Rapoport’s Rule, the Ecotone Concept, and Salinity Gradient Predict the Distribution of Benthic Foraminifera in a Southeastern Pacific Estuary
Abstract
:1. Introduction
2. Materials and Methods
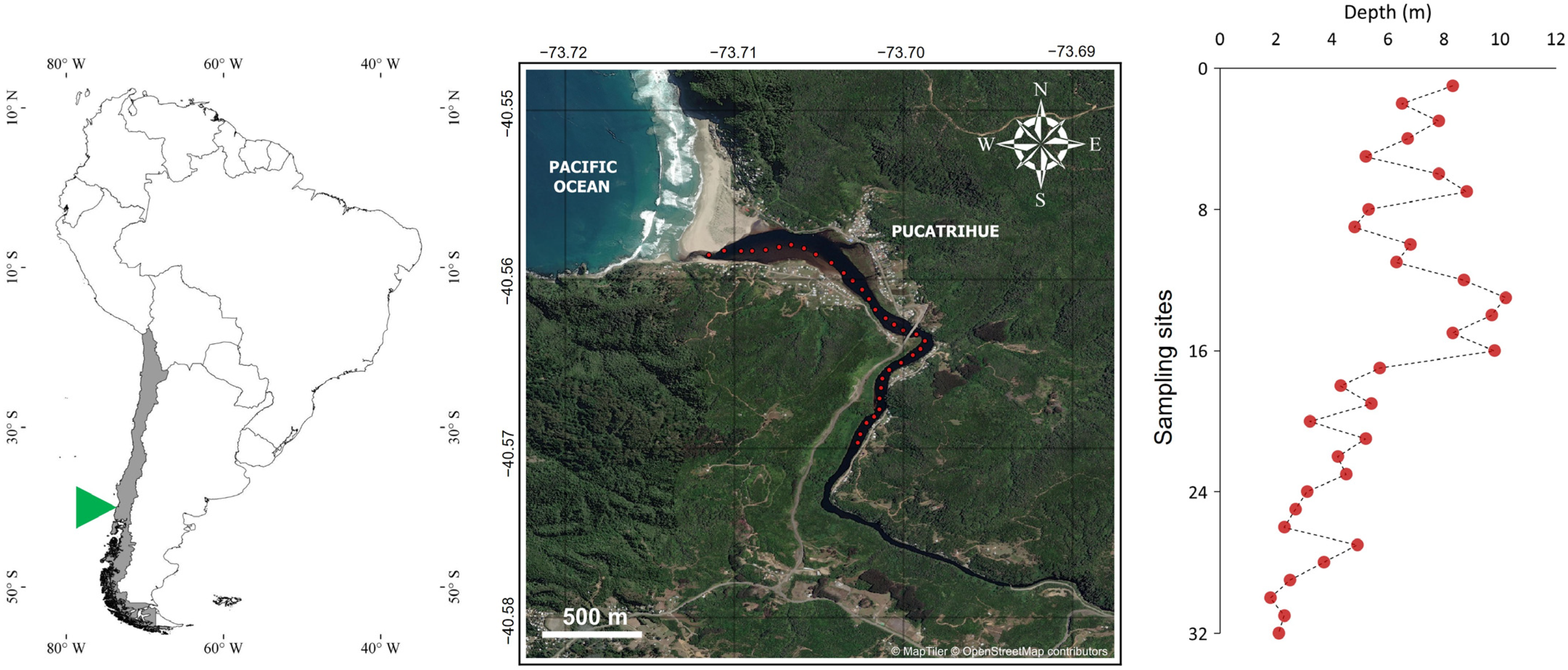
2.1. Study Area
2.2. Sampling of Benthic Foraminifera
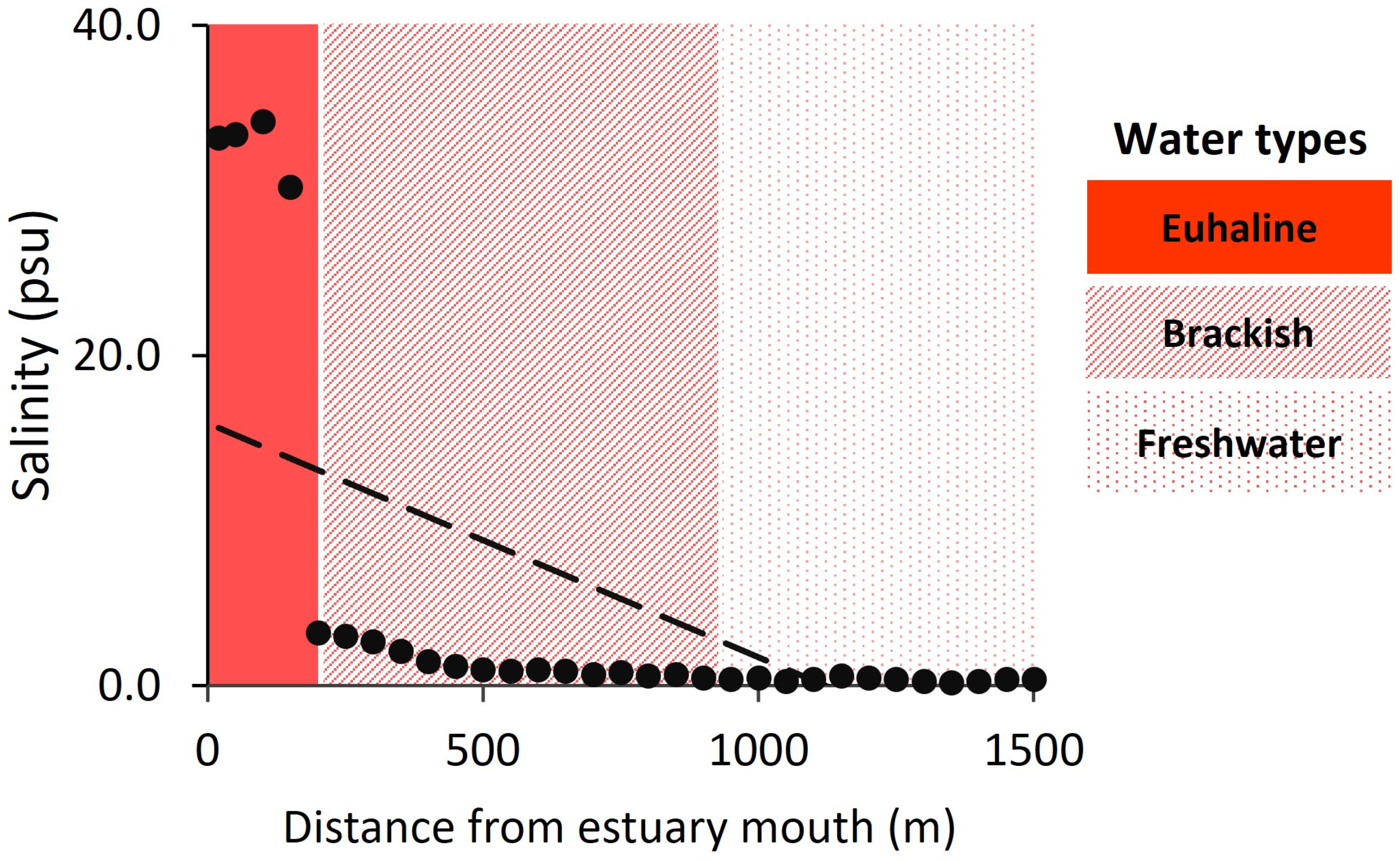
2.3. Salinity Measurements
2.4. Evaluation of Sampling Artifacts
2.5. Assessing Rapoport’s Longitudinal Rule and Mid-Domain Effect
2.6. Testing the TA-Pattern and Source–Sink Dynamics
3. Results
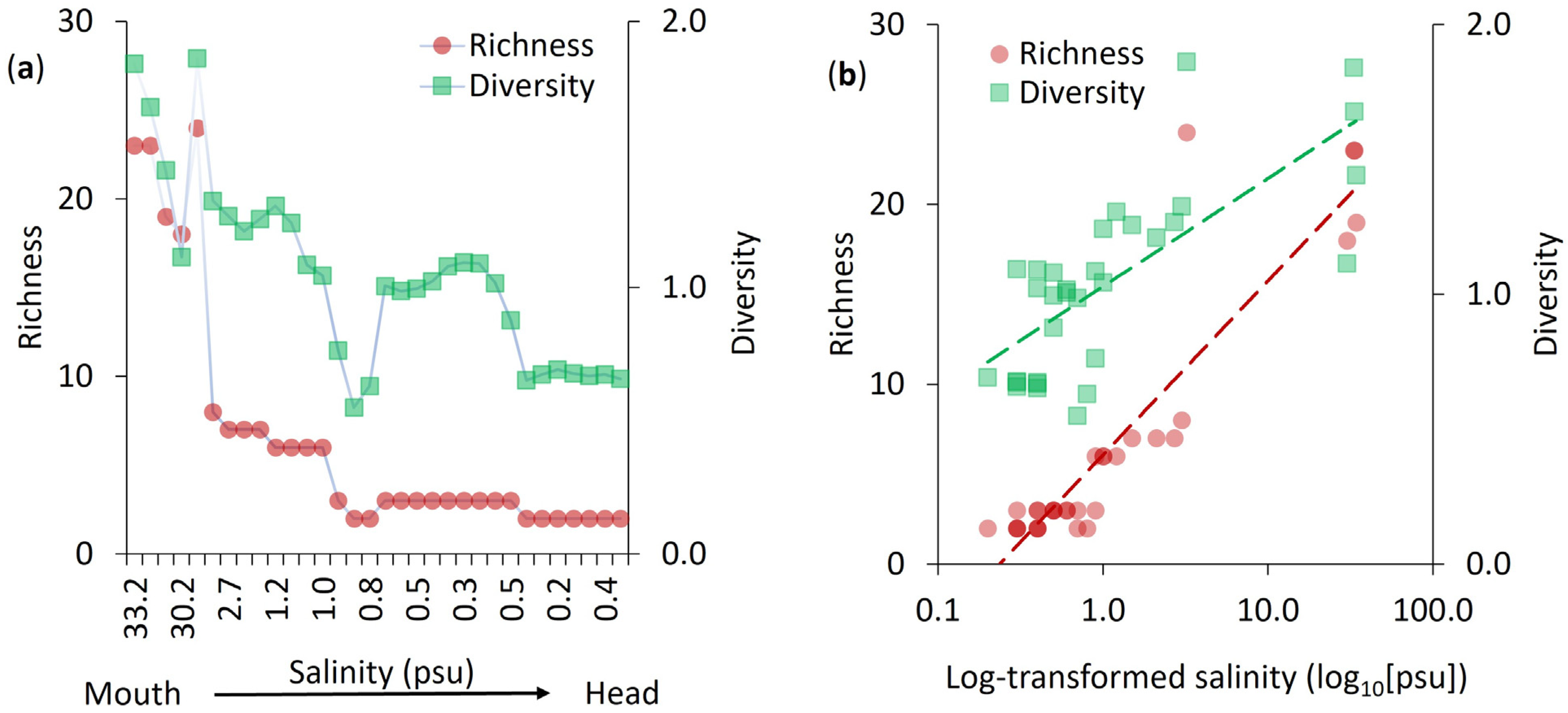
3.1. Salinity Gradient and Foraminiferal Diversity
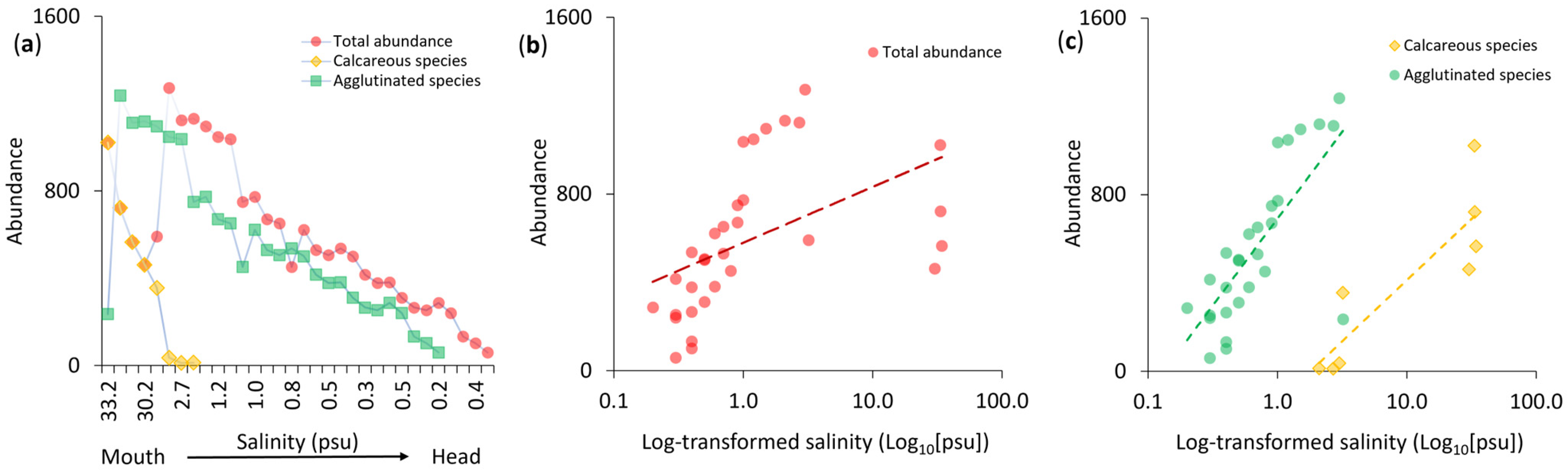
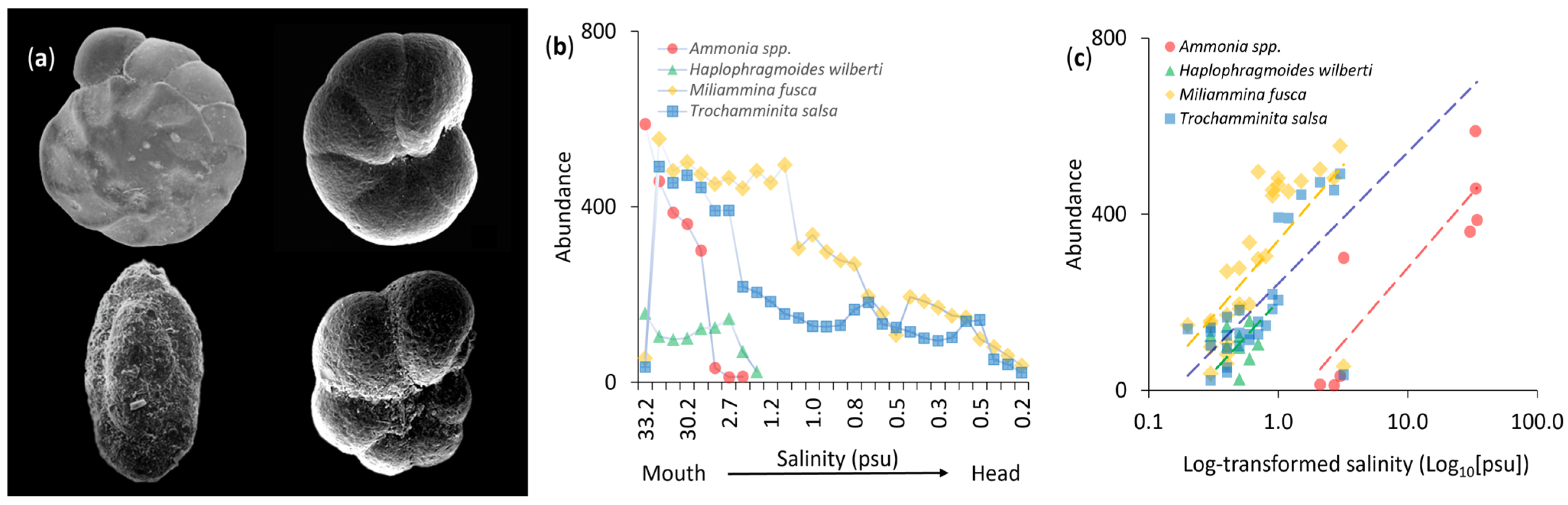
3.2. Abundance Patterns
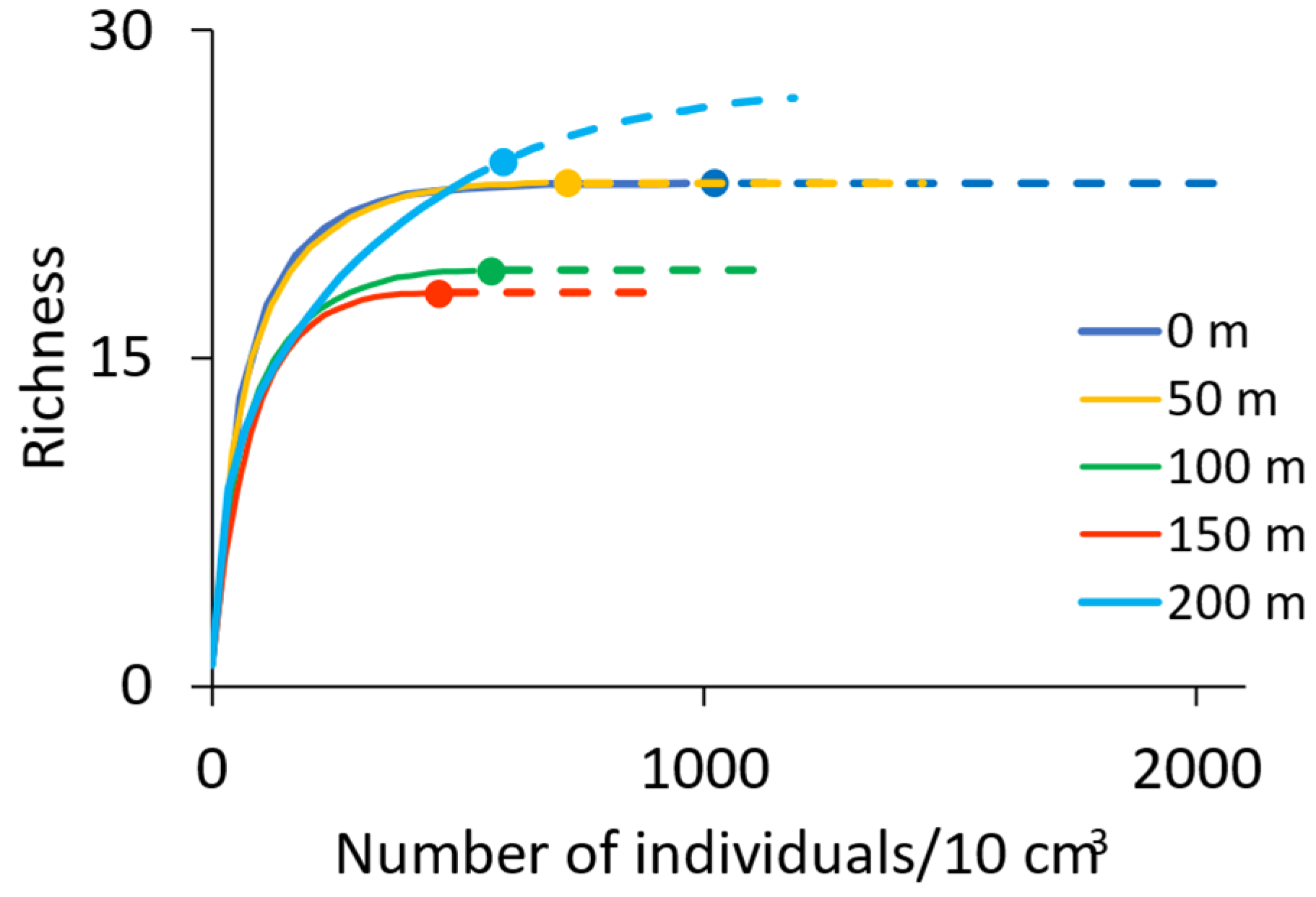
3.3. Sampling Artifact Analysis
3.4. Rapoport’s Rule and Mid-Domain Effect
3.5. TA-Pattern vs. Source–Sink Dynamics
4. Discussion
4.1. Diversity and Richness Along the Estuarine Gradient
4.2. Abundance and Salinity Tolerance
4.3. Longitudinal Variation and Ecotonal Dynamics in Species Composition
4.4. Rapoport’s Rule and Mid-Domain Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittelbach, G.G.; Schemske, D.W.; Cornell, H.V.; Allen, A.P.; Brown, J.M.; Bush, M.B.; Harrison, S.P.; Hurlbert, A.H.; Knowlton, N.; Lessios, H.A.; et al. Evolution and the latitudinal diversity gradient: Speciation, extinction and biogeography. Ecol. Lett. 2007, 10, 315–331. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.E.; Moreno, R.A.; Rozbaczylo, N. Biogeographical patterns and Rapoport’s rule in southeastern Pacific benthic polychaetes of the Chilean coast. Ecography 2005, 28, 363–373. [Google Scholar] [CrossRef]
- Dunn, R.R.; Colwell, R.K.; Nilsson, C. The river domain: Why are there more species halfway up the river? Ecography 2006, 29, 251–259. [Google Scholar] [CrossRef]
- Moreno, R.A.; Rivadeneira, M.M.; Hernández, C.E.; Sampértegui, S.; Rozbaczylo, N. Do Rapoport’s rule, the mid-domain effect or the source-sink hypotheses predict bathymetric patterns of polychaete richness on the Pacific coast of South America? Glob. Ecol. Biogeogr. 2008, 17, 415–423. [Google Scholar] [CrossRef]
- Beketov, M.A. The Rapoport effect is detected in a river system and is based on nested organization. Glob. Ecol. Biogeogr. 2009, 18, 498–506. [Google Scholar] [CrossRef]
- McCain, C.M. Global analysis of bird elevational diversity. Glob. Ecol. Biogeogr. 2009, 18, 346–360. [Google Scholar] [CrossRef]
- Wiens, J.J.; Ackerly, D.D.; Allen, A.P.; Anacker, B.L.; Buckley, L.B.; Cornell, H.V.; Damschen, E.I.; Jonathan Davies, T.; Grytnes, J.A.; Harrison, S.P.; et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 2010, 13, 1310–1324. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Ulrich, W. A straightforward computational approach for quantifying nestedness using abundance data. Environ. Modell. Softw. 2011, 26, 173–178. [Google Scholar] [CrossRef]
- El Yaagoubi, S.; Edegbene, A.O.; El Haissoufi, M.; Harrak, R.; El Alami, M. Odonata, Coleoptera, and Heteroptera (OCH) Trait-Based Biomonitoring of Rivers within the Northwestern Rif of Morocco: Exploring the Responses of Traits to Prevailing Environmental Gradients. Ecologies 2024, 5, 132–154. [Google Scholar] [CrossRef]
- Fernández, L.D. Source–sink dynamics shapes the spatial distribution of soil protists in an arid shrubland of northern Chile. J. Arid Environ. 2015, 113, 121–125. [Google Scholar] [CrossRef]
- Fernández, L.D.; Fournier, B.; Rivera, R.; Lara, E.; Mitchell, E.A.D.; Hernández, C.E. Water–energy balance, past ecological perturbations and evolutionary constraints shape the latitudinal diversity gradient of soil testate amoebae in south-western South America. Glob. Ecol. Biogeogr. 2016, 25, 1216–1227. [Google Scholar] [CrossRef]
- Schiaffino, M.R.; Lara, E.; Fernández, L.D.; Balagué, V.; Singer, D.; Seppey, C.C.W.; Massana, R.; Izaguirre, I. Microbial Eukaryote Communities Exhibit Robust Biogeographical Patterns along a Gradient of Patagonian and Antarctic Lakes. Environ. Microbiol. 2016, 18, 5249–5264. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.D.; Hernández, C.E.; Schiaffino, M.R.; Izaguirre, I.; Lara, E. Geographical Distance and Local Environmental Conditions Drive the Genetic Population Structure of a Freshwater Microalga (Bathycoccaceae; Chlorophyta) in Patagonian Lakes. FEMS Microbiol. Ecol. 2017, 93, fix125. [Google Scholar] [CrossRef] [PubMed]
- Geisen, S.; Mitchell, E.A.; Wilkinson, D.M.; Adl, S.; Bonkowski, M.; Brown, M.W.; Fiore-Donno, A.M.; Heger, T.J.; Jassey, V.E.; Krashevska, V.; et al. Soil protistology rebooted: 30 fundamental questions to start with. Soil Biol. Biochem. 2017, 111, 94–103. [Google Scholar] [CrossRef]
- Singer, D.; Mitchell, E.A.D.; Payne, R.J.; Blandenier, Q.; Duckert, C.; Fernández, L.D.; Fournier, B.; Hernández, C.E.; Granath, G.; Rydin, H.; et al. Dispersal limitations and historical factors determine the biogeography of specialized terrestrial protists. Mol. Ecol. 2019, 28, 3089–3100. [Google Scholar] [CrossRef] [PubMed]
- Adl, S.M.; Simpson, A.G.B.; Lane, C.E.; Lukes, J.; Bass, D.; Bowser, S.S.; Brown, M.; Burki, F.; Dunthorn, M.; Hampl, V.; et al. The Revised Classification of Eukaryotes. J. Eukaryot. Microbiol. 2012, 59, 429–493. [Google Scholar] [CrossRef]
- Boltovskoy, E.; Lena, H. Foraminíferos del Río de la Plata. Serv. Hidrogr. Naval. 1974, 661, 3–22. [Google Scholar]
- Scott, D.B.; Medioli, F.S. Quantitative studies of marsh foraminiferal distributions in Nova Scotia: Implications for sea level studies. J. Foraminifer. Res. 1980, 17, 58. [Google Scholar]
- Boltovskoy, E.; Giussani, G.; Watanabe, S.; Wright, R. Atlas of Benthic Shelf Foraminifera of the Southwest Atlantic; Junk by Publications: London, UK, 1980. [Google Scholar]
- Hayward, B.W.; Grenfell, H.R.; Reid, C.M.; Hayward, K.A. Recent New Zealand Shallow-Water Benthic Foraminifera: Taxonomy, Ecologic Distribution, Biogeography, and Use in Paleoenvironmental Assessmen; Institute of Geological and Nuclear Sciences Monograph 21: Lower Hutt, New Zealand, 1999. [Google Scholar]
- Scott, D.B.; Medioli, F.S.; Schafer, C.T. Monitoring in Coastal Environments Using Foraminifera and Thecamoebian Indicators; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Wang, P. Distribution of foraminifera in estuarine deposits: A comparison between Asia, Europe, and Australia. In Centenary of Japanese Micropaleontology; Ishizaki, K., Saito, T., Eds.; Terra Scientific Publishing Company: Tokyo, Japan, 1992; pp. 71–83. [Google Scholar]
- Zapata, J.; Álvarez, P.; Cea, C. Tecamebas del Río Contaco (40°33′12″ S; 73°43′00″ W), Osorno, Chile. Bol. Soc. Biol. Concepción 2002, 73, 17–35. [Google Scholar]
- Fernández, L. Foraminíferos (Protozoa: Foraminiferida) del estuario del río Contaco (40°33′ S; 73°43′ O), Chile. Bol. Biodivers. Chile 2010, 4, 18–62. [Google Scholar]
- Fernández, L.; Zapata, J. Registro tafonómico de Ammonia beccarii (Linné, 1758) (Protozoa: Foraminiferida) en la ensenada Quillaipe (41°32′ S; 72°44′ O), Chile. Lat. Am. J. Aquat. Res. 2010, 38, 286–291. [Google Scholar] [CrossRef]
- Fernández, L.; Zapata, J. Distribución de foraminíferos bentónicos (Protozoa: Foraminiferida) en la ensenada Quillaipe (41°32′ S; 72°44′ O), Chile: Implicaciones para el estudio del nivel del mar. Rev. Chil. Hist. Nat. 2010, 83, 567–583. [Google Scholar] [CrossRef]
- Stevens, G.C. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Stevens, G.C. Extending Rapoport’s rule to Pacific marine fishes. J. Biogeogr. 1996, 23, 149–154. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources, sinks, and population regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Almeida-Neto, M.; Guimarães, P.; Guimarães, P.R., Jr.; Loyola, R.D.; Ulrich, W. A consistent metric for nestedness analysis in ecological systems: Reconciling concept and measurement. Oikos 2008, 117, 1227–1239. [Google Scholar] [CrossRef]
- Colwell, R.K.; Lees, D.C. The mid-domain effect: Geometric constraints on the geography of species richness. Trends Ecol. Evol. 2000, 15, 70–76. [Google Scholar] [CrossRef]
- Xu, M.; Du, R.; Li, X.; Yang, X.; Zhang, B.; Yu, X. The mid-domain effect of mountainous plants is determined by community life form and family flora on the Loess Plateau of China. Sci. Rep. 2021, 11, 10974. [Google Scholar] [CrossRef] [PubMed]
- Fernández, L.D.; Seppey, C.V.W.; Singer, D.; Fournier, B.; Tatti, D.; Mitchell, E.A.D.; Lara, E. Niche conservatism drives the elevational diversity gradient in major groups of free-living soil unicellular eukaryotes. Microb. Ecol. 2022, 83, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Hewson, I.; Fuhrman, J.A. Richness and diversity of bacterioplankton species along an estuarine gradient in Moreton Bay, Australia. Appl. Environ. Microbiol. 2004, 70, 3425–3433. [Google Scholar] [CrossRef]
- Fernández, L.D.; Lara, E.; Mitchell, E.A.D. Checklist, diversity and distribution of testate amoebae in Chile. Eur. J. Protistol. 2015, 51, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Campello-Nunes, P.H.; Woelfl, S.; da Silva-Neto, I.D.; Paiva, T.d.S.; Fernández, L.D. Checklist, diversity and biogeography of ciliates (Ciliophora) from Chile. Eur. J. Protistol. 2022, 84, 125892. [Google Scholar] [CrossRef] [PubMed]
- Zapata, J.; Zapata, C.; Gutiérrez, A. Foraminíferos bentónicos recientes del sur de Chile. Gayana Zool. 1995, 59, 25–40. [Google Scholar]
- Zapata, J.; Moyano, H. Distribution of benthic foraminifera collected by the Akebono Maru “72” in southern Chile. Gayana Zool. 1996, 60, 89–98. [Google Scholar]
- Zapata, J.; Moyano, H. Foraminíferos bentónicos recientes de Chile Austral. Bol. Soc. Biol. Concepción 1997, 68, 27–37. [Google Scholar]
- Zapata, J. Foraminíferos bentónicos recientes de bahía Cumberland (33°41′ S; 78°50′ W), Archipiélago de Juan Fernández, Chile: Aspectos zoogeográficos. Bol. Soc. Biol. Concepción 1999, 70, 21–35. [Google Scholar]
- Marchant, M.; Hebbeln, D.; Wefer, G. High-resolution planktic foraminiferal record of the last 13,300 years from the upwelling area off Chile. Mar. Geol. 1999, 161, 115–128. [Google Scholar] [CrossRef]
- Hromić, T. Distribución latitudinal de foraminíferos bentónicos (Protozoa: Foraminiferida) a nivel de subórdenes y familias, en canales y fiordos patagónicos chilenos. Investig. Mar. 2006, 34, 71–81. [Google Scholar] [CrossRef]
- Hromić, T.; Ishman, S.; Silva, N.; Hromić, T.; Ishman, S.; Silva, N. Benthic foraminiferal distributions in Chilean fjords: 47° S to 54° S. Mar. Micropaleontol. 2006, 59, 115–134. [Google Scholar] [CrossRef]
- Marchant, M.; Hebbeln, D.; Giglio, S.; Coloma, C.; González, H.E. Seasonal and interannual variability in the flux of planktic foraminifera in the Humboldt Current System off central Chile (30° S). Deep-Sea Res. 2004, 51, 2441–2455. [Google Scholar] [CrossRef]
- Hromić, T.; Montiel, A. Foraminíferos bentónicos de Seno Gallegos y Bahía Brookes (54.5° S–69.5°S), Chile: Patrones de distribución y diversidad. An. Inst. Patagon. 2011, 39, 33–46. [Google Scholar] [CrossRef]
- Tavera Martínez, L.; Marchant, M.; Muñoz, P.; Abdala Díaz, R.T. Spatial and vertical benthic foraminifera diversity in the oxygen minimum zone of Mejillones Bay, northern Chile. Front. Mar. Sci. 2022, 9, 821564. [Google Scholar] [CrossRef]
- Tavera, L.; Fernández, L.; Marchant, M.; Hromic, T. The biogeography of benthic foraminifera is driven by ecological and historical processes in the Humboldt Current System-Southeastern Pacific. SSRN 2024, Preprint, 1–26. [Google Scholar] [CrossRef]
- Páez, M.; Zúñiga, O.; Valdés, J.; Ortlieb, L. Foraminíferos bentónicos recientes en sedimentos micróxicos de la bahía Mejillones del Sur (23º S), Chile. Rev. Biol. Mar. Oceanogr. 2001, 36, 129–139. [Google Scholar] [CrossRef]
- Cienfuegos, R.; Campino, J.R.; Gironás, J.; Almar, R.; Villagrán, M. River mouths and coastal lagoons in Central Chile. In The Ecology and Natural History of Chilean Saltmarshes; Fariña, J.M., Camaño, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 29–50. [Google Scholar]
- Scott, D.B.; Medioli, F.S. Living vs. total foraminiferal populations: Their relative usefulness in paleoecology. J. Paleontol. 1980, 4, 814–831. [Google Scholar]
- Horton, B.P.; Edwards, R.J. Quantifying Holocene sea-level change using intertidal foraminifera: Lessons from the British Isles. J. Foraminifer. Res. 2006, 40, 1–97. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.; Ryan, P. PAST: Paleontological statistics software for education and data analysis. Paleontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Boltovskoy, E. Distribution of recent foraminifera in the South America region. In Foraminifera; Hedley, R.H., Adams, C.G., Eds.; Academic Press: London, UK, 1976; pp. 273–276. [Google Scholar]
- Gotelli, N.; Entsminger, G. EcoSim: Null Models Software for Ecology, Version 7.72; Acquired Intelligence Incorporated and Kesey-Bear: Jericho, VT, USA, 2009. [Google Scholar]
- Chao, A.; Gotelli, N.J.; Hsieh, T.C.; Sander, E.L.; Ma, K.H.; Colwell, R.K.; Ellison, A.M. Rarefaction and extrapolation with Hill numbers: A framework for sampling and estimation in species diversity studies. Ecol. Monogr. 2014, 84, 45–67. [Google Scholar] [CrossRef]
- Rohde, K.; Heap, M.; Heap, D. Rapoport’s rule does not apply to marine teleosts and cannot explain latitudinal gradients in species richness. Am. Nat. 1993, 142, 1–16. [Google Scholar] [CrossRef]
- McCain, C.M. The mid-domain effect applied to elevational gradients: Species richness of small mammals in Costa Rica. J. Biogeogr. 2004, 31, 19–31. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 17 November 2024).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. R Package Version 2.2-0. 2014. Available online: http://CRAN.R-project.org/package=vegan (accessed on 17 November 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 17 November 2024).
- Bruni, E.P.; Rusconi, O.; Broennimann, O.; Adde, A.; Jauslin, R.; Krashevska, V.; Kosakyan, A.; Armynot du Châtelet, E.; Alcino, J.P.B.; Collart, F.; et al. Global distribution modelling of a conspicuous Gondwanian soil protist reveals latitudinal dispersal limitation and range contraction in response to climate warming. Divers. Distrib. 2024, 30, e13779. [Google Scholar] [CrossRef]
- Fernández, L.D.; Rau, J.; Arriagada, A. Calidad de la vegetación ribereña del Río Maullín, utilizando el índice QBR. Gayana Bot. 2009, 62, 269–278. [Google Scholar] [CrossRef]
- Attrill, M.J.; Rundle, S.D. Ecotone or ecocline: Ecological boundaries in estuaries. Estuar. Coast. Shelf Sci. 2002, 55, 929–936. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, D.; Li, Z.; Fernández, L.D. Editorial: Microbial Diversity and Ecosystem Functioning in Fragmented Rivers Worldwide. Front. Microbiol. 2023, 14, 1253190. [Google Scholar] [CrossRef] [PubMed]
- Parra-Gómez, A.; Fernández, L.D. Filling gaps in the diversity and biogeography of Chilean millipedes (Myriapoda: Diplopoda). Arthropod Syst. Phylogeny 2022, 80, 561–573. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, L.D.; Marchant, M. Rapoport’s Rule, the Ecotone Concept, and Salinity Gradient Predict the Distribution of Benthic Foraminifera in a Southeastern Pacific Estuary. Ecologies 2025, 6, 11. https://doi.org/10.3390/ecologies6010011
Fernández LD, Marchant M. Rapoport’s Rule, the Ecotone Concept, and Salinity Gradient Predict the Distribution of Benthic Foraminifera in a Southeastern Pacific Estuary. Ecologies. 2025; 6(1):11. https://doi.org/10.3390/ecologies6010011
Chicago/Turabian StyleFernández, Leonardo D., and Margarita Marchant. 2025. "Rapoport’s Rule, the Ecotone Concept, and Salinity Gradient Predict the Distribution of Benthic Foraminifera in a Southeastern Pacific Estuary" Ecologies 6, no. 1: 11. https://doi.org/10.3390/ecologies6010011
APA StyleFernández, L. D., & Marchant, M. (2025). Rapoport’s Rule, the Ecotone Concept, and Salinity Gradient Predict the Distribution of Benthic Foraminifera in a Southeastern Pacific Estuary. Ecologies, 6(1), 11. https://doi.org/10.3390/ecologies6010011





