Abstract
The fusion reactions involving deuterium are of great interest for the exploitation of the fusion energy via magnetic-confinement devices. In classical thermodynamics, the spontaneity of a process is established through the assessment of the change in Gibbs free energy. So far, the feasibility of nuclear reactions has been characterized in terms of cross section and Q-value while the entropic term (T ΔS) has been neglected. Such an assumption is always justified for fission reactions where the term ΔS is positive. In the case of fusion reactions that operate at very high temperatures (106–107 K) and where ΔS is negative, the change in Gibbs free energy may be positive, making the reaction non-spontaneous. This paper proposes a classical thermodynamic analysis of D-based reactions of interest for the magnetic-confinement fusion applications. The entropy contribution was evaluated via the Sackur–Tetrode equation while the change in enthalpy was considered constant and as corresponding to the Q-value of the fusion reaction. The results of the thermodynamic analysis are compared with nuclear reaction feasibility criteria based on the reaction reactivity. The DT and D3He reactions show a high degree of spontaneity although the second one presents a lower reactivity. An increase in temperature could enhance the reactivity of the D3He reaction at the cost of decreasing its thermodynamic spontaneity. Both branches of the DD reaction are characterized by a much lower thermodynamic spontaneity than that of the DT and D3He reactions. Furthermore, at the temperature of their maximum cross section, the DD reactions exhibit a largely positive change in Gibbs free energy and, therefore, are not spontaneous. At the temperature of magnetic-confinement fusion machines (1.5 × 108 K), among the D-based reactions studied, the DT one exhibits the highest degrees of spontaneity and reactivity.
1. Introduction
Although the COVID-19 pandemic is still subduing the economy, in 2021 the global energy demand is come back above the 2019 levels and exhibits still a significant share of fossil fuels with, in particular, the coal demand approaching its 2014 peak [1]. Under this scenario, in order to control the emissions of CO2 and other greenhouse gases (GHG) the national and worldwide energy strategies are aimed at advancing the penetration of renewable sources (solar, photovoltaic, biomass) and the use of cleaner energy vectors (namely hydrogen) [2,3,4,5,6].
Fusion energy has zero-carbon emissions and is considered capable of contributing to the decarbonization of the energy systems along the lines established by international agreements [7]. In this light, the exploitation of nuclear fusion reactions on Earth may provide humankind with a sustainable, safe and environmentally friendly source of energy [8,9]. Since its inception in the 1940s, nuclear fusion research is mainly based on the magnetic confinement, an approach that uses magnetic fields to confine a plasma of light atoms that react and produce energy in devices called tokamak [10,11,12,13]. The plasma, which reaches very high temperatures (about 150 million °C for the D-T reaction), has to be confined to relatively small distances and, therefore, in these machines the resulting very large temperature gradients represent the main difficulties in fusion research. In fact, these temperature gradients induce large turbulence in the plasma making its control difficult from a physical point of view, while the high temperatures and thermal fluxes generated have to be withstood by the plasma facing components.
In a nuclear fusion reaction, two nuclei, or a nucleus and an external subatomic particle, merge to produce a heavier nucleus and a lighter particle [14].
Following the classical thermodynamic approach based on the evaluation of the change in Gibbs free energy, the main objective of this work is to establish when a fusion process or reaction is “spontaneous”, as will be discussed in the next Section.
Presently, some criteria based on the energy balance at the plasma level have been proposed for the assessment of the scientific feasibility of magnetic nuclear fusion [15]. In order to produce electricity, a plasma has to operate at a high temperature with a sufficient density and for sufficient time so that the released fusion energy compensates for the power losses from the plasma itself. These conditions are determined through the assessment of three parameters: the plasma density (np), the temperature (T) and the energy confinement time (τE). Their product is called the “triple product”, a parameter used to rank the performance of the tokamaks existing in the laboratories or designed for future research. Based on the balance between the thermonuclear power generated (A) and the power lost from the plasma (B), Lawson’s criterion has been introduced [16]. This criterion has been given in several versions (e.g., “plasma breakeven”, “ignition”) that differ each other for the fraction of B considered to be useful for providing energy to the plasma (e.g., that carried by alpha particles) or that may be converted to electricity. For instance, for the DT reaction, under the assumption that the fusion power carried out by the alpha particles is equal to the losses for conduction, Lawson’s criterion leads to calculating the following triple product:
np T τE ≥ 3 × 1021 m−3 keV s
In a more general approach, the analysis of a nuclear reaction relies on the assessment of its exothermicity (amount of energy released) and reaction rate. The amount of energy released is expressed by the Q-value that corresponds, through the Einstein’s equation, to the mass defect calculated as the difference between the total mass of reactants and that of the products of the reaction. The Q-values of nuclear reactions are of the order of 1 MeV per nucleon, much larger than those of the chemical reactions (~1 eV per nucleon). The reaction rate, i.e., the number of reactions per unit time and per unit volume, is proportional to the cross section (σ) and the reactivity (<σ v>), as will explained in detail.
The fusion reactions involving deuterium are of great interest for the exploitation of fusion energy via magnetic-confinement devices. Their feasibility is in general characterized in terms of cross section and Q-value. The most studied reaction is that between deuterium and tritium that react to form 4He and a neutron. This reaction exhibits a high value of maximum cross section at a relatively low temperature:
with a Q-value of 17.586 MeV and a maximum cross section of 5.0 barn at 64 keV.
The fusion reaction of two deuterium atoms has two branches, both with branching ratio 0.50, characterized by cross sections lower and achieved at higher temperature with respect to reaction (1). The first branch of the DD reaction is:
with a Q-value of 3.267 MeV and a maximum cross section of 0.096 barn at 1250 keV, while the second branch is:
with a Q-value of 4.032 MeV and a maximum cross section of 0.11 barn at 1750 keV.
The reaction between deuterium and 3Helium, then, is:
with a Q-value of 18.351 MeV and a maximum cross section of 0.9 barn at 250 keV.
It is noteworthy that, since the reacting particles are characterized by a given velocity distribution, their temperature is expressed in terms of the energy that corresponds to the centre-of-mass of the distribution. Hence, in nuclear physics the temperature is usually measured in keV (1 keV/kB = 1.16 × 107 K, where kB is the Boltzmann constant).
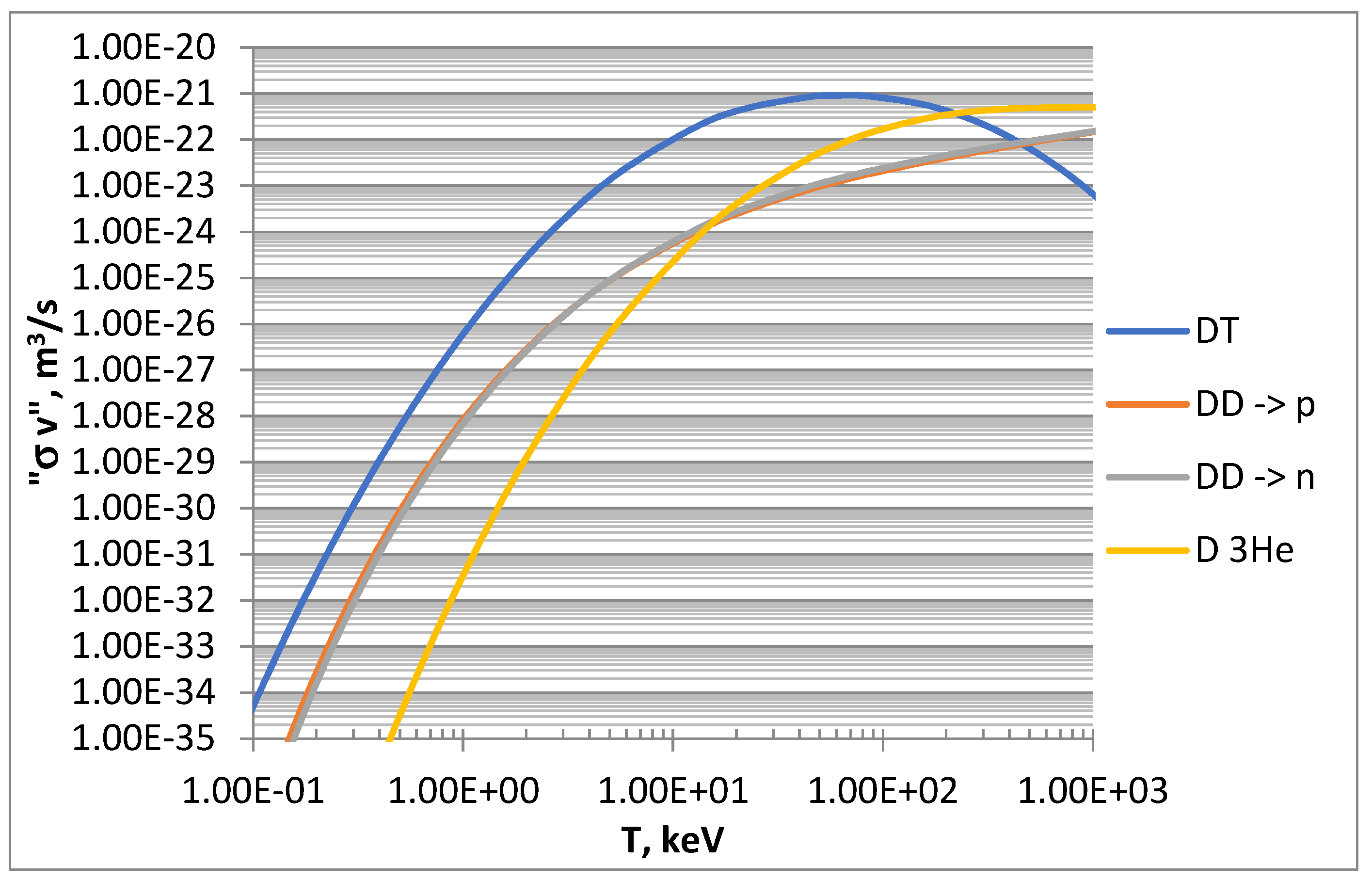
As introduced above, the effectiveness of a fusion reaction can be described by its reactivity, a parameter giving the probability of reaction per unit time and per unit density of the target nuclei. Reactivity is the result of the product of the cross section (σ) and the particle velocity (v) and therefore is expressed in m3/s. The reaction rate, i.e., the number of reactions per unit time and volume, is then obtained from the reactivity via its product with the density of the particles involved. Once the distribution of the velocities of the reacting nuclei is defined, it is possible to evaluate an average reactivity <σ v> through the parameterization of experimental data [17]. For the scope of this work, the average reactivities of the D-based fusion reactions, shown in Figure 1, have been calculated by considering Maxwellian velocity distributions and using simplified formulas from the literature [18,19].
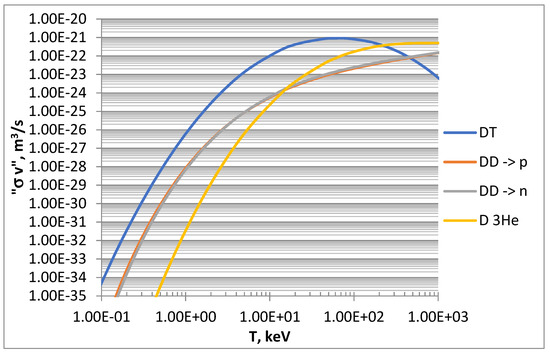
Figure 1.
Maxwell-averaged reactivities of D-based reactions calculated from literature [18,19].
Compared to a classical thermodynamic analysis, the approach so far adopted to study the feasibility of fusion reactions misses the evaluation of the entropic contribution which, indeed, will be the main innovative aspect introduced by this paper. While the entropic contribution can be neglected in the feasibility analysis of fission reactions, it can assume a key role for determining the spontaneity of fusion processes [20]. Accordingly, this paper aims to perform a classical thermodynamic analysis of D-based nuclear fusion reactions and establish their spontaneity level by calculating the change in Gibbs free energy. The results of this analysis are compared with the feasibility criteria used thus far to study the nuclear fusion reactions which are based on the reaction reactivity.
2. Process Analysis via Classical Thermodynamics
In classical thermodynamics, the criterion of predicting the spontaneity of a reaction or, more generally, of a process relies on the assessment of the change in Gibbs free energy (ΔG). From the thermodynamic point of view, a process, intended as the transition from an initial to a final state, occurs spontaneously if ΔG < 0, while the condition ΔG > 0 indicates that the reverse process or reaction is proceeding. When ΔG = 0 the system is at equilibrium, that is, no net exchange of matter or energy takes place. It is important to recall that, once an initial and final state have been defined, a thermodynamic analysis is uniquely determined, and, therefore, the change in Gibbs free energy (between these initial and final states) does not depend on the intermediate states or the reaction pattern followed. Such a concept will be exploited in the following calculations of the change in entropy in which the final state of the fusion reactions will be selected properly.
The Gibbs free energy, G (J mol−1), is expressed by:
where H is the enthalpy (J mol−1), T (K) the temperature and S the entropy (J mol−1 K−1). The change in G can be calculated at constant temperature and pressure by:
where the change in enthalpy ΔH corresponds to the energy released by the system that, for a nuclear fusion reaction, could be expressed to a good approximation by the “Q-value” as discussed in a previous work [20]. According to the different rules adopted in nuclear physics and classical thermodynamics, the “Q-value” is positive for exothermal reactions and negative for endothermal reactions, while the opposite sign is adopted for the change in enthalpy ΔH. That is, we can write:
G = H − T S
ΔG = ΔH − T ΔS
Q-value = −ΔH
According to Equations (6) and (7), ΔG expresses the energy released by the system (ΔH) minus the entropic contribution “T ΔS”. When negative, the change in G corresponds to the maximum work that a system can do on the surroundings at constant temperature and pressure, and vice versa. Generally, in nuclear fusion reactions, where light atoms merge into heavier elements, the change in entropy is supposed to be negative [20]. In this sense, the expression in (6) is telling us that only a part of all the energy released by an exothermic fusion reaction (Q-value = −ΔH) is made available to perform work (−ΔG) since some of it (−T ΔS) is spent to make the system more ordered. In nuclear physics, due to the enormous amount of energy released by a nuclear reaction (Q-value >> 0, ΔH << 0), the entropic term (T ΔS) has been so far neglected by the feasibility analyses of both fission and fusion reactions. Such an assumption is always justified for fission reactions where the ΔS term is positive. In the case of fusion reactions that operate at very high temperatures (106–107 K) and where ΔS is negative, the change in Gibbs free energy may be positive (i.e., when |T ΔS| > |ΔH|) making the reaction non-spontaneous above the temperature at which the condition ΔG = 0 occurs. Such a temperature, that establishes the achievement of the equilibrium conditions and then the passage from a spontaneous (ΔG < 0) to a non-spontaneous (ΔG > 0) reaction, was introduced and defined as follows under the hypothesis of constant pressure and temperature [20]:
For the scope of this work, the change in enthalpy is assumed to be constant (ΔH = −Q-value) while the change in entropy has been evaluated through statistical thermodynamic methods. For instance, such an approach has been used to calculate the entropy of “compound nuclei”, intermediate compounds consisting of target nuclei and the projectiles of nuclear reactions in excited states [21,22]. Specifically, it is possible to calculate statistical analogues of thermodynamic properties through the assessment of the partition function of the system (Q) at constant temperature and volume [23].
The entropy (S) is related to Q by the following expression:
where k is the Boltzmann constant and T the absolute temperature.
For a perfect gas, the partition function is given by:
where N is the number of particles, m their mass, h the Plank constant and fint the partition function of each particle (or molecule). For monoatomic compounds fint is equal to 1.
Combining the above expressions, the Sackur–Tetrode equation is obtained:
where n is the number of moles (1 mole = 6.02214076 × 1023 particles), P is the pressure (atm), M the molecular weight and R the gas constant.
The theory behind the Sackur–Tetrode equation could justify its application for assessing the entropy of atoms, while its use for the sub-nuclear particles involved in fusion reactions (neutrons, protons, etc.) is questionable. For this reason, in order to apply formula (11) correctly, the initial and final states of the fusion reactions must be selected among those in which only particles in the form of atoms are present. For the reactions (1)–(4), the initial state consists of two monatomic atoms whose entropy can easily evaluated via the Sackur–Tetrode expression. The final state has to be specifically chosen in correspondence with the condition in which all the subatomic particles (i.e., neutrons and protons) present in the second term of Formulas (1)–(4) have disappeared and their energy has been transferred to the surrounding system. In this way, the products of these reactions consist only of mono-atomic particles (4He, tritium and 3He) plus an amount of heat corresponding to the Q-value. Such an assumption complies with the architecture of the tokamak machines presently studied where the energy carried by the neutrons is changed into heat at the level of the shielding and/or blanket systems.
Accordingly, the fusion reactions (1)–(4) can be rewritten adopting the style typical of chemical reactions by obtaining the following expressions:
In this vein, the temperature adopted in the calculations is that commonly referred as the ions temperature. The pressure of the reacting matter used for this entropy assessment, namely, the plasma one, is 5 × 10−5 atm (≈5 Pa), a value in agreement with both experiments and designs of magnetic fusion devices [24,25]. According to Formula (11), the change of pressure has a modest influence on the entropy assessment and, therefore, the variation of this parameter has not been considered.
With this selection of the initial and final states, ΔG is calculated through the following expression that takes into account the temperature variation:
where the initial state corresponds to the reactants at the plasma temperature and the final one to the reaction products at 700 K, a temperature that is representative of the tokamak shielding or blanket systems.
In this approach, T* corresponding to the value of temperature at which ΔG = 0 can be evaluated by solving the following expression:
Q-value = [T S]700 K − [T S]T*
In summary, the main hypotheses considered henceforth for the assessment of the fusion reactions are:
- -
- the reacting particles, at plasma state, behave like a perfect gas;
- -
- the change in enthalpy is assumed to be constant and is defined by Formula (7);
- -
- according to the initial and final states defined for fusion reactions (12–15), the contribution of the sub-nuclear particles (neutrons, protons, etc.) to the assessment of the thermodynamic functions is neglected.
3. Results and Discussion
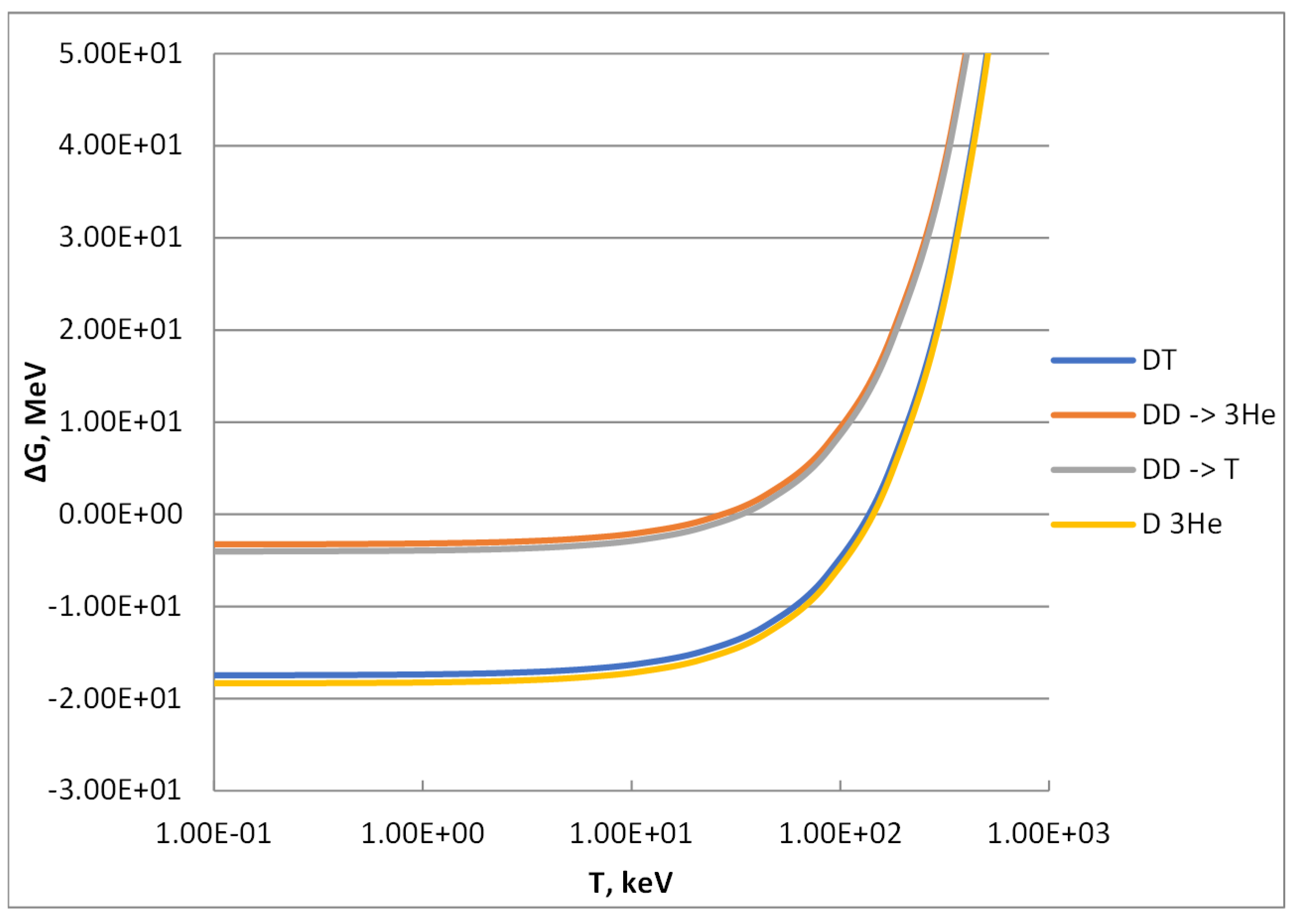
The values of entropy calculated through formula (11) mainly depend on the molecular weight of the particles, and, therefore, they are identical for the reactants and products of reactions (1) and (4) and for those of reactions (2) and (3), respectively. Accordingly, the ΔG values of reactions (1) and (4) and those of reactions (2) and (3) are very close each other and differ only for the contribution to this parameter coming from the Q-values. In particular, these are 17.586 MeV vs. 18.351 MeV for reactions (1) and (4) and 3.267 MeV vs. 4.032 MeV for reactions (2) and (3). The resulting ΔG values calculated through formula (16) are reported in Figure 2.
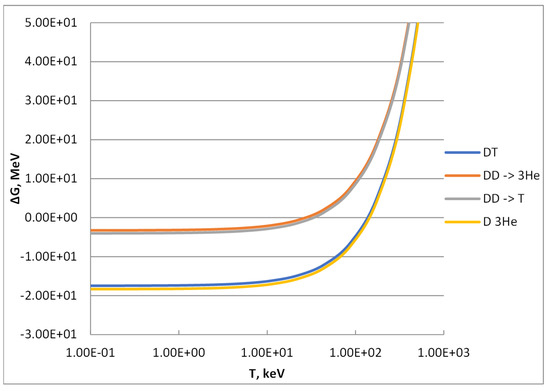
Figure 2.
Change of Gibbs free energy of the D-based reactions reported in the graph legend as follows: (1) = DT, (2) = DD -> T, (3) DD -> 3He and (4) = D3He.
Hereafter, the assessment of the change of Gibbs free energy for the D-based reactions is discussed in the relationship with the reactivity, the parameter used so far to evaluate the feasibility of the nuclear fusion processes.
At low temperature, the contribution of the entropic term (TS) is negligible, and the change of Gibbs free energy is almost equal to the change in enthalpy (i.e., the opposite of the Q-value). As shown in Figure 2, at low temperature reactions (1) and (4) exhibit the minimum values of ΔG, around minus 17–18 MeV (i.e., minus 1.6–1.7 × 1012 J/mol), and, therefore, these reactions are very spontaneous from the thermodynamic point of view. Both branches of the DD reaction, formulas (2) and (3), show quite similar values of ΔG along the T as well: these values at low temperature are about minus 3.2 and 4.0 MeV (i.e., minus 3.1–3.9 × 1011 J/mol), indicating a spontaneity level 4–5 times lower than those of reactions (1) and (4).
The values of the limit temperature T* at which the equilibrium conditions are achieved (ΔG = 0) by the D-based reactions considered in this study and calculated through expression (17) are reported in Table 1 together with the values of ΔG calculated at the temperature of interest for the feasibility analysis of fusion reactions. In this table, the average reactivities <σ v> at 13 keV have been calculated using simplified formulas from the literature [18,19].

Table 1.
Values of ΔG and temperatures of interest for the analysis of the reactions (1–4).
Reaction (1) is studied to create fusion on the Earth in devices that through magnetic fields confine plasma at around 1.5 × 108 K (≈13 keV). At this temperature the reactivity <σ v> of the reaction (1) is 1.91 × 10−22 m3/s and the ΔG is about −16.0 MeV. These values indicate a high degree of thermodynamic spontaneity of the DT reaction when carried out in the magnetic-confinement devices existing or under design.
Although reaction (4) exhibits at 1.5 × 108 K a high spontaneity level (ΔG = −16.8 MeV), it appears to be less practicable since its reactivity is about 7.40 × 10−25 m3/s, i.e., three orders of magnitude lower than that of reaction (1). On the contrary, at a temperature of 2.9 × 109 K (around 250 keV), corresponding to its maximum cross section, the ΔG reaction is positive (9.81 MeV), showing that the process is not spontaneous. This is a clear example of conflict between thermodynamic and kinetic optimization: best values of ΔG occur at temperature (1.5 × 108 K) quite below its maximum reactivity <σ v> that instead takes place at 2.9 × 109 K. In other words, by operating the reaction (4) at temperatures higher than 1.5 × 108 K the thermodynamics foresees a reduction of the amount of D and 3He converted although its reaction probability increases.
Similar considerations can be done for both branches (3) and (4) of the DD reaction. Below 3.10 and 3.88 × 108 K, their thermodynamic spontaneity is, respectively, verified (ΔG < 0) although at these temperatures their reactivity is very poor. For instance, at 1.5 × 108 K, ΔG is −1.77 and −2.53 MeV for reactions (2) and (3), respectively. At this temperature, the reactivity of these reactions is about 10−24 m3/s against the value of 10−22 m3/s exhibited by reaction (1), as verified in fusion experimental devices where the amount of DD fuel reacting is at least two orders of magnitude lower than that of the DT reaction.
4. Conclusions
This work analyses, through a classical thermodynamic approach, the D-based reactions of interest for magnetic-confinement fusion applications. In an innovative way, this analysis introduces the evaluation of the entropic contribution, a factor so far neglected by the feasibility evaluations of nuclear reactions which were based on the evaluation of the Q value (enthalpy contribution) and of the reaction kinetics (reactivity). As verified in this work, the entropic contribution can control the change in Gibbs free energy occurring in fusion reactions and then determine the temperature limits for having spontaneous processes.
From the thermodynamic point of view, at the temperature of magnetic-confinement fusion machines (1.5 × 108 K), both reactions DT and D3He exhibit a high level of spontaneity, although the last one presents a lower reactivity. An increase in temperature could enhance the reactivity of reaction D3He despite, however, decreasing its thermodynamic spontaneity since it results in a ΔG = 0 at 1.65 × 109 K.
Both branches of the DD reaction are not of practical interest because they are characterized by a lower thermodynamic spontaneity than that of the DT and D3He reactions. In particular, at the temperature of their maximum cross section where the reaction kinetics is favoured, the DD reactions exhibit a largely positive change in Gibbs free energy since their entropic contribution (TΔS) is not balanced by the Q-value (−ΔH).
In practice, the results of this study confirm that among the D-based fusion reactions the DT reaction exhibits the best feasibility even when the entropic contribution to the change in Gibbs free energy is considered.
Author Contributions
Conceptualization, S.T. and L.M.; methodology, S.T. and L.M.; validation, S.T. and L.M.; formal analysis, S.T. and L.M.; investigation, S.T. and L.M.; writing—original draft preparation, S.T.; writing—review and editing, S.T.; supervision, S.T.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been carried out within the framework of the EUROfusion Consortium and has received funding from the Euratom research and training programme 2014–2018 and 2019–2020 under Grant agreement No 633053. The views and opinions expressed herein do not necessarily reflect those of the European Commission.
Acknowledgments
The authors thank Antonino Pietropaolo for his suggestions and comments during a revision of this paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Global Energy Review 2021, IEA Report. April 2021. Available online: https://www.iea.org/reports/global-energy-review-2021?mode=overview (accessed on 19 January 2021).
- Marseglia, G.; Rivieccio, E.; Medaglia, C.M. The dynamic role of Italian Energy strategies in the worldwide scenario. Kybernetes 2019, 48, 636–649. [Google Scholar] [CrossRef]
- Capurso, T.; Stefanizzi, M.; Torresi, M.; Camporeale, S.M. Perspective of the role of hydrogen in the 21st century energy transition. Energy Convers. Manag. 2022, 251, 114898. [Google Scholar] [CrossRef]
- Maestre, V.M.; Ortiz, A.; Ortiz, I. Challenges and prospects of renewable hydrogen-based strategies for full decarbonization of stationary power applications. Renew. Sustain. Energy Rev. 2021, 152, 111628. [Google Scholar] [CrossRef]
- Trelles, J.P. Solar-plasma reactors for CO2 conversion. J. Phys. D Appl. Phys. 2022, 55, 103001. [Google Scholar] [CrossRef]
- Tosti, S.; Pozio, A.; Farina, L.; Santucci, A. Hydrogen and oxygen production via water splitting in a solar-powered membrane reactor—A conceptual study. Hydrogen 2021, 2, 18–32. [Google Scholar] [CrossRef]
- Gi, K.; Sano, F.; Akimoto, K.; Hiwatari, R.; Tobita, K. Potential contribution of fusion power generation to low-carbon development under the Paris Agreement and associated uncertainties. Energy Strategy Rev. 2020, 27, 100432. [Google Scholar] [CrossRef]
- Ongena, J. Fusion: A true challenge for an enormous reward. EPJ Web Conf. 2018, 189, 00015. [Google Scholar] [CrossRef][Green Version]
- Ongena, J.; Koch, R.; Wolf, R.; Zohm, H. Magnetic-confinement fusion. Nat. Phys. 2016, 12, 398–410. [Google Scholar] [CrossRef]
- Post, R.F. Controlled fusion research—An application of the physics of high temperature plasmas. Rev. Mod. Phys. 1956, 28, 338. [Google Scholar] [CrossRef]
- Artsimovich, L. The road to controlled nuclear fusion. Nature 1972, 239, 18–22. [Google Scholar] [CrossRef]
- Toschi, R. Nuclear fusion, an energy source. Fusion Eng. Des. 1997, 36, 1–8. [Google Scholar] [CrossRef]
- Sheffield, J. Physics requirements for an attractive magnetic fusion reactor. Nucl. Fusion. 1985, 25, 1733. [Google Scholar] [CrossRef]
- Atzeni, S.; Meyer-ter-Vehn, J. The Physics of Inertial Fusion: Beam Plasma Interaction, Hydrodynamics, Hot Dense Matter; Oxford University Press: Oxford, UK, 2008. [Google Scholar] [CrossRef]
- Jackson, T. Is fusion feasible? An assessment of the methodology and policy implications. Energy Policy 1989, 17, 407–412. [Google Scholar] [CrossRef]
- Hartwig, S.Z.; Podpaly, A.Y.; Magnetic Fusion Energy Formulary, MIT Plasma Science and Fusion Center, Revision. February 2014. Available online: https://www-internal.psfc.mit.edu/research/MFEFormulary/files/MFEFormulary.pdf (accessed on 19 January 2021).
- Bosch, H.-S.; Hale, G.M. Improved formulas for fusion cross-sections and thermal reactivities. Nucl. Fusion 1992, 32, 611. [Google Scholar] [CrossRef]
- Hively, L.M. Convenient computational forms for Maxwellian reactivities. Nucl. Fusion 1977, 17, 873. [Google Scholar] [CrossRef]
- Hively, L.M. A simple computational form for Maxwellian reactivities. Nucl. Technol. Fusion 1983, 3, 199–200. [Google Scholar] [CrossRef]
- Tosti, S. Spontaneity of nuclear fusion: A qualitative analysis via classical thermodynamics. Open Res. Eur. 2021, 1, 67. [Google Scholar] [CrossRef]
- Isvoranu, D.; Badescu, V. Radiation exergy: The case of thermal and nuclear energy. Int. J. Nucl. Gov. Econ. Ecol. 2008, 2, 90–112. [Google Scholar] [CrossRef]
- Badescu, V. Exergy of nuclear radiation—A quantum statistical thermodynamics approach. Cent. Eur. J. Phys. 2009, 7, 141–146. [Google Scholar] [CrossRef]
- Denbigh, K.G. The Principles of Chemical Equilibrium: With Applications in Chemistry And Chemical Engineering; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Garcia, J.; Challis, C.; Gallart, D.; Garzotti, L.; Görler, T.; King, D.; Mantsinen, M.; JET Contributors. Challenges in the extrapolation from DD to DT plasmas: Experimental analysis and theory based predictions for JET-DT. Plasma Phys. Control. Fusion 2017, 59, 014023. [Google Scholar] [CrossRef]
- Giegerich, T.; Day, C.; Gliss, C.; Luo, X.; Strobel, H.; Wilde, A.; Jimenez, S. Preliminary configuration of the torus vacuum pumping system installed in the DEMO lower port. Fusion Eng. Des. 2019, 146, 2180–2183. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).