the differences in H-values obtained for the sign and no-sign plots. The Importance Value Index (IVI) of each tree species was calculated for both the sign and no-sign plots to identify the important tree species. IVI was calculated as the sum of relative density, relative frequency, and relative dominance of trees [39]. A digital elevation model (DEM) was created using SRTM-DEM data (https://www.earthdata.nasa.gov/sensors/srtm, accessed on 10 March 2024) in ArcGIS 10.2. The slope, aspect, and distance to water sources were measured in the ArcGIS 10.2 version. Twelve different environmental variables were recorded as follows: elevation, aspect, bamboo cover, bamboo density, bamboo height, bamboo cutting, distance to water source, effects of livestock grazing, shrub density, slope, and tree diameter at breast height. A multicollinearity test was performed among the environmental variables and because none of the variable pairs were highly correlated r ≥ |0.7| (Figure S1), all of them were used for further analysis [43]. Firstly, we performed the generalized linear model in the global environment using Himalayan red panda signs as a response and all the environmental variables as predictor variables. Secondly, logistic regression modeling using a binomial distribution was performed to model the red panda habitat use as a function of habitat covariates that were significant in the global model. The best-fit model was investigated by multi-model inference using a ‘dredge’ function in the ‘MuMIN’ package [44]. The fit of the candidate models was examined by selecting the lowest Akaike’s information criterion corrected (AICc) for small sample sizes, and the final models were restricted to ΔAICc < 2 for habitat use variables before model averaging with the Akaike weights [45]. All the analyses were performed using R 4.3.1.
3. Results
3.1. Distribution and Abundance of the Himalayan Red Panda
The Himalayan red panda occurrence was confirmed in Gorkhe and Jogmai in the Ilam district, which is a major portion of the PIT Corridor in Eastern Nepal. Out of the total of 92 plots, Himalayan red panda signs were recorded in 41 plots across the 23 transects. Three instances of Himalayan red panda sightings and 32 fecal pellet groups were encountered in the transects (approximately 15 km) of the 41 plots, totaling 51.56% of detected signs. Eight transects (approximately 8 km) in the upper temperate mixed broad-leaf forest (2500 m–2741 m) yielded 48.43% of the detected signs (31 fecal pellet groups). Fecal pellet groups were encountered at an altitude range of between 2200 m and 2700 m asl, with a higher frequency (68.25%) between 2400 and 2500 m (Figure S2). The highest encounter rate was 5.75/km between 2500 and 2599 m, and no fecal pellet groups were recorded in the lower altitudinal range (between 2100 and 2199 m) (Figure S3). Fecal pellet groups illustrated a clumped distribution (S2/X− = 3.91 > 1, χ2 = 0.031 < χ20.05) pattern. Among 133 plots, 30.83% (n = 41) contained at least one fecal pellet group. The average sign encounter rate ranged from 0.4/km in Kalikhop-dadheli to 4/km in Chitre-Hile and Kalikhop-dadheli.
3.2. Vegetation Composition of the Himalayan Red Panda Habitat
A total of 32 species of trees including 20 species in sign plots and 31 species in no-sign plots were recorded from the 133 plots in the study area. Lithocarpus pachyphylla (IVI = 45.05), Symplocus theifolia (IVI = 37.19), Symplocus pyrifolia (IVI = 20.99), Quercus lamellosa (IVI = 19.25), and Magnolia campbellii (IVI = 17.25) were the dominant tree species in the sign plots. Lithocarpus pachyphylla (IVI = 36.83), Symplocus theifolia (IVI = 31.79), Quercus lamellosa (IVI = 23), Quercus glauca (IVI = 17.07), and Castanopsis hystrix (IVI = 14.8) were recorded in the no-sign plots. Bamboo Arundinaria maling, with a frequency of 92.48%, was the dominant plant in all the plots and was present in all sign plots at a density of 20/m2 and a mean number of culms of 175 per sign plot. Other shrub species including Daphne bholua, Viburnum erubescens, Eupatorium adenophorum, and Sarcococca coriacea were observed at a higher density and frequency in the sign plots. Arundinaria maling was present with a frequency of 89.13% and density of 14.32/m2 in no-sign plots, and the mean number of culms was 144.17 per plot. Shrub species including Daphne bholua, followed by Viburnum erubescens, had the highest density, and Viburnum erubescens, Daphne bholua, and Pteris sp. had the highest frequency in the no-sign plots. A total of 20 species of herbs were present in the study area. The herbs with the highest density and frequency in the sign plots were Pteris sp., Rubus sp., Viota sp., and Elatostema sessile. Herbs including Pteris sp., Elastotema sessile, Rubus sp. had a higher density and frequency in all the no-sign plots.
The Shannon–Wiener index of tree diversity was higher in no-sign plots (2.71) than in sign plots (2.46). The t-test showed no significant difference between the diversity indices of systematic and random plots (t = 1.84 < t0.05). Jacob’s coefficient of evenness indicated that tree species were more evenly distributed in the sign plots (E = 0.1232) than in no-sign plots (E = 0.0876). Similarly, the dominance index was higher in sign plots than in no-sign plots. Sorensen’s Similarity Index indicated a 70.58% similarity in vegetation between the systematic and sign plots (Table 1).

Table 1.
Comparison of different indices (dominance index, diversity index, evenness, species richness, variance in density (H) and similarity index) between systematic and sign plots.
3.3. Factors Associated with Himalayan Red Panda Distribution
The Himalayan red panda fecal pellets (Figure 2a) were observed more frequently in tree branches (78.12%), followed by the forest floor (15.62%) and rocks (6.25%). Lithocarpus pachyphylla (28.3%), Symplocus pyrifolia (13.3%), Rhododendron grande (13.3%), and Eurya acuminate (8.3%) were the tree species used frequently for defecation. A total of 39.02% of the Himalayan red panda signs were recorded on slight slopes (10–20°), followed by 31.70% signs on moderate slopes (20–30°), and 25% on steep slopes (30–40°); however, no sign was recorded on very steep slopes (>40%). Most of the Himalayan red panda signs were recorded on the southeast aspect (Figure 2b–d) of the slopes (43.90%), followed by the west (26.82%), southwest (12.19%), east (9.75%), and south (7.31%). None of the Himalayan red panda signs were recorded in the north, northeast, or northwest aspects.

Figure 2.
Himalayan red panda fecal pellets and different types of habitats in the Panchthar–Ilam–Taplejung Corridor; (a) fecal pellets of Himalayan red panda from the study area; (b) temperate forest habitat of red panda; (c,d) different densities of bamboo in red panda habitats.
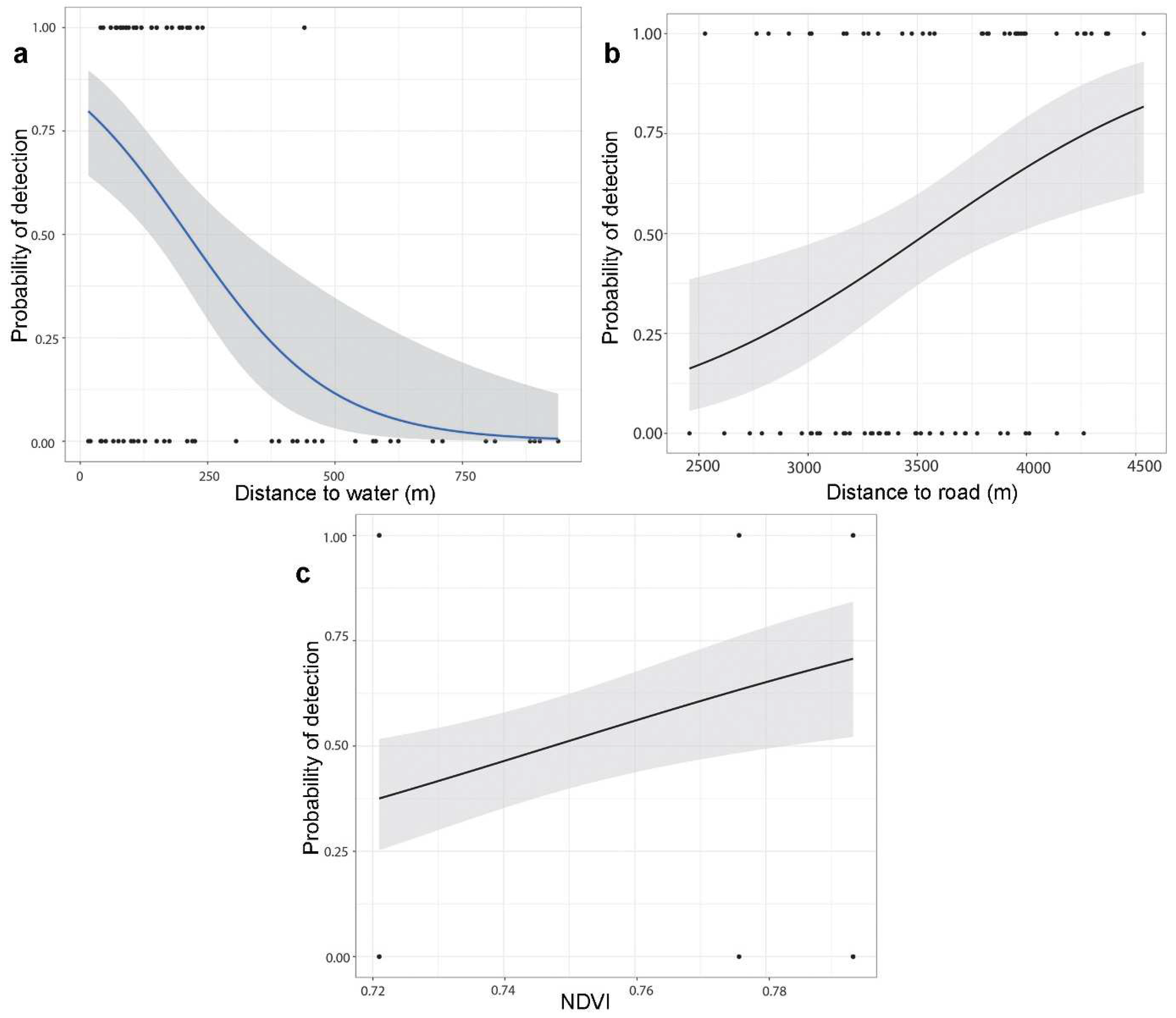
Of the thirteen environmental variables, distance to water body, distance to road, bamboo density, and NDVI significantly influenced the global environment model on the occurrence of the Himalayan red panda. The logistic regression models revealed that distance to the water body had a significant negative effect (Figure 3), and distance to the road had a significant positive effect on the occurrence of the Himalayan red panda signs (Table 2). The bamboo density had a marginal positive influence on habitat use. The best-fit model for habitat use by the Himalayan red panda was described by the combined effects of the distance to the water body, distance to the road, and NDVI (Table 3).
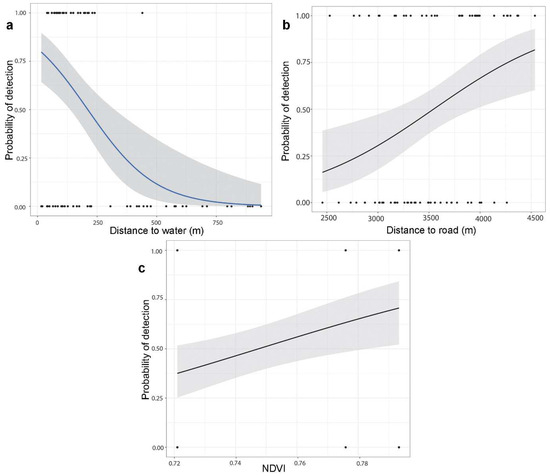
Figure 3.
Probability of the Himalayan red panda distribution in relation to (a) distance to water; (b) distance to road; and (c) Normalized Difference Vegetation Index.

Table 2.
Summary of the logistic regression (binomial distribution) models showing degrees of freedom, AICc, ∆AICc, and model weight for habitat covariates influencing red panda habitat use in Panchthar–ILam–Taplejung (PIT) Corridor.

Table 3.
Summary of the best-fit model coefficients and standard errors (SE) from the analysis of the influence of habitat covariates on red panda habitat use.
4. Discussion
This study assessed the distribution of the Himalayan red pandas in the community forests of the Panchthar–Ilam–Taplejung (PIT) Corridor of Eastern Nepal and identified the environmental variables affecting their distribution. A clumped distribution pattern of fecal pellets was observed in the study area. The clumped distribution pattern commonly occurs in nature [42]. The clumped distribution of the Himalayan red panda has been reported by several studies from various parts of Nepal, including the buffer zone of Sagarmatha National Park [46], Ilam [47], the Dhorpatan Hunting Reserve [48], and the Gaurishankar Conservation Area [49,50]. Distribution of habitat resources, such as the availability of food, water, and vegetation cover, could contribute to uneven distribution of the Himalayan red pandas in their habitats. In the PIT Corridor, the elevational distribution of the Himalayan red panda ranged between 2200 m and 2700 m, with a relatively higher abundance in elevation between 2400 m and 2500 m. Previous studies revealed that the Himalayan red panda is restricted within a narrow elevational range [11,12,15,16,19,24,25,27,33,39,40,46,47,48,51,52,53,54,55,56,57], indicating that resource availability, local micro-environmental conditions, and anthropogenic factors play an important role in their distribution. They are more abundant in elevations between 2400 m and 3700 m in Bhutan [54]. The Himalayan red panda sign has been recorded at 2400 m in Singalila National Park [15] and at 2442 m in Eastern Ilam [39]. This study detected the Himalayan red panda sign at a lower elevation (2245 m) than those from other range counties. Kandel [47] recorded signs at 2280 m in Hangetham and Choyatar community forests in Ilam. Sightings of this species are infrequent, likely due to their solitary nature [11,58]; however, they may be observed more frequently during mating season or when mothers have cubs. We observed three direct sightings and 32 fecal pellet groups of the Himalayan red pandas in the study area. Williams [39] observed only five direct sightings in Ilam; Bhatta et al. [51] recorded a single direct sighting with 28 indirect observations in Jumla; and Bista et al. [25] directly sighted 2.4% of all observations in the Chitwan–Annapurna Landscape (CHAL), with 132 indirect observations.
Human activities, including the collection of malingo (Arundinaria maling), firewood, fodder, and grazing, increase habitat disturbance and may have been attributed to the fewer signs of Himalayan red pandas at lower elevations. Furthermore, very steep slopes and areas with low vegetation cover foster unsuitable habitats in the upper altitudinal range. There is minimal human disturbance and a high degree of vegetation cover, particularly malingo, in the middle elevation range (2400–2500 m) of the Gorkhe and Jogmai community forests. Our findings contrast with those of William [33] and Kandel [47], which indicated that elevation between 2800 m and 3000 m in Jamuna and Mabu, and 2700 m and 2900 m in Hangetham and Choyatar, in the community forests were the preferred altitudinal range for the Himalayan red pandas in Ilam. Variations in the preferred altitudinal ranges of the Himalayan red panda might be attributed to multiple factors such as varied micro-habitats, the level of anthropogenic activities, and variability of food and water sources of the study sites.
Similar types of plant communities were found in sign plots and no-sign plots. Related types of tree species had been observed in the Chhipchhipe and Singhadevi community forests by Lama et al. [32] and Jamuna and Marbu in Ilam by Williams [39] and Kandel [47]. The forest types that support the majority of the Himalayan red pandas in Eastern and Western Nepal consist of different vegetation compositions [26]. Important forest types that are used by the Himalayan red panda include mixed broad-leaved forests and east Himalayan oak–laurel forests in Eastern Nepal; temperate mountain oak forest in central Nepal; and temperate juniper forest and blue pine–spruce forests in Western Nepal [26]. Distribution of the Himalayan red panda has a strong association with old-growth Bhutan fir forests [21,54].
In the field, fecal pellet groups were observed in the branches of mature trees of Lithocarpus pachyphylla, Rhododendron sp., and Symplocus pyrifolia. The tree trunks contain holes which provide space for resting, breeding, and escape from predators [11,18,25,26,39,58]. The fecal pellet groups were reported occasionally on the ground, possibly being deposited when red pandas were in search of water or when foraging for bamboo shoots. Pradhan et al. [15] reported the position of pellet groups on trees (81.25%), indicating them as a preferred defecation site in the pre-monsoon season. Other studies have also found tree branches as the preferred defecation substrate [11,19,25,26,39]. Thapa et al. [26] observed the ground or forest floor as the preferred site for defecation, which contradicts with our findings, that may be due the local scale of the study. Season, tree species, tree inclination, availability of fallen logs, bamboo height, and growth of bamboo shoots could influence substrate use [26].
The best-fit model showed that distance to water, distance to the road, and NDVI are factors associated with the distribution of the Himalayan red panda. Proximity to water was scored as an important habitat parameter to assess habitat suitability mapping of the Himalayan red panda [11,18]. We found that fecal pellet groups of the Himalayan red panda occurred frequently in proximity to water. Habitats close to water sources are important for conserving energy, as the red pandas are not required to travel long distances in search of water [11,15,25]. This study supports the statement that Himalayan red pandas prefer habitats near water resources. Bista et al. [29] found that movement activity collared Himalayan red pandas showed 79.6% of the locations within 100 m distance to water sources. Lama et al. [32] recorded most of the red panda signs at a distance of 100 m from the water source. Also, similar results were further supported by the studies of Yonzon and Hunter [18], Kandel [47], Dorji et al. [21], Zhou et al. [59], Bhatta et al. [51], and Bista et al. [25]. Proximity to water sources could provide favorable environment for the vegetation growth, including bamboo which is a main food source for the Himalayan red panda.
The Normalized Difference Vegetation Index (NDVI) serves as an indicator for describing the primary productivity of vegetation and is commonly used as a proxy for canopy cover in studying animal ecology [60]. Habitat occupancy of the Himalayan red panda was influenced by the distance to water and NDVI at the landscape level [23]. NDVI is negatively associated with the habitat use of the Himalayan red panda in our study area. Similarly, Thapa et al. [23] found a negative influence of NDVI on the detectability of the Himalayan red panda with an increase in NDVI. Habitats with more open canopies might contribute to the growth performance of the understory vegetation like bamboo. Similarly, Bist et al. [19] found that the Himalayan red panda preferred forests consisting of high trees with low canopy cover in the eastern part of Nepal. Low shrub and tree covers are associated with the mating season when the temperature is low in the winter season [29]. Bist et al. [29] found that NDVI showed a negative response to habitat use of the Himalayan red panda during the cub-rearing season. This could be related to their adaptation for conserving energy and thermoregulation by maximizing sun exposure in temperate habitats. In contrast, the Chinese red panda showed a strong habitat preference for the deep slope facing the south with dense canopy in their easternmost distribution range in China [17].
Human-induced disturbances, particularly road and cattle-herding activities influenced habitat utilization of the Himalayan red panda throughout the year [29]. The road provides access to collected resources from the forest and increases activities in the habitats. Livestock grazing and human activities frequently occur in the grassland, creating disturbances in the Himalayan red panda habitat. We found that observed signs of the Himalayan red panda were decreased with proximity to the road, indicating species preferred less disturbances habitats. Disturbance from these activities may have caused the absence of signs of the Himalayan red pandas [15,23,26,30,32,39,48,51,59].
The community forests in the PIT Corridor of Eastern Nepal provide a suitable habitat for the endangered Himalayan red panda. Such habitats are outside the protected area system of the country; hence, human activities are deteriorating the habitat quality posing serious threats to wildlife including the Himalayan red panda. Therefore, the community forest user groups should formulate habitat management strategies according to the specific needs of the keystone species like the Himalayan red panda. Such management strategies should focus on habitat management, considering the availability of bamboo and easy access to water resources. Additionally, as the habitats in Eastern Nepal share a border between Nepal and India, a transboundary management approach is urgently needed.
5. Conclusions
The occurrence of the Himalayan red pandas was found in all the studied community forests in the Panchthar–Ilam–Taplejung Corridor. The evidence (direct and indirect) of the Himalayan red panda distribution was found between 2245 m and 2715 m elevation. The branches of trees were the primary defecation substrates. Himalayan red pandas preferred forests with tree species such as Lithocarpus pachyphylla, Symplocus theifolia, Symplocus pyrifolia, and Quercus lamellose in the sites. Distance to water bodies, distance to road, NDVI and bamboo density have impacts on the occurrence of the Himalayan red panda. Most of the habitats of the Himalayan red panda lie beyond the protected area i.e., community forestry. So, community forest users should consider these environmental factors when managing the Himalayan red panda habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecologies5030021/s1, Figure S1: Correlation matrix of factors associated with Himalayan red panda distribution; Figure S2: Sign counter rate along the elevation of the study area; Figure S3: Sign encounter rate in different community forests of the study area; Table S1: Summary of the global models.
Author Contributions
A.L. and T.B.T. conceptualized the research and T.B.T. supervised it; A.L. and S.G. collected data in the field; A.T., A.L. and L.K. processed and analyzed data; A.L. and A.T. prepared the original draft with input from T.B.T.; A.T., L.K., N.J.C. and T.B.T. reviewed the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the MSc Research Grant of the University Grants Commission Nepal to AL.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This study was financially supported in part by the University Grant Commission, Nepal. We thank the Central Department of Zoology, Tribhuvan University for providing field equipment, and the Divisional Forest Office, Ilam, for providing research permission. We would like to thank the members of the community forest user committee and forest guardians for their assistance in conducting field surveys in the community forests.
Conflicts of Interest
No conflicts of interest exist among the authors.
References
- Wei, F.W.; Feng, Z.J.; Wang, Z.W.; Zhou, A.; Hu, J.C. Use of the nutrients in bamboo by the red panda (Ailurus fulgens). J. Zool. 1999, 248, 535–541. [Google Scholar] [CrossRef]
- Yonzon, P.B.; Hunter, M.L., Jr. Ecological Study of the Red Panda in the Nepal-Himalaya; Glatson, A.R., Ed.; Red Panda Biology, Academic Publication: The Hauge, The Netherlands, 1989; pp. 1–7. [Google Scholar]
- Hu, Y.; Hu, Y.; Zhou, W.; Wei, F. Conservation genomics and metagenomics of Giant and Red pandas in the Wild. Annu. Rev. Anim. Biosci. 2024, 12, 69–89. [Google Scholar] [CrossRef]
- Hu, Y.; Wei, F.; Thapa, A. Red panda genomics and the evidence for two species. In Red Panda; Elsevier: Amsterdam, The Netherlands, 2022; pp. 413–420. [Google Scholar] [CrossRef]
- Hu, Y.; Thapa, A.; Fan, H.; Ma, T.; Wu, Q.; Ma, S.; Zhang, D.; Wang, B.; Li, M.; Yan, L.; et al. Genomic evidence for two phylogenetic species and long-term population bottlenecks in red pandas. Sci. Adv. 2020, 6, eaax5751. [Google Scholar] [CrossRef]
- Hu, Y.; Thapa, A.; Wei, F. Ailurus fulgens (Himalayan red panda) and Ailurus styani (Chinese red panda). Trends Genet. 2020, 36, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Thapa, A.; Bista, D.; Robinson, N.; Sherpa, A.P.; Acharya, K.P.; Jnawali, S.R.; Lama, S.T.; Lama, S. Distribution and habitat attributes associated with the Himalayan red panda in the westernmost distribution range. Ecol. Evol. 2021, 11, 4023–4034. [Google Scholar] [CrossRef] [PubMed]
- Dalui, S.; Singh, S.K.; Joshi, B.D.; Ghosh, A.; Basu, S.; Khatri, H.; Sharma, L.K.; Chandra, K.; Thakur, M. Geological and pleistocene glaciations explain the demography and disjunct distribution of red panda (A. fulgens) in eastern Himalayas. Sci. Rep. 2021, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Glatston, A.; Wei, F.; Zaw, T.; Sherpa, A. Ailurus fulgens (Errata Version Published in 2017); The IUCN Red List of Threatened Species: Cambridge, UK, 2015; E.T714A110023718. [Google Scholar]
- Jnawali, S.; Leus, K.; Molur, S.; Glatston, A.; Walker, S. Population and Habitat Viability Assessment (PHVA) and Species Conservation Strategy (SCS) Workshop Report; National Trust for Nature Conservation: Kathmandu, Nepal; Conservation Breeding Specialist Group and Zoo Outreach Organization: Coimbatore, India, 2012. [Google Scholar]
- Thapa, A.; Hu, Y.; Wei, F. The endangered red panda (Ailurus fulgens): Ecology and conservation approaches across the entire range. Biol. Conserv. 2018, 220, 112–121. [Google Scholar] [CrossRef]
- Thapa, A.; Wu, R.; Hu, Y.; Nie, Y.; Singh, P.B.; Khatiwada, J.R.; Yan, L.; Gu, X.; Wei, F. Predicting the potential distribution of the endangered red panda across its entire range using MaxEnt modeling. Ecol. Evol. 2018, 8, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- DNPWC; DFSC. Red Panda Action Plan for Nepal (2019–2013); Department of National Park and Wildlife Conservation and Department of Forest and Soil Conservation: Kathmandu, Nepal, 2018.
- Jnawali, S.; Baral, H.; Lee, S.; Acharya, K.; Upadhyay, G.; Pandey, M.; Griffiths, J. The Status of Nepal Mammals: The National Red List Series; Preface by Simon M. Stuart Chair IUCN Species Survival Commission; Department of National Parks and Wildlife Conservation: Kathmandu, Nepal, 2011; Volume 4.
- Pradhan, S.; Saha, G.K.; Khan, J.A. Ecology of the red panda Ailurus fulgens in the Singhalila National Park, Darjeeling, India. Biol. Conserv. 2001, 98, 11–18. [Google Scholar] [CrossRef]
- Choudhury, A. An overview of the status and conservation of the red panda Ailurus fulgens in India, with reference to its global status. Oryx 2001, 35, 250–259. [Google Scholar] [CrossRef]
- Wei, F.W.; Feng, Z.J.; Wang, Z.W.; Hu, J.C. Current distribution, status and conservation of wild red pandas Ailurus fulgens in China. Biol. Conserv. 1999, 89, 285–291. [Google Scholar] [CrossRef]
- Yonzon, P.B.; Hunter, M.L. Conservation of the red panda Ailurus fulgens. Biol. Conserv. 1991, 57, 1–11. [Google Scholar] [CrossRef]
- Bista, D.; Paudel, P.K.; Jnawali, S.R.; Sherpa, A.P.; Shrestha, S.; Acharya, K.P. Red panda fine-scale habitat selection along a Central Himalayan longitudinal gradient. Ecol. Evol. 2019, 9, 5260–5269. [Google Scholar] [CrossRef] [PubMed]
- Dendup, P.; Humle, T.; Bista, D.; Penjor, U.; Lham, C.; Gyeltshen, J. Habitat requirements of the Himalayan red panda (Ailurus fulgens) and threat analysis in Jigme Dorji National Park, Bhutan. Ecol. Evol. 2020, 10, 9444–9453. [Google Scholar] [CrossRef] [PubMed]
- Dorji, S.; Vernes, K.; Rajaratnam, R. Habitat correlates of the red panda in the temperate forests of Bhutan. PLoS ONE 2011, 6, e26483. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.W.; Feng, Z.J.; Wang, Z.W.; Hu, J.C. Habitat use and separation between the giant panda and the red panda. J. Mammal. 2000, 81, 448–455. [Google Scholar] [CrossRef]
- Thapa, K.; Thapa, G.J.; Bista, D.; Jnawali, S.R.; Acharya, K.P.; Khanal, K.; Kandel, R.C.; Thapa, M.K.; Shrestha, S.; Lama, S.T.; et al. Landscape variables affecting the Himalayan red panda Ailurus fulgens occupancy in wet season along the mountains in Nepal. PLoS ONE 2020, 15, e0243450. [Google Scholar] [CrossRef] [PubMed]
- Panthi, S.; Khanal, G.; Acharya, K.P.; Aryal, A.; Srivathsa, A. Large anthropogenic impacts on a charismatic small carnivore: Insights from distribution surveys of red panda Ailurus fulgens in Nepal. PLoS ONE 2017, 12, e0180978. [Google Scholar] [CrossRef]
- Bista, D.; Shrestha, S.; Sherpa, P.; Thapa, G.J.; Kokh, M.; Lama, S.T.; Khanal, K.; Thapa, A.; Jnawali, S.R. Distribution and habitat use of red panda in the Chitwan-Annapurna Landscape of Nepal. PLoS ONE 2017, 12, e0178797. [Google Scholar] [CrossRef]
- Thapa, A.; Hu, Y.; Aryal, P.C.; Singh, P.B.; Shah, K.B.; Wei, F. The endangered red panda in Himalayas: Potential distribution and ecological habitat associates. Glob. Ecol. Conserv. 2020, 21, e00890. [Google Scholar] [CrossRef]
- Bista, D.; Paudel, R. An overview of the status and conservation initiatives of Red Panda Ailurus fulgens (Cuvier, 1825) in Nepal. Initiation 2014, 5, 171–181. [Google Scholar] [CrossRef]
- Bista, D.; Baxter, G.S.; Hudson, N.J.; Murray, P.J. Red panda tourism gives hope in the mid-mountain range of the Eastern Himalaya, yet inappropriate practices may lead to failure. Conserv. Sci. Pract. 2023, 5, e13036. [Google Scholar] [CrossRef]
- Bista, D.; Baxter, G.S.; Hudson, N.J.; Murray, P.J. Seasonal resource selection of an arboreal habitat specialist in a human-dominated landscape: A case study using red panda. Curr. Zool. 2023, 69, 1–11. [Google Scholar] [CrossRef]
- Bista, D.; Baxter, G.S.; Hudson, N.J.; Lama, S.T.; Murray, P.J. Effect of disturbances and habitat fragmentation on an arboreal habitat specialist mammal using GPS telemetry: A case of the red panda. Landsc. Ecol. 2022, 37, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Bista, D.; Lama, S.T.; Weerman, J.; Sherpa, A.P.; Pandey, P.; Thapa, M.K.; Acharya, H.; Hudson, N.J.; Baxter, G.S.; Murray, P.J. Improved trapping and handling of an arboreal, montane mammal: Red panda Ailurus fulgens. Animals 2021, 11, 921. [Google Scholar] [CrossRef]
- Lama, S.; Shrestha, S.; Koju, N.P.; Sherpa, A.P.; Tamang, M. Assessment of the impacts of livestock grazing on endangered red panda (Ailurus fulgens) habitat in Eastern Nepal. Open J. Ecol. 2020, 10, 97–110. [Google Scholar] [CrossRef]
- Williams, B.H. Red panda in eastern Nepal: How do they fit into ecoregional conservation of the eastern Himalaya. Conserv. Biol. Asia 2003, 16, 236–250. [Google Scholar]
- Bista, D.; Baxter, G.S.; Murray, P.J. What is driving the increased demand for red panda pelts? Hum. Dimens. Wildl. 2020, 25, 324–338. [Google Scholar] [CrossRef]
- Karki, S.; Maraseni, T.; Mackey, B.; Bista, D.; Lama, S.T.; Gautam, A.P.; Sherpa, A.P.; Koju, U.; Shrestha, A.; Cadman, T. Reaching over the gap: A review of trends in and status of red panda research over 193 years (1827–2020). Sci. Total Environ. 2021, 781, 146659. [Google Scholar] [CrossRef]
- Acharya, K.P.; Shrestha, S.; Paudel, P.K.; Sherpa, A.P.; Jnawali, S.R.; Acharya, S.; Bista, D. Pervasive human disturbance on habitats of endangered red panda Ailurus fulgens in the central Himalaya. Glob. Ecol. Conserv. 2018, 15, e00420. [Google Scholar] [CrossRef]
- Lama, S.T.; Ross, J.G.; Bista, D.; Sherpa, A.P.; Regmi, G.R.; Suwal, M.K.; Sherpa, P.; Weerman, J.; Lama, S.S.; Thapa, M. First photographic record of marbled cat Pardofelis marmorata Martin, 1837 (Mammalia, Carnivora, Felidae) in Nepal. Nat. Conserv. 2019, 32, 19–34. [Google Scholar] [CrossRef]
- Grimmett, R.; Inskipp, C.; Inskipp, T.; Baral, H.S. Birds of Nepal; Bloomsbury Publishing: London, UK, 2016. [Google Scholar]
- Mueller-Dumbois, D.; Ellenberg, H. Aims and Methods in Vegetation Ecology; John Wiley and Sons: New York, NY, USA, 1974; p. 547. [Google Scholar]
- Williams, B.H. The Status of Red Panda in Jamuna and Mabu Village of Eastern Nepal. Master’s Thesis, The Faculty of the Department of Environmental Studies, San Jose State University, San Jose, CA, USA, 2004. [Google Scholar]
- Polunin, O.; Stainton, A. Flowers of the Himalaya; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Odum, E.P.; Barrett, G.W. Fundamentals of Ecology; Saunders: Philadelphia, PA, USA, 1971; Volume 3. [Google Scholar]
- Panthi, S.; Wang, T.; Sun, Y.; Thapa, A. An assessment of human impacts on endangered red pandas (Ailurus fulgens) living in the Himalaya. Ecol. Evol. 2019, 9, 13413–13425. [Google Scholar] [CrossRef] [PubMed]
- Barton, K. MuMIn: Multi-Model Inference, R package version 1.15. 6 CRAN; 2020. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 25 May 2024). [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer: New York, NY, USA, 2002. [Google Scholar]
- Mahato, N. Status of Red Panda Ailurus fulgens (Cuvier, 1825) in the Kanchanjungha Conservation Area. Bachelor’s Thesis, Institute of Forestry, Tribhuvan University, Pokhara, Nepal, 2003. Volume 40. [Google Scholar]
- Kandel, K. Distribution and habitat use of red panda (Ailurus fulgen) in Eastern Nepal. Master’s Thesis, Central Department of Zoology, Tribhuvan University, Kathmandu, Nepal, 2009. [Google Scholar]
- Panthi, S.; Aryal, A.; Raubenheimer, D.; Lord, J.; Adhikari, B. Summer diet and distribution of the red panda (Ailurus fulgens fulgens) in Dhorpatan Hunting Reserve, Nepal. Zool. Stud. 2012, 51, 701–709. [Google Scholar]
- Thapa, A.; Basnet, K. Seasonal diet of wild red panda (Ailurus fulgens) in Langtang National Park, Nepal Himalaya. Int.J. Conserv. Sci. 2015, 6, 261–270. [Google Scholar]
- Thapa, A.; Thapa, S.; Poudel, S. Gaurishankar Conservation Area-A prime habitat for red panda (Ailurus fulgens) in Central Nepal. Initiation 2013, 5, 43–49. [Google Scholar] [CrossRef]
- Bhatta, M.; Shah, K.B.; Devkota, B.; Paudel, R.; Panthi, S. Distribution and habitat preference of red panda (Ailurus fulgens fulgens) in Jumla District, Nepal. Open J. Ecol. 2014, 4, 989–1001. [Google Scholar] [CrossRef]
- Mallick, J.K. Status of red panda Ailurus fulgens in Neora Valley National Park, Darjeeling District, West Bengal, India. Small Carniv. Conserv. 2010, 43, 30–36. [Google Scholar]
- Wei, F.; Zhang, Z. Red Panda Ecology; William Andrew: Norwich, NY, USA, 2011; pp. 193–212. [Google Scholar]
- Dorji, S.; Rajaratnam, R.; Vernes, K. The Vulnerable red panda Ailurus fulgens in Bhutan: Distribution, conservation status and management recommendations. Oryx 2012, 46, 536–543. [Google Scholar] [CrossRef]
- Kandel, K.; Huettmann, F.; Suwal, M.K.; Regmi, G.R.; Nijman, V.; Nekaris, K.; Lama, S.T.; Thapa, A.; Sharma, H.P.; Subedi, T.R. Rapid multi-nation distribution assessment of a charismatic conservation species using open access ensemble model GIS predictions: Red panda (Ailurus fulgens) in the Hindu-Kush Himalaya region. Biol. Conserv. 2015, 181, 150–161. [Google Scholar] [CrossRef]
- Panthi, S.; Coogan, S.C.; Aryal, A.; Raubenheimer, D. Diet and nutrient balance of red panda in Nepal. Naturwissenschaften 2015, 102, 54. [Google Scholar] [CrossRef]
- Bista, D.; Paudel, P.K.; Ghimire, S.; Shrestha, S. National Survey of Red Panda to Assess Its Status, Habitat and Distribution in Nepal; Final report submitted to WWF/USAID/Hariyo Ban Program; Baluwatar: Kathmandu, Nepal, 2016. [Google Scholar]
- Yonzon, P.B.; Hunter, M.L., Jr. Cheese, tourists, and red pandas in the Nepal Himalayas. Conserv. Biol. 1991, 5, 196–202. [Google Scholar] [CrossRef]
- Zhou, X.; Jiao, H.; Dou, Y.; Aryal, A.; Hu, J.; Hu, J.; Meng, X. The winter habitat selection of red panda (Ailurus fulgens) in the Meigu Dafengding National Nature Reserve, China. Curr. Sci. 2013, 105, 1425–1429. [Google Scholar]
- Pettorelli, N.; Ryan, S.; Mueller, T.; Bunnefeld, N.; Jedrzejewska, B.; Lima, M.; Kausrud, K. The Normalized Difference Vegetation Index (NDVI): Unforeseen successes in animal ecology. Clim. Res. 2011, 46, 15–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).