Formation and Detection of Hydrogen by Oxygen Discharge Using Oxygen Pump-Sensor
Abstract
:1. Introduction
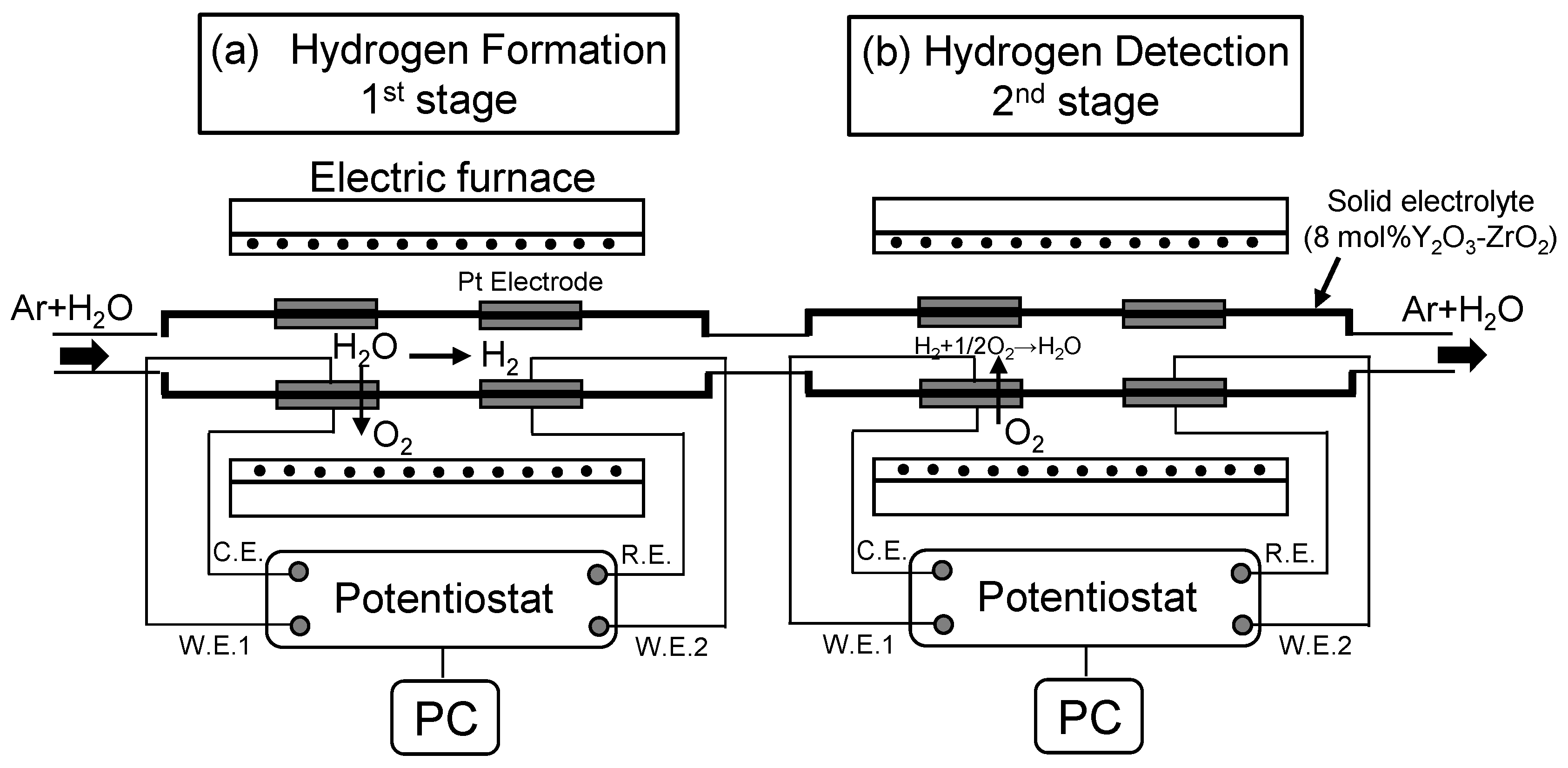
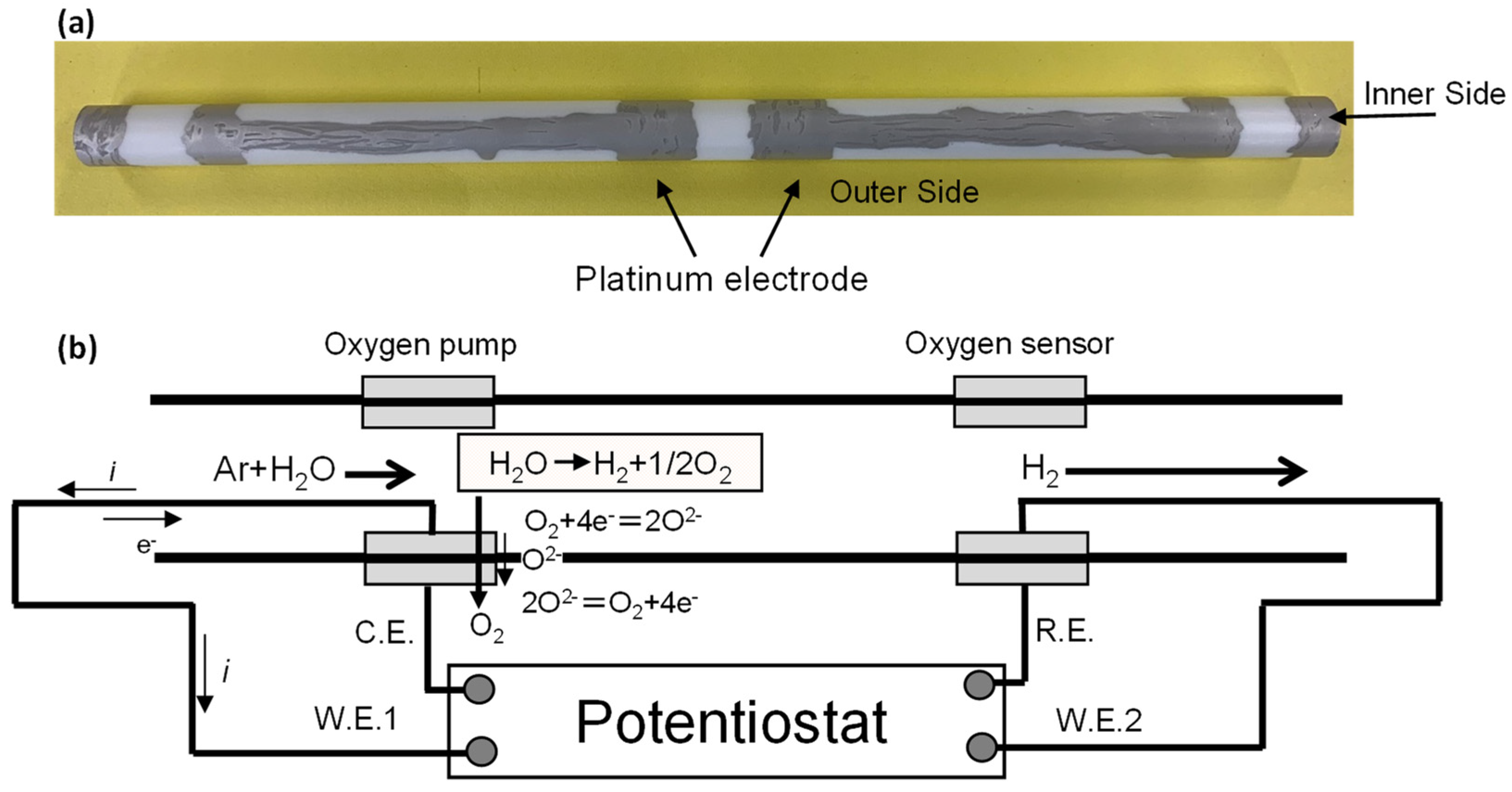
2. Experimental Procedure
3. Results and Discussion
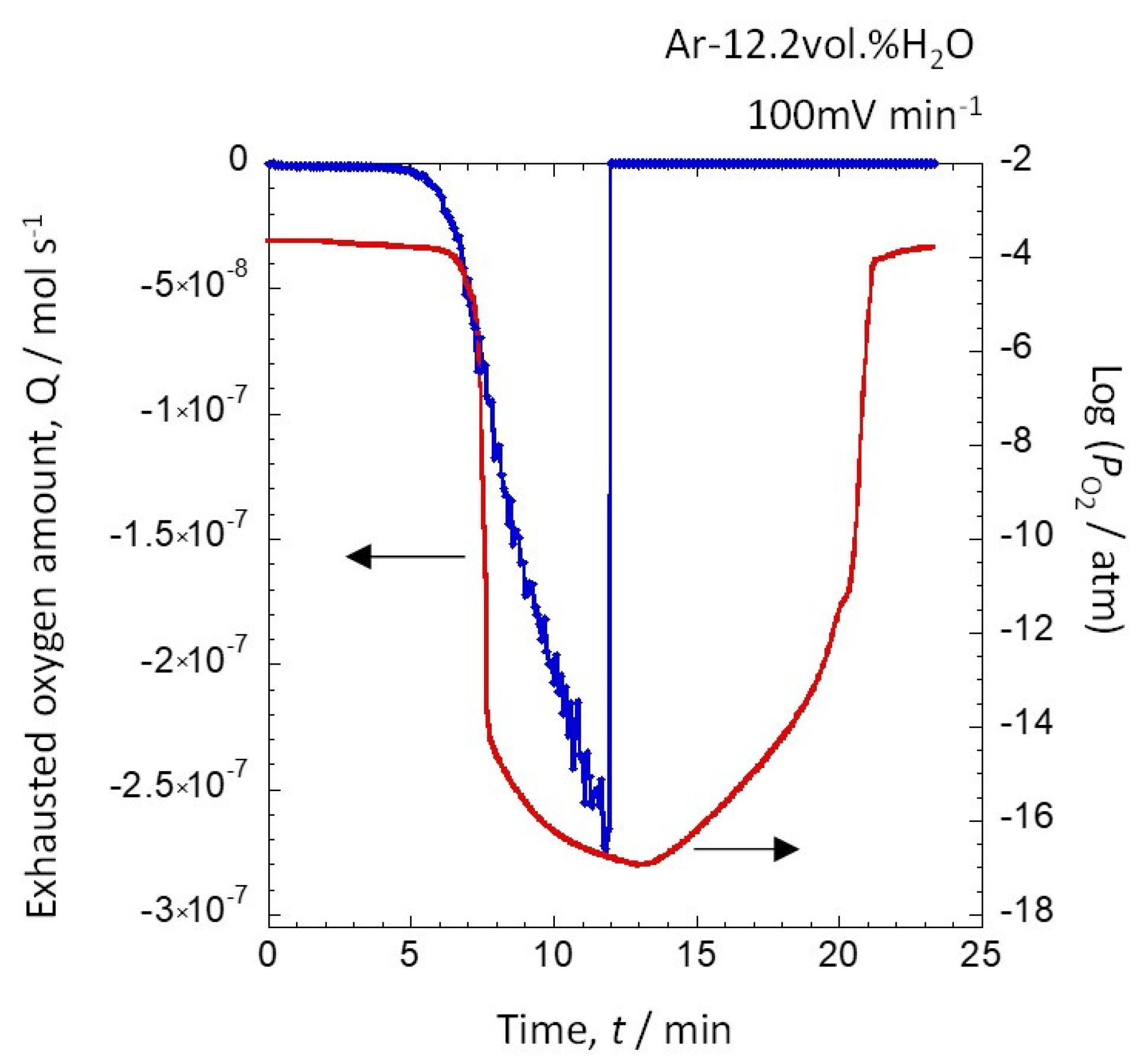
3.1. Relationship between Discharged Oxygen, Oxygen Partial Pressure, and the Amount of Generated Hydrogen due to a Potential Change
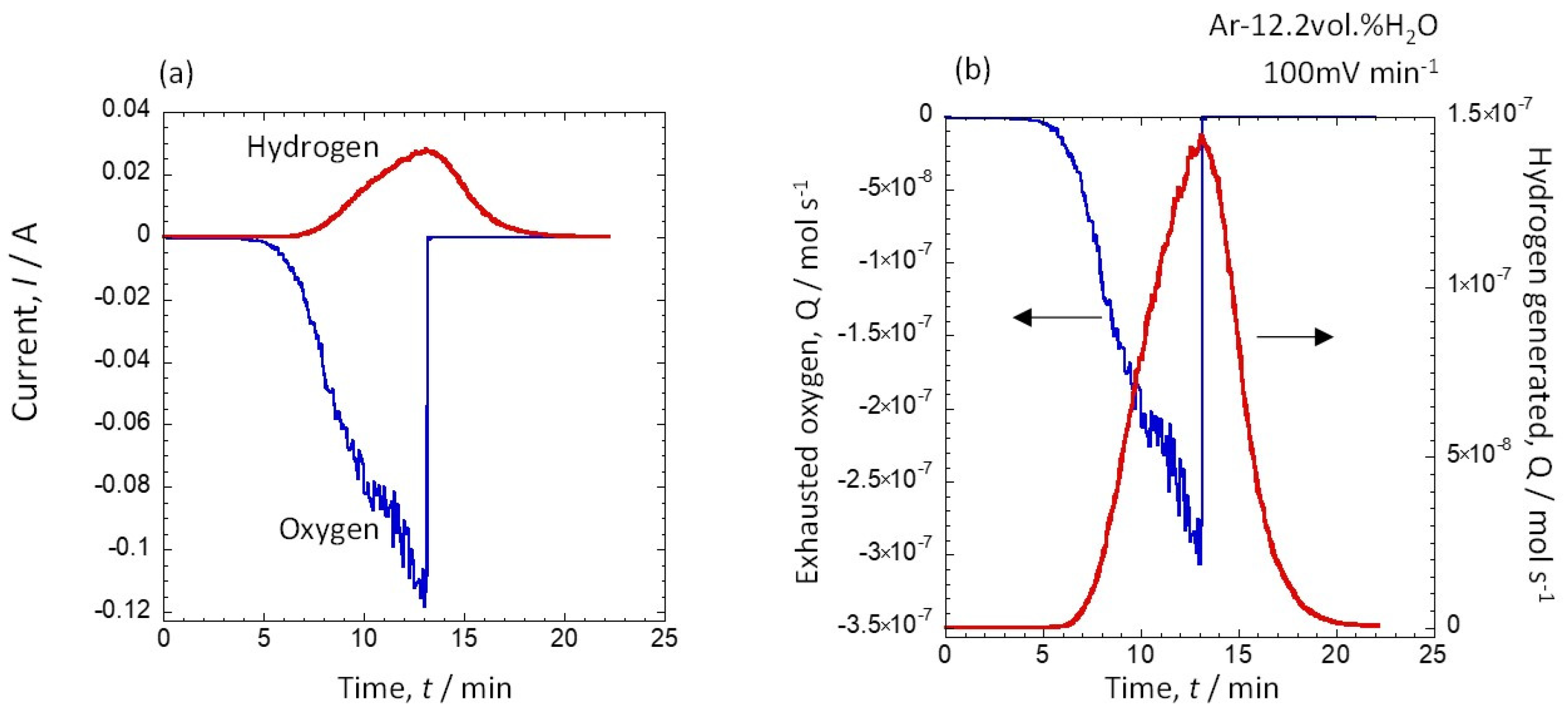
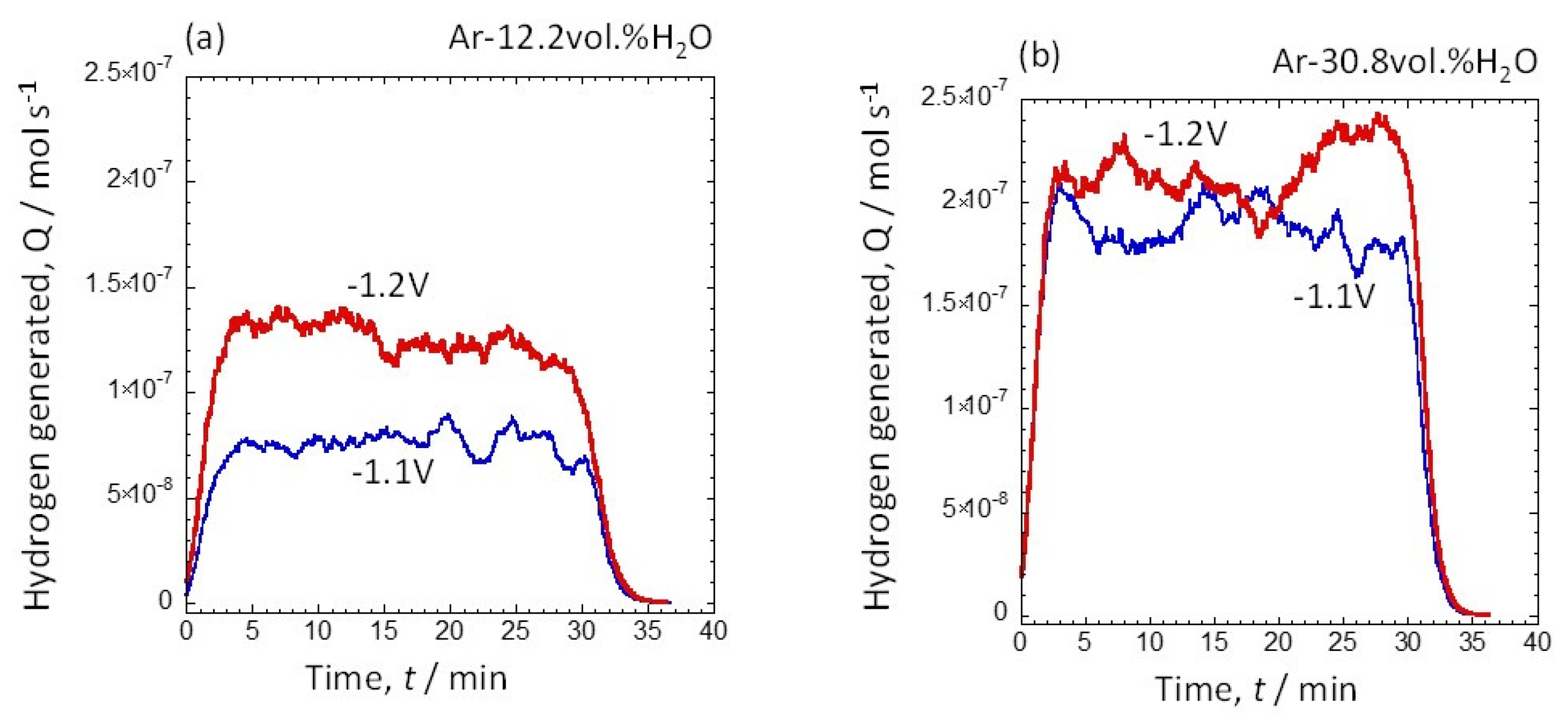
3.2. Amount of Hydrogen Generated at Water Vapor Amounts of 12.2 vol.% and 30.8 vol.%
3.3. Potential Dependence of the Amount of Hydrogen Generated at 12.2 vol.% and 30.8 vol.% Water Vapor
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrogen Energy 2005, 30, 765–802. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis developments for hydrogen evolutuion reaction in alkaline solutions—A Review. Int. J. Hydrogen Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Mauer, A.; Kirk, D.W.; Thorpe, S.J. The role of iron in the prevention of nickel electrode deactivation in alkaline electrolysis. Electrochim. Acta 2007, 52, 3505–3509. [Google Scholar] [CrossRef]
- Brown, I.J.; Sotiropoulos, S. Preparation and characterization of microporous Ni coatings as hydrogen evolving cathodes. J. Appl. Electrochem. 2000, 30, 107–111. [Google Scholar] [CrossRef]
- Chade, D.; Berlouis, L.; Infield, D.; Cruden, A.; Nielsen, P.N.; Mathiesen, T. Evaluation of Raney nickel electrodes prepared by atmospheric plasma spraying for alkaline water electrolysers. Int. J. Hydrogen Energy 2013, 38, 14380–14390. [Google Scholar] [CrossRef]
- Chen, L.; Lasia, A. Study of the kinetics of hydrogen evolution reaction on nickel-zinc alloy electrodes. J. Electrochem. Soc. 1991, 138, 3321–3328. [Google Scholar] [CrossRef]
- Chen, L.; Lasia, A. Study of the kinetics of hydrogen evolution reaction on nickel-zinc powder electrodes. J. Electrochem. Soc. 1992, 139, 3214–3219. [Google Scholar] [CrossRef]
- Rami, A.; Lasia, A. Kinetics of hydrogen evolution on Ni-Al alloy electrodes. J. Appl. Electrochem. 1992, 22, 376–382. [Google Scholar] [CrossRef]
- Birry, L.; Lasia, A. Studies of the hydrogen evolution reaction on Raney nickel-molybdenum electrodes. J. Appl. Electrochem. 2004, 34, 735–749. [Google Scholar] [CrossRef]
- Dong, H.; Lei, T.; He, Y.; Xu, N.; Huang, B.; Liu, C.T. Electrochemical performance of porous Ni3Al electrodes for hydrogen evolution reaction. Int. J. Hydrogen Energy 2011, 36, 12112–12120. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Chong, Z.; Omanovic, S. Characterization of Ni, NiMo, NiW and NiFe electroactive coatings as electrocatalysts for hydrogen evolution in an acidic medium. J. Mol. Catal. A Chem. 2005, 226, 179–197. [Google Scholar] [CrossRef]
- Mullis, A.M.; Bigg, T.D.; Adkins, N.J. A microstructural investigation of gas atomized Raney type Al-27.5at.% Ni catalyst precursor alloys. J. Alloys Compd. 2015, 648, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Hirose, N.; Tanaki, T.; Ogata, Y.H. The effect of tin ingredients on electrocatalytic activity of Raney-Ni prepared by mechanical alloying. Int. J. Hydrogen Energy 2001, 26, 47–53. [Google Scholar] [CrossRef]
- Yu, L.; Lei, T.; Nao, B.; Jiang, Y.; He, Y.; Liu, C.T. Characteristics of a sintered porous Ni-Cu alloy cathode for hydrogen production in a potassium hydroxide solution. Energy 2016, 97, 498–505. [Google Scholar] [CrossRef]
- Yüce, A.O.; Döner, A.; Kardaş, G. NiMn composite electrodes as cathode material for hydrogen evolution reaction in alkaline solution. Int. J. Hydrogen Energy 2013, 38, 4466–4473. [Google Scholar] [CrossRef]
- Lupi, C.; Dell’Era, A.; Pasquali, M. Nickel-cobalt electrodeposited alloys for hydrogen evolution in alkaline media. Int. J. Hydrogen Energy 2009, 34, 2101–2106. [Google Scholar] [CrossRef]
- González-Buch, C.; Herraiz-Cardona, I.; Ortega, E.; García-Antón, J.; Pérez-Herranz, V. Synthesis and characterization of macroporous Ni, Co and Ni-Co electrocatalytic deposits for hydrogen evolution reaction in alkaline media. Int. J. Hydrogen Energy 2013, 38, 10157–10169. [Google Scholar] [CrossRef]
- Domínguez-Crespo, M.A.; Ramírez-Meneses, E.; Torres-Huerta, A.M.; Garibay-Febles, V.; Philippot, K. Kinetics of hydrogen evolution reaction on stabilized Ni, Pt and Ni-Pt nanoparticles obtained by an organometallic approach. Int. J. Hydrogen Energy 2012, 37, 4798–4811. [Google Scholar] [CrossRef]
- Fiameni, S.; Herraiz-Cardona, I.; Musiani, M.; Pérez-Herranz, V.; Vázquez-Gómez, L.; Verlato, E. The HER in alkaline media on Pt-modified three-dimensional Ni cathode. Int. J. Hydrogen Energy 2012, 37, 10507–10516. [Google Scholar] [CrossRef]
- Eiler, K.; Suriñach, S.; Sort, J.; Pellicer, E. Mesoporous Ni-rich Ni-Pt thin films: Electrodeposition, characterization and performance toward hydrogen evolution reaction in acidic media. Appl. Catal. B 2020, 265, 118597. [Google Scholar] [CrossRef]
- Fukumoto, M.; Sugiuchi, K.; Nakajima, K. Formation of porous Ni surface by electrodeposition and dissolution in molten salt. Int. J. Hydrogen Energy 2020, 45, 28252–28259. [Google Scholar] [CrossRef]
- Nakajima, K.; Yamada, T.; Fukumoto, M. Formation of porous stainless steel surface by Al electrodeposition and dissolution in the molten salt and sliding properties. ISIJ Int. 2021, 61, 945–952. [Google Scholar] [CrossRef]
- Nakajima, K.; Fukumoto, M. Porous Ni-Co surface formation and analysis of hydrogen generation by gas sensor. Int. J. Hydrogen Energy 2021, 46, 26263–26271. [Google Scholar] [CrossRef]
- Lessing, P.A. A review of sealing technologies applicable to solid oxide electrolysis cells. J. Mater. Sci. 2007, 42, 3465–3476. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, Y.C. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Jensen, S.H.; Sun, X.; Ebbesen, S.D.; Knibbe, R.; Mogensen, M. Hydrogen and synthetic fuel production using pressurized solid oxide electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 9544–9549. [Google Scholar] [CrossRef]
- Tietz, F.; Sebold, D.; Brisse, A.; Schefold, J. Degradation phenomena in a solid oxide electrolysis cell after 9000 h of operation. J. Power Sources 2013, 223, 129–135. [Google Scholar] [CrossRef]
- Moçoteguy, P.; Brisse, A. A review and comprehensive analysis of degradation mechanisms of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 15887–15902. [Google Scholar] [CrossRef]
- Im-orb, K.; Visitdumrongkul, N.; Saebea, D.; Patcharavorachot, Y.; Arpornwichanop, A. Flowsheet-based model and energy analysis of solid oxide electrolysis cells for clean hydrogen production. J. Clean. Prod. 2018, 170, 1–13. [Google Scholar] [CrossRef]
- Momma, A.; Kato TKaga, Y.; Nagata, S. Polarization Behavior of High Temperature Solid Oxide Electrolysis Cells (SOEC). J. Ceram. Soc. Jpn. 1997, 105, 369–373. [Google Scholar] [CrossRef]
- Stephen Herring, J.; O’Brien, J.E.; Stoots, C.M.; Hawkes, G.L.; Hartvigsen, J.J.; Shahnam, M. Progress in high-temperature electrolysis for hydrogen production using planar SOFC technology. Int. J. Hydrogen Energy 2007, 32, 440–450. [Google Scholar] [CrossRef] [Green Version]
- Brisse, A.; Schefold, J.; Zahid, M. High temperature water electrolysis in solid oxide cells. Int. J. Hydrogen Energy 2008, 33, 5375–5382. [Google Scholar] [CrossRef]
- Ebbesen, S.D.; Graves, C.; Hauch, A.; Jensen, S.H.; Mogensen, M. Poisoning of Solid Oxide Electrolysis Cells by Impurities. J. Electrochem. Soc. 2010, 157, B1419–B1429. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Yu, B.; Zhang, W.; Chen, J.; Luo, X.; Stephan, K. Microstructure modification of the anode / electrolyte interface of SOEC for hydrogen production. Int. J. Hydrogen Energy 2012, 37, 12833–12838. [Google Scholar] [CrossRef]
- Kim, J.; Jun, A.; Gwon, O.; Yoo, S.; Liu, M.; Shin, J.; Lim, T.H.; Kim, G. Hybrid-solid oxide electrolysis cell: A new strategy for efficient hydrogen production. Nano Energy 2018, 44, 121–126. [Google Scholar] [CrossRef]
- Vialetto, G.; Noro, M.; Colbertaldo, P.; Rokni, M. Enhancement of energy generation efficiency in industrial facilities by SOFE-SOEC systems with additional hydrogen production. Int. J. Hydrogen Energy 2019, 44, 9608–9620. [Google Scholar] [CrossRef]
- Fukumoto, M.; Kawamori, Y.; Sonobe, H.; Hara, M.; Kaneko, H. Investigation of high-temperature oxidation in steam for Ni-Al alloys using the combination of a hydrogen sensor and an oxygen pump-sensor. Oxid. Met. 2018, 89, 357–373. [Google Scholar] [CrossRef]
- Fukumoto, M.; Kawamori, Y.; Hara, M. Investigation of cyclic oxidation in Ar-H2O for NiAl containing Hf or Zr by the combination of a hydrogen sensor and an oxygen pump-sensor. Corros. Sci. 2019, 149, 68–74. [Google Scholar] [CrossRef]
- Fukumoto, M.; Nakajima, K.; Kawamori, Y. Investigation of alumina formation and oxidation rate of Ni-5wt%Al-Xwt%Cr alloy using hydrogen sensor and oxygen pump sensor. Oxid. Met. 2020, 94, 191–204. [Google Scholar] [CrossRef]
- Ferlazzo, A.; Espro, C.; Iannazzo, D.; Moulaee, K.; Neri, G. A novel yttria-doped ZrO2 based conductometric sensor for hydrogen leak monitoring. Int. J. Hydrogen Energy 2022, 47, 9819–9828. [Google Scholar] [CrossRef]
- Shinoda, T.; Ozawa, S.; Kawashima, K.; Kuribayashi, K.; Yamayoshi, T.; Itoh, Y. Flux-Free Brazing of Aluminum Alloys under Ultra-Low Oxygen Partial Pressure through a Zirconia Oxygen Pump. Mater. Trans. 2022, 63, 1477–1483. [Google Scholar] [CrossRef]
- Fukumoto, M.; Sakuraba, T.; Hara, M.; Kaneko, H. Hydrogen generation monitoring and mass gain analysis during the steam oxidation for zircaloy using hydrogen and oxygen sensors. J. Jpn. Inst. Met. Mater. 2015, 79, 362–371. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, M.; Sonobe, H.; Hara, M.; Kaneko, H. Investigation of high temperature oxidation in steam containing O2 for zircaloy using the combination of a hydrogen sensor and an oxygen sensor. J. Jpn. Inst. Met. Mater. 2016, 80, 231–239. [Google Scholar] [CrossRef]
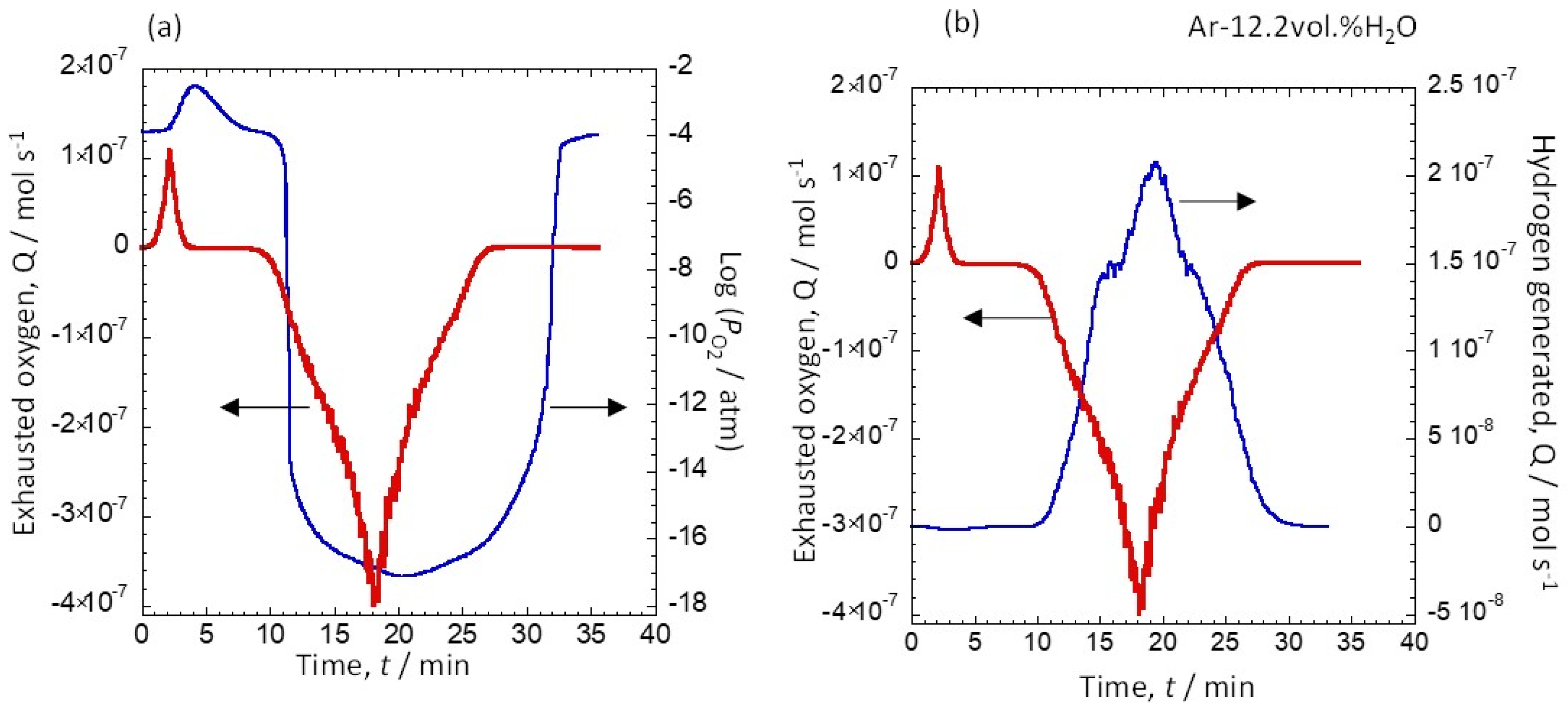
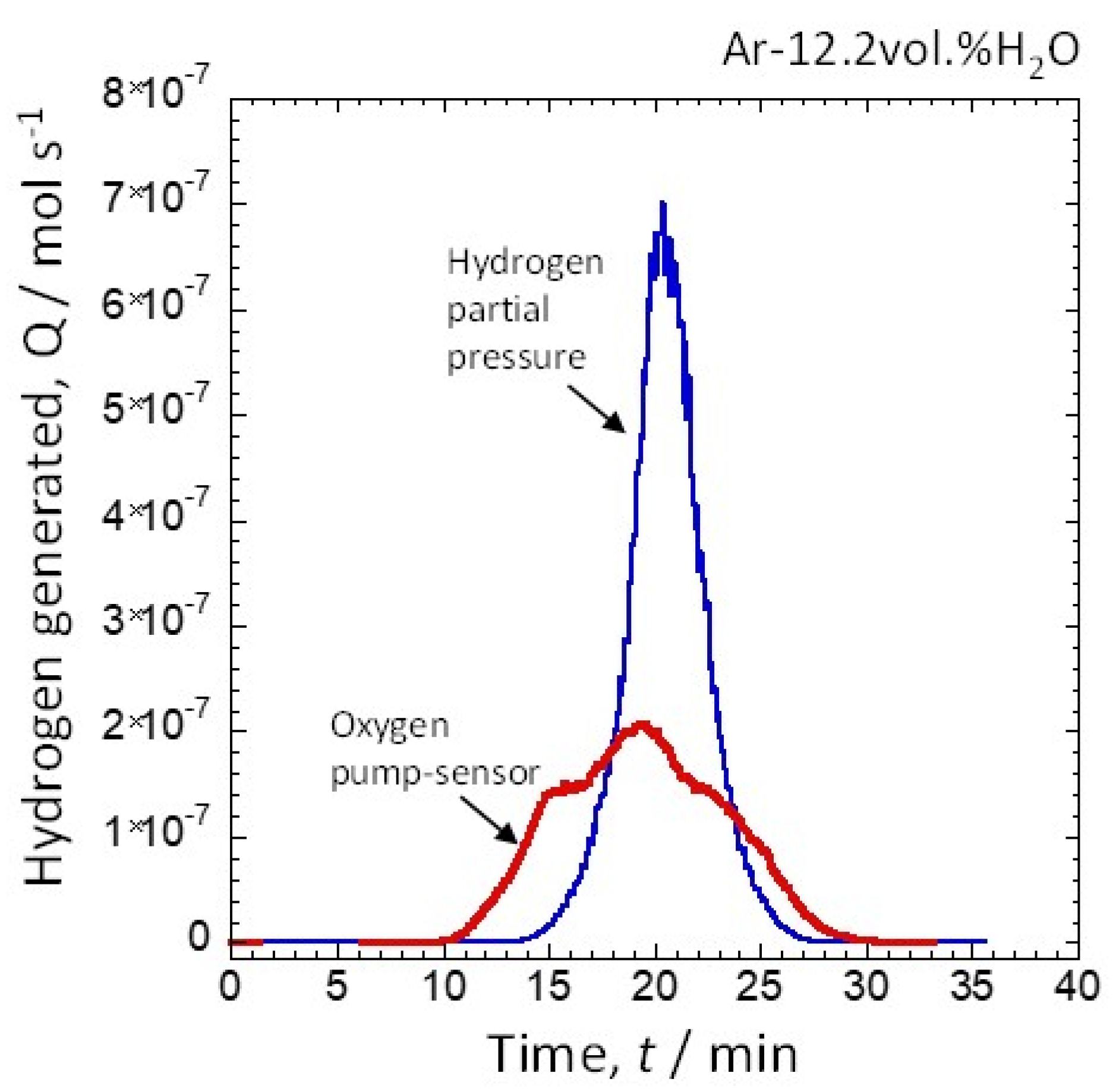
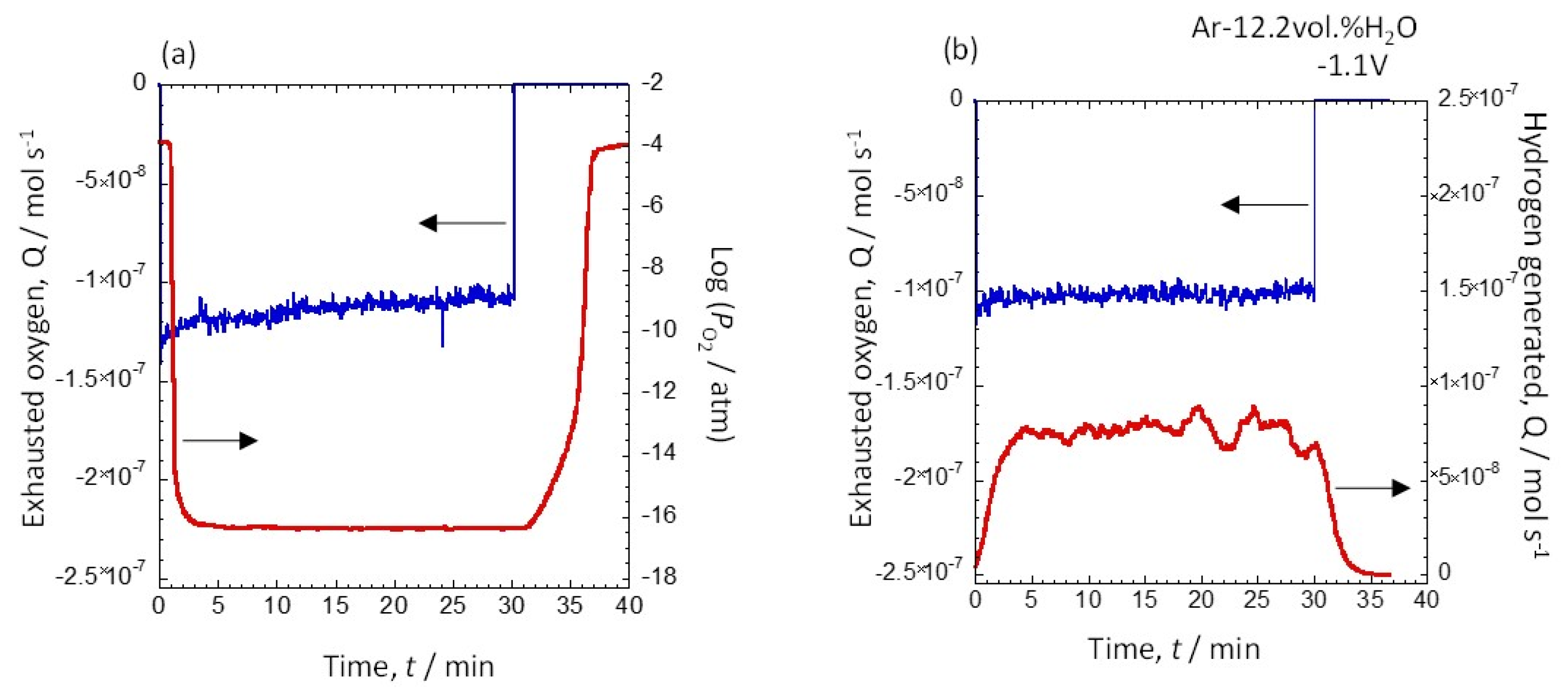
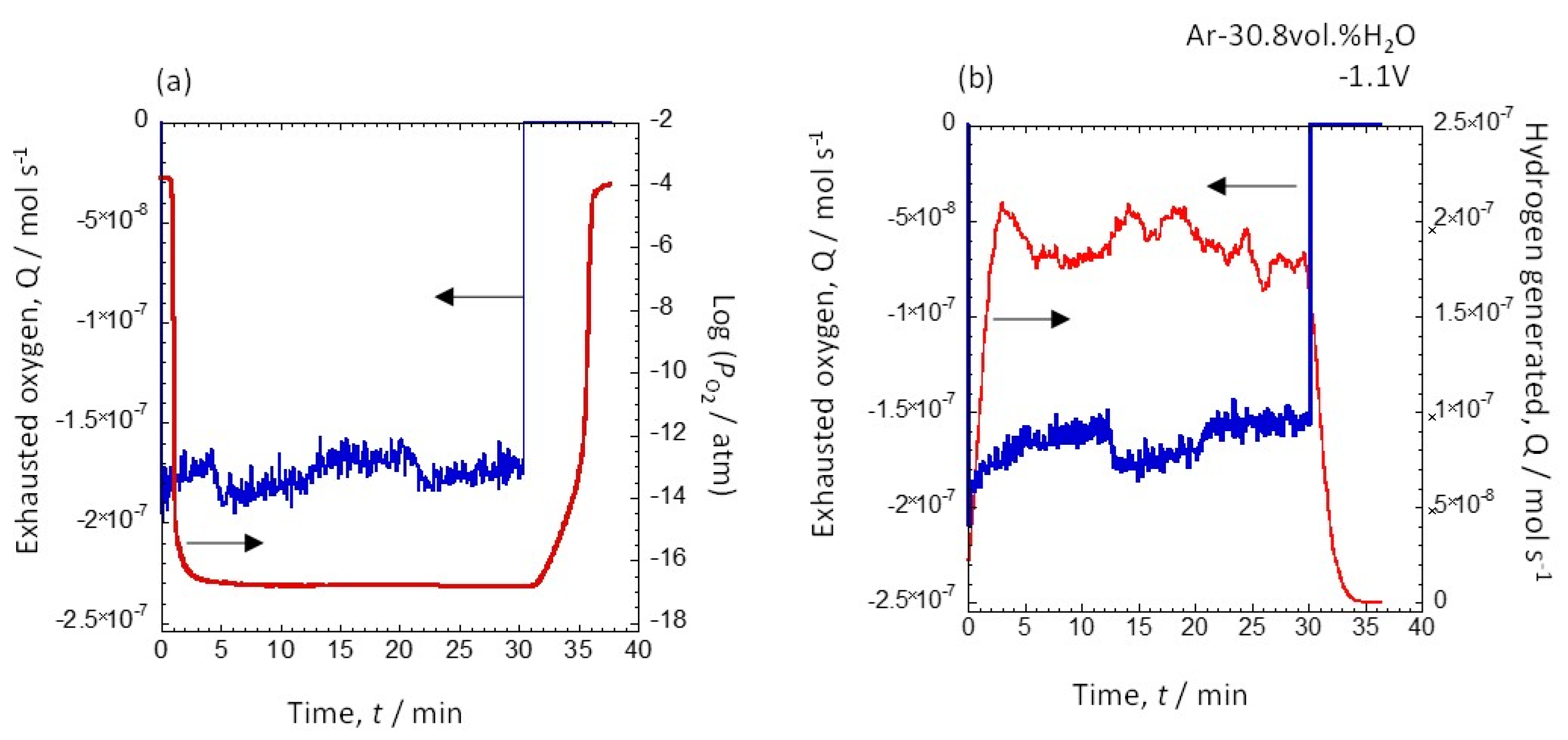
| Exhausted Oxygen (mol) | Generated Hydrogen (mol) | Hydrogen Production Efficiency (%) | |
|---|---|---|---|
| 12.2 vol.% H2O (−1.1V) | 1.84 × 10−4 | 1.38 × 10−4 | 37.5 |
| 12.2 vol.% H2O (−1.2V) | 2.86 × 10−4 | 2.25 × 10−4 | 39.3 |
| 30.8 vol.% H2O (−1.1V) | 3.00 × 10−4 | 3.42 × 10−4 | 57.2 |
| 30.8 vol.% H2O (−1.2V) | 2.99 × 10−4 | 3.93 × 10−4 | 65.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukumoto, M.; Nakajima, K. Formation and Detection of Hydrogen by Oxygen Discharge Using Oxygen Pump-Sensor. Hydrogen 2022, 3, 463-473. https://doi.org/10.3390/hydrogen3040029
Fukumoto M, Nakajima K. Formation and Detection of Hydrogen by Oxygen Discharge Using Oxygen Pump-Sensor. Hydrogen. 2022; 3(4):463-473. https://doi.org/10.3390/hydrogen3040029
Chicago/Turabian StyleFukumoto, Michihisa, and Kano Nakajima. 2022. "Formation and Detection of Hydrogen by Oxygen Discharge Using Oxygen Pump-Sensor" Hydrogen 3, no. 4: 463-473. https://doi.org/10.3390/hydrogen3040029
APA StyleFukumoto, M., & Nakajima, K. (2022). Formation and Detection of Hydrogen by Oxygen Discharge Using Oxygen Pump-Sensor. Hydrogen, 3(4), 463-473. https://doi.org/10.3390/hydrogen3040029









