Strength, Hardness, and Ductility Evidence of Solid Solution Strengthening and Limited Hydrogen Embrittlement in the Alloy System Palladium-Copper (Cu wt. % 5–25)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
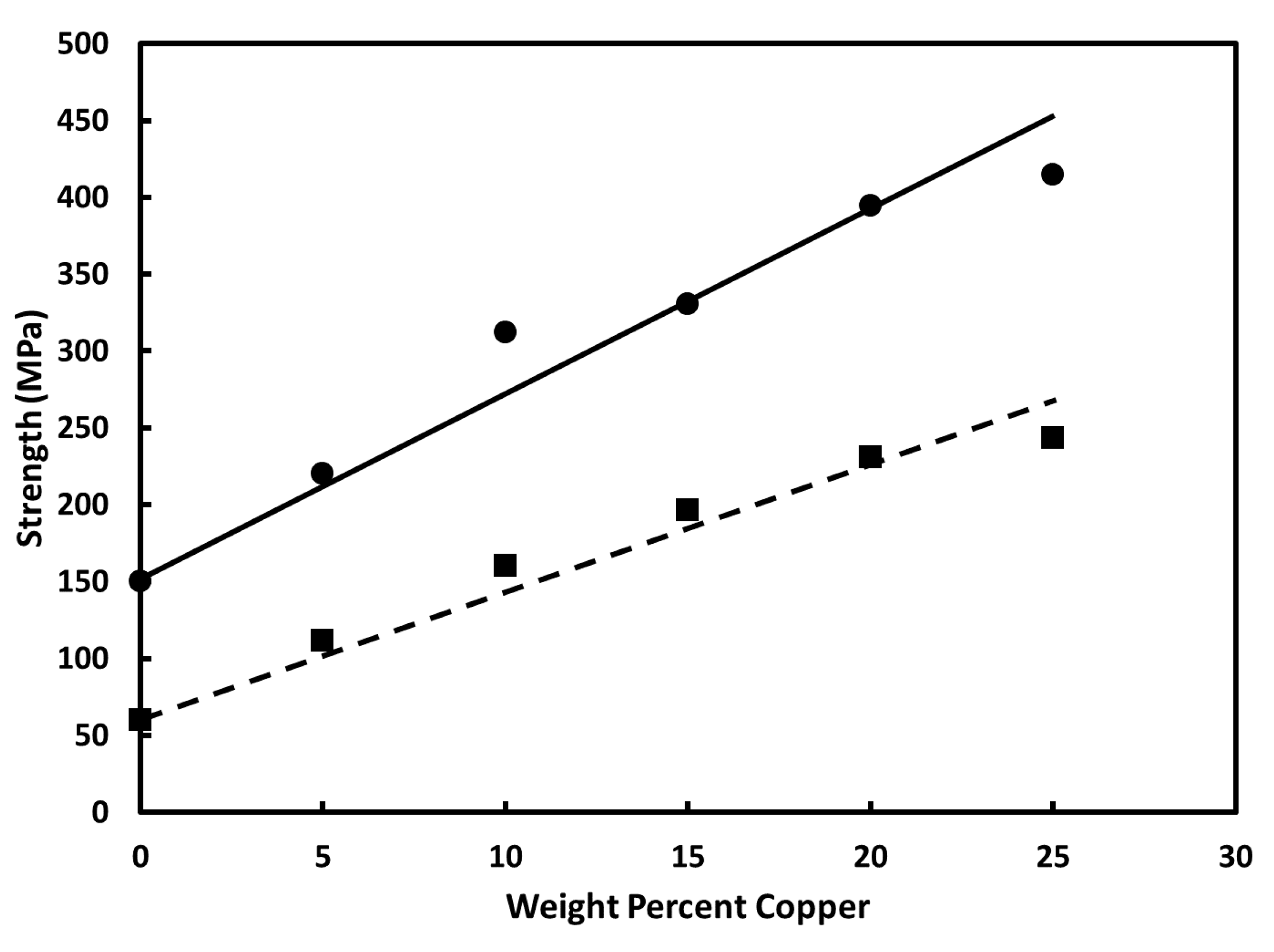
3.1. Vacuum-Annealed Alloys
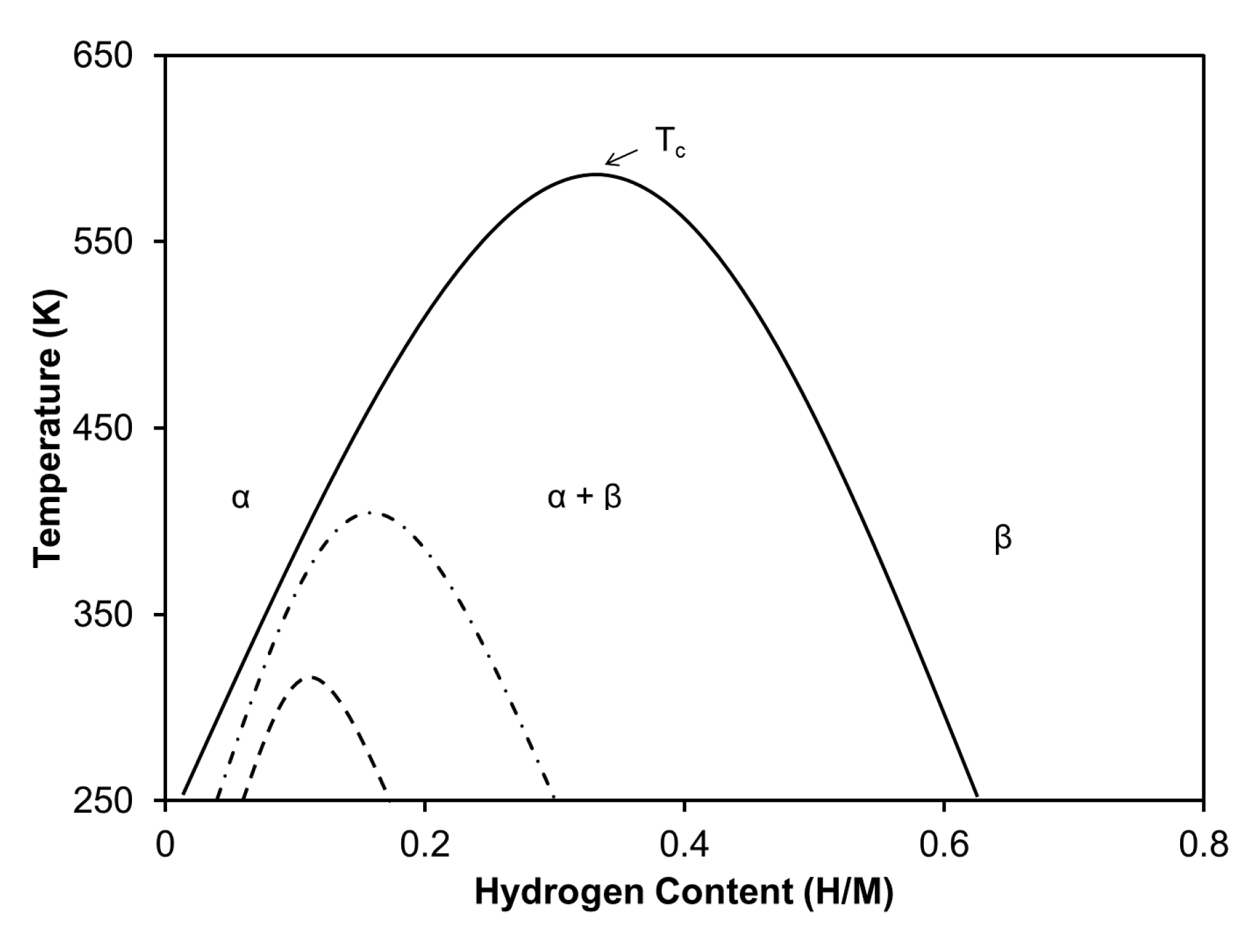
3.2. Hydrogen-Cycled Alloys
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grashoff, G.J.; Pilkington, C.E.; Corti, C.W. A Review of the Technology Emphasising the Current Status of Palladium Membrane Diffusion. Platinum Met. Rev. 1983, 27, 158–169. [Google Scholar]
- Ryi, S.K.; Park, J.S.; Kim, S.H.; Cho, S.H.; Park, J.S.; Kim, D.W. Development of a New Porous Metal Support of Metallic Dense Membrane for Hydrogen Separation. J. Membr. Sci. 2006, 279, 439–445. [Google Scholar] [CrossRef]
- Hatlevik, O.; Gade, S.K.; Keeling, M.K.; Thoen, P.M.; Davidson, A.P.; Way, J.D. Palladium and Palladium Alloy Membranes for Hydrogen Separation and Production: History, Fabrication Strategies, and Current Performance. Sep. Purif. Technol. 2010, 73, 59–64. [Google Scholar] [CrossRef]
- Burkhanov, G.S.; Gorina, N.B.; Kolchugina, N.B.; Roshan, N.R.; Slovetsky, D.I.; Chistov, E.M. Palladium-Based Alloy Membranes for Separation of High Purity Hydrogen from Hydrogen-Containing Gas Mixtures. Platinum Metals Rev. 2011, 55, 3–12. [Google Scholar] [CrossRef]
- Conde, J.J.; Marono, M.; Sanchez-Hervas, J.M. Pd-Based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement, 1st ed.; Springer: Singapore, 2016; pp. 103–135. [Google Scholar]
- Owen, C.V.; Scott, T.E. Relation between Hydrogen Embrittlement and the Formation of Hydride in the Group V Transition Metals. Metall. Mater. Trans. B 1972, 3, 1715–1726. [Google Scholar] [CrossRef]
- Dillon, E.; Jimenez, G.; Davie, A.; Bulak, J.; Nesbit, S.; Craft, A. Factors Influencing the Tensile Strength, Hardness, and Ductility of Hydrogen-Cycled Palladium. Mater. Sci. Eng. A 2009, 524, 89–97. [Google Scholar] [CrossRef]
- Musket, R.G. Effects of Contamination on the Interaction of Hydrogen gas with palladium: A Review. J. Less Common Met. 1976, 45, 173–183. [Google Scholar] [CrossRef]
- Okazaki, J.; Tanaka, D.; Tanco, M.; Wakui, Y.; Mizukami, F.; Suzuki, T. Hydrogen Permeability Study on the Thin Pd-Ag Alloy Membranes in the Temperature Range Across the α-β Phase Transition. J. Membr. Sci. 2006, 282, 370–374. [Google Scholar] [CrossRef]
- McLeod, L.; Degertekin, F.; Fedorov, A. Non-Ideal Absorption Effects on Hydrogen Permeation Through Palladium-Silver Membranes. J. Membr. Sci. 2009, 339, 109–114. [Google Scholar] [CrossRef]
- Millet, P.; Ngameni, R.; Decaux, C.; Grogoriev, S. Hydrogen Sorption by Pd77Ag23 Metalic Membranes. Role of Hydrogen Content, Temperature, and Sample Microstructure. Int. J. Hydrog. Energy 2011, 36, 4262–4269. [Google Scholar] [CrossRef]
- Pinto, F.; Andre, R.; Franco, C.; Carolino, C.; Gulyurtlu, I. Effect of Syngas Composition on Hydrogen Permeation through a Pd-Ag Membrane. Fuel 2013, 103, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Vadrucci, M.; Borgognoni, F.; Moriani, A.; Santucci, A.; Tosti, S. Hydrogen Permeation through Pd-Ag Membranes: Surface Effects and Sieverts’ Law. Int. J. Hydrog. Energy 2013, 38, 4144–4152. [Google Scholar] [CrossRef]
- Dahlmeyer, J.; Garrison, T.; Garrison, T.; Darkey, S.; Massicote, F.; Rebeiz, K.; Nesbit, S.; Craft, A. Effects of Hydrogen Exposure Temperature on the Tensile Strength, Microhardness and Ductility of Pd/Ag (25 wt.%) Alloy. Scr. Mater. 2011, 64, 789–792. [Google Scholar] [CrossRef]
- Rebeiz, K.; Dahlmeyer, J.; Garrison, T.; Garrison, T.; Darkey, S.; Paciulli, D.; Talukder, M.; Kubik, J.; Wald, K.; Massicote, F.; et al. Tensile Properties of a Series of Palladium-Silver Alloys Exposed to Hydrogen. J. Energy Eng. 2014, 141, 04014029. [Google Scholar] [CrossRef]
- Wald, K.; Kubik, J.; Paciulli, D.; Talukder, M.; Nott, J.; Massicote, F.; Rebeiz, K.; Nesbit, S.; Craft, A. Effects of Multiple Hydrogen Absorption/Desorption Cycles on the Mechanical Properties of the Alloy System Palladium/Silver (wt% 10–25). Scr. Mater. 2016, 117, 6–10. [Google Scholar] [CrossRef]
- Jimenez, G.; Dillon, E.; Dahlmeyer, J.; Garrison, T.; Garrison, T.; Darkey, S.; Paciulli, D.; Talukder, M.; Nott, J.; Ferrer, M.; et al. A Comparative Assessment of Hydrogen Embrittlement: Palladium and Palladium-Silver (25 Weight% Silver) Subjected to Hydrogen Absorption/Desorption Cycling. Adv. Chem. Eng. Sci. 2016, 6, 246–261. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Way, J.D. Palladium-copper membranes for hydrogen separation. Sep. Purif. Technol. 2017, 186, 39–44. [Google Scholar] [CrossRef]
- Rebeiz, K.; Craft, A. Tensile Characteristics of Palladium Exposed to Hydrogen (Deuterium). ASCE J. Energy Eng. 2000, 126, 95–106. [Google Scholar] [CrossRef]
- Subramanian, P.; Laughlin, D. Cu-Pd (copper-palladium). J. Phase Equilib. 1991, 12, 231–243. [Google Scholar] [CrossRef]
- Askeland, D.; Wright, W. The Science and Engineering of Materials, 7th ed.; Cengage: Boston, MA, USA, 2015; pp. 357–359. [Google Scholar]
- Timofeev, N.; Berseneva, F.; Makarov, M. New Palladium-based Membrane Alloys for Separation of Gas Mixtures to Generate Ultrapure Hydrogen. Int. J. Hydrog. Energy 1994, 19, 895–898. [Google Scholar] [CrossRef]
- Manchester, F.D.; San-Martin, A.; Pitre, J.M. The H-Pd (hydrogen-palladium) System. J. Phase Equilib. 1994, 15, 62–83. [Google Scholar] [CrossRef]
- Wang, D.; Flanagan, T.B.; Balasubramaniam, B. Hydrogen Solubility as a Probe for Dislocation Formation, Rearrangement, and Annihilation in Pd and Pd/Al2O3 Composites. Scr. Mater. 1999, 41, 517–521. [Google Scholar] [CrossRef]
- Wise, M.L.; Farr, J.P.; Harris, I.R. X-ray Studies of the α/β Miscibility Gaps of Some Palladium Solid Solution-Hydrogen Systems. J. Less Common Met. 1975, 41, 115–127. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Baba, K.; Flanagan, T.B. The Effect of Alloying of Palladium on the Hydrogen-Palladium Miscibility Gap. Zeitschrift fuer Physikalische Chemie 1988, 158, 223–235. [Google Scholar] [CrossRef]
- Fazle Kibria, A.K.M.; Sakamoto, Y. The Effect of Alloying of Palladium with Silver and Rhodium on the Hydrogen Solubility, Miscibility Gap and Hysteresis. Int. J. Hydrog. Energy 2000, 25, 853–859. [Google Scholar] [CrossRef]
- Burch, R.; Buss, R.G. Pressure-Composition Isotherms in the Palladium-Copper-Hydrogen System. Solid State Commun. 1974, 15, 407–409. [Google Scholar] [CrossRef]
- Burch, R.; Buss, R.G. Absorption of Hydrogen by Palladium-Copper Alloys, Part 1 Experimental Measurements. J. Chem. Soc. Faraday Trans. 1 1975, 71, 913–921. [Google Scholar] [CrossRef]
- Flanagan, T.B.; Chisdes, D.M. Solubility of Hydrogen (1 atm, 298 K) in Some Copper/Palladium Alloys. Solid State Commun. 1975, 16, 532–592. [Google Scholar] [CrossRef]
- Fisher, D.; Chisdes, D.M.; Flanagan, T.B. Solution of Hydrogen in Palladium/Copper Alloys. J. Solid State Chem. 1977, 20, 149–158. [Google Scholar] [CrossRef]






| % Solute | 5% Ag | 5% Cu | 15% Ag | 15% Cu | 25% Ag | 25% Cu | |
|---|---|---|---|---|---|---|---|
| Property | |||||||
| Yield Strength (MPa) | 18 | 85 | 90 | 226 | 107 | 305 | |
| Ultimate Strength (MPa) | 16 | 46 | 56 | 120 | 83 | 176 | |
| Vickers Microhardness (VHN) | 9 | 36 | 43 | 72 | 74 | 111 | |
| Total Elongation (%) | 5 | 5 | 5 | 5 | 5 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DiMauro, S.; Legall, G.; Lubinsky, C.; Nadeau, M.; Tait, R.; Miller, W.; Adutwum, A.; Portal, I.; Roy, B.; Nesbit, S.; et al. Strength, Hardness, and Ductility Evidence of Solid Solution Strengthening and Limited Hydrogen Embrittlement in the Alloy System Palladium-Copper (Cu wt. % 5–25). Hydrogen 2021, 2, 262-272. https://doi.org/10.3390/hydrogen2030014
DiMauro S, Legall G, Lubinsky C, Nadeau M, Tait R, Miller W, Adutwum A, Portal I, Roy B, Nesbit S, et al. Strength, Hardness, and Ductility Evidence of Solid Solution Strengthening and Limited Hydrogen Embrittlement in the Alloy System Palladium-Copper (Cu wt. % 5–25). Hydrogen. 2021; 2(3):262-272. https://doi.org/10.3390/hydrogen2030014
Chicago/Turabian StyleDiMauro, Sebastian, Gabrielle Legall, Coleman Lubinsky, Monica Nadeau, Renee Tait, William Miller, Abena Adutwum, Isabella Portal, Brandon Roy, Steve Nesbit, and et al. 2021. "Strength, Hardness, and Ductility Evidence of Solid Solution Strengthening and Limited Hydrogen Embrittlement in the Alloy System Palladium-Copper (Cu wt. % 5–25)" Hydrogen 2, no. 3: 262-272. https://doi.org/10.3390/hydrogen2030014
APA StyleDiMauro, S., Legall, G., Lubinsky, C., Nadeau, M., Tait, R., Miller, W., Adutwum, A., Portal, I., Roy, B., Nesbit, S., & Craft, A. (2021). Strength, Hardness, and Ductility Evidence of Solid Solution Strengthening and Limited Hydrogen Embrittlement in the Alloy System Palladium-Copper (Cu wt. % 5–25). Hydrogen, 2(3), 262-272. https://doi.org/10.3390/hydrogen2030014






