Effect of Ti-Based Additives on the Hydrogen Storage Properties of MgH2: A Review
Abstract
:1. Introduction
2. Fundamentals of the MgH2 System
2.1. Crystal Structure of MgH2 System
2.2. Thermodynamics of the MgH2 System
2.3. Kinetics of MgH2 System
- (i)
- Surface assimilation of molecular hydrogen:
- (ii)
- Hydrogen molecules convert into atoms:
- (iii)
- Penetration of hydrogen atoms on the surface:
- (iv)
- Diffusion of atomic hydrogen:
- (v)
- Formation of hydride at the metal/hydride interface:
- k = β/Tp2;
- β = heating rate;
- Tp = peak temperature;
- Ea = activation energy of desorption;
- R = gas constant.
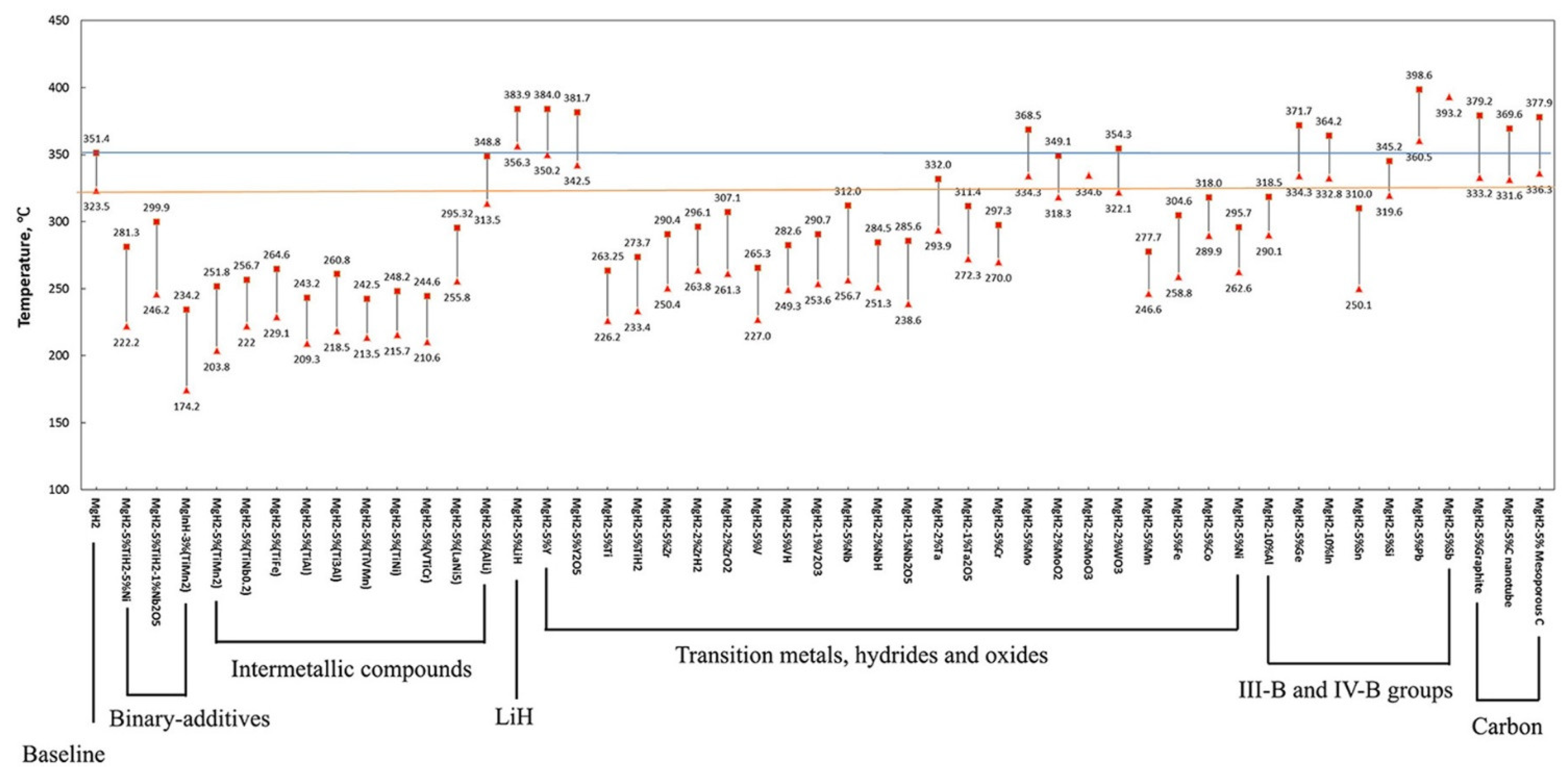
3. Effect of Catalyst
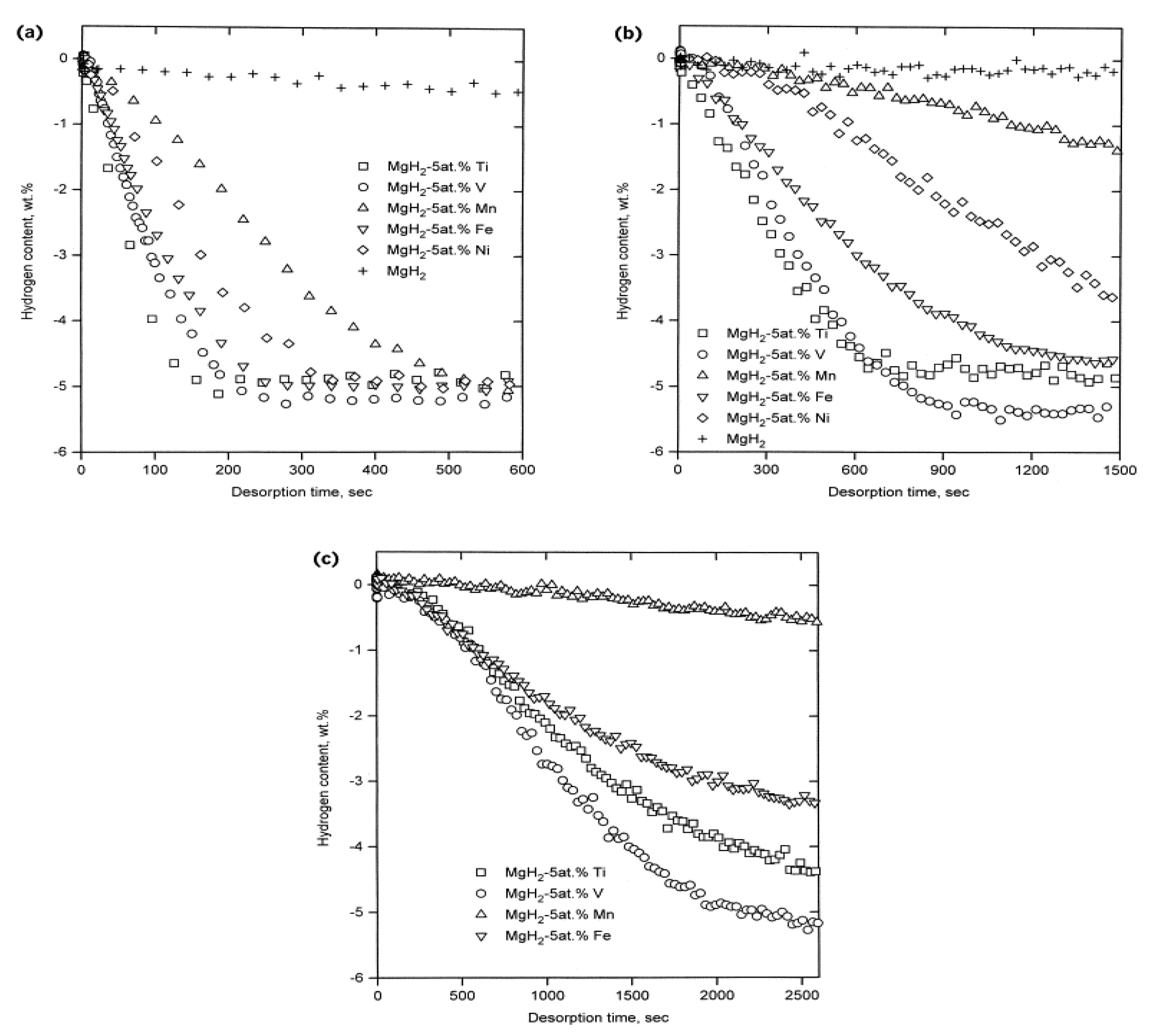
3.1. Transition Metal Catalyst or Additives
3.2. Titanium-Based Additives
3.2.1. Titanium or Titanium Hydride
3.2.2. Titanium Oxide
3.2.3. Titanium Halide
3.2.4. Ti-Based Intermetallics
3.2.5. Titanium Carbides and Carbonitrides
3.3. Other Catalysts and Additives
4. Discussion
5. Future Prospects and Challenges
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Schlapbach, L.; Zuttel, A. Hydrogen storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sust. Energ. Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogenstorage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Principi, G.; Agresti, F.; Maddalena, A.; Russo, S.L. The problem of solid state hydrogenstorage. Energy 2009, 34, 2087–2091. [Google Scholar] [CrossRef]
- Berube, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Huot, J.; Liang, G.; Boily, S.; Van Neste, A.; Schulz, R.J. Structural study and hydrogen sorption kinetics of ball-milled magnesium hydride. J. Alloys Compd. 1999, 293, 495–500. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Strom-Olsen, J.O.J. Nanocrystalline magnesium for hydrogen storage. J. Alloys Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Wagemans, R.W.P.; Lenth, J.H.V.; de Jongh, P.E.; Dillen, A.J.V.; de Jong, K.P. Hydrogen storage in magnesium clusters: Quantum chemical study. J. Am. Chem. Soc. 2005, 127, 16675–16680. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Zang, L.; Huang, Y.; Gao, P.; Jiao, L.; Yuan, H.; Wang, Y. Improved hydrogen storage properties of MgH2 with Ni-based compounds. Int. J. Hydrog. Energy 2017, 42, 24247–24255. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Wu, Y.; Guo, X.; Ye, J.; Yuan, B.; Wang, S.; Jiang, L. Recent advances on the thermal destabilization of Mg-based hydrogen storage materials. RSC Adv. 2019, 9, 408–428. [Google Scholar] [CrossRef] [PubMed]
- Dornheim, M.; Doppiu, S.; Barkhordarian, G.; Boesenberg, U.; Klassen, T.; Gutfleisch, O. Hydrogen storage in magnesium-based hydrides and hydride composites. Scr. Mater. 2007, 56, 841–846. [Google Scholar] [CrossRef]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solid. 2015, 85, 96–106. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Shao, H.; Li, W.; Lin, H. Catalysis and downsizing in Mg-based hydrogen storage materials. Catalysts 2018, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Chen, M.; Hu, M.; Wang, B.; Yu, R.; Liu, T. Recent advances in magnesium-based hydrogen storage materials with multiple catalysts. Int. J. Hydrog. Energy 2019, 44, 10694–10712. [Google Scholar] [CrossRef]
- Danaie, M.; Tao, S.X.; Kalisvaart, P.; Mitlin, D. Analysis of deformation twins and the partially dehydrogenated microstructure in nanocrystalline magnesium hydride (MgH2) powder. Acta Mater. 2010, 58, 3162–3172. [Google Scholar] [CrossRef]
- Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. Catalytic effect of transition metals on hydrogen sorption in nanocrystalline ball milled MgH2-Tm (Tm = Ti, V, Mn, Fe and Ni) systems. J. Alloys Compd. 1999, 292, 247–252. [Google Scholar] [CrossRef]
- Hanada, N.; Ichikawa, T.; Fujii, H. Catalytic effect of nanoparticle 3d- transition metals on hydrogen storage properties in magnesium hydride MgH2 prepared by mechanical milling. J. Phys. Chem. B 2005, 109, 7188–7194. [Google Scholar] [CrossRef]
- Mohsen, D.; Cesario Asselli, A.A.; Huot, J.; Botteon, G.A. Formation of the ternary complex hydride Mg2FeH6 from magnesium hydride (β-MgH2) and Iron: An electron microscopy and energy-loss spectroscopy study. J. Phys. Chem. C 2012, 116, 25701–25714. [Google Scholar]
- Tan, Z.; Chun, C.; Heilweil, E.J.; Bendersky, L.A. Thermodynamics, kinetics and microstructural evolution during hydrogenation of iron- doped magnesium thin films. Int. J. Hydrog. Energy 2011, 36, 9702–9713. [Google Scholar] [CrossRef]
- Zahiri, B.; Harrower, C.T.; Amirkhiz, B.S.; Mitlin, D. Rapid and reversible hydrogen sorption in Mg-FeTi thin films. Appl. Phys. Lett. 2009, 95, 103114. [Google Scholar] [CrossRef]
- Gasan, H.; Celik, O.N.; Aydinbeyli, N.; Yaman, Y.M. Effect of V, Nb, Ti graphite addition on the hydrogen desorption temperature of magnesium hydride. Int. J. Hydrog. Energy 2012, 37, 1912–1918. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Emami, S. Kinetics of dehydrogenation of the Mg-Ti-H hydrogen storage system. Int. J. Hydrog. Energy 2011, 36, 8344–8350. [Google Scholar] [CrossRef]
- Stampfer, J.F.; Holley, C.E.; Suttle, J.F. The Magnesium-hydrogen system. J. Am. Chem. Soc. 1960, 82, 3504–3508. [Google Scholar] [CrossRef] [Green Version]
- Bastide, J.P.; Bonnetot, B.; Letoffe, J.M.; Claudy, P. Polymorphisme de l’hydrure de magnesium sous haute pression. Mater. Res. Bull. 1980, 15, 1215–1224. [Google Scholar] [CrossRef]
- Bortz, M.; Bertheville, B.; Bottger, G.; Yvon, K. Structure of the high pressure phase γ-MgH2 by neutron powder diffraction. J. Alloys Compd. 1999, 287, L4–L6. [Google Scholar] [CrossRef]
- Magnesium-hydride-unit-cell-3D-balls. Available online: https://commons.wikimedia.org/wiki/File:Magnesium-hydride-unit-cell-3D-balls.png (accessed on 8 August 2022).
- Vajeeston, P.; Ravindran, P.; Hauback, B.C.; Fjellvåg, H.; Kjekshus, A.; Furuseth, S.; Hanfland, M. Structural stability and pressure-induced phase transitions in MgH2. Phys. Rev. B 2006, 73, 224102. [Google Scholar] [CrossRef]
- Dornheim, M. Thermodynamics of Metal Hydrides: Tailoring Reaction Enthalpies of Hydrogen Storage Materials. In Thermodynamics-Interaction Studies-Solids, Liquids and Gases; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The U.S. Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Kohno, T.; Tsuruta, S.; Kanda, M. The hydrogen storage properties of new Mg2Ni alloy. J. Electrochem. Soc. 1996, 143, 198–199. [Google Scholar] [CrossRef]
- Shao, H.; Wang, Y.; Xu, H.; Li, X. Preparation and hydrogen storage properties of nanostructured Mg2Cu alloy. J. Solid. State Chem. 2005, 178, 2211–2217. [Google Scholar] [CrossRef]
- Bououdina, M.; Guo, Z.X. Comparative study of mechanical alloying of (Mg+ Al) and (Mg+ Al+ Ni) mixtures for hydrogen storage. J. Alloys Compd. 2002, 336, 222–231. [Google Scholar] [CrossRef]
- Chaudhary, A.-L.; Sheppard, D.A.; Paskevicius, M.; Webb, C.J.; Gray, E.M.; Buckley, C.E. Mg2Si Nanoparticle Synthesis for High Pressure Hydrogenation. J. Phys. Chem. C 2014, 118, 1240–1247. [Google Scholar] [CrossRef]
- Asano, K.; Enoki, H.; Akiba, E. Effect of Li Addition on Synthesis of Mg-Ti BCC Alloys by means of Ball Milling. Mater. Trans. 2007, 48, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, P.; van Thiel, E.F.; Notten, P.H. Ternary MgTiX-alloys: A promising route towards low-temperature, high-capacity, hydrogen-storage materials. Chemistry 2007, 13, 9892–9898. [Google Scholar] [CrossRef]
- Jain, A.; Miyaoka, H.; Ichikawa, T. Destabilization of lithium hydride by the substitution of group 14 elements: A review. Int. J. Hydrog. Energy 2016, 41, 5969–5978. [Google Scholar] [CrossRef]
- Bloch, J. The kinetics of a moving metal hydride layer. J. of Alloys Compd. 2000, 312, 135–153. [Google Scholar] [CrossRef]
- Wang, C.S.; Wang, X.H.; Lei, Y.Q.; Chen, C.P.; and Wang, Q.D. Hydriding Kinetics of M1Ni-I Development of the model. Int. J. Hydrog. Energy 1996, 21, 471–478. [Google Scholar] [CrossRef]
- Du, A.J.; Smith, S.C.; Yao, X.D.; Lu, G.Q. Hydrogen spillover mechanism on a Pd-doped Mg surface as revealed by ab initio density functional calculation. J. Am. Chem. Soc. 2007, 129, 10201–10204. [Google Scholar] [CrossRef]
- Yao, X.; Wu, C.; Du, A.; Zou, J.; Zhu, Z.; Wang, P.; Cheng, H.; Smith, S.C.; Lu, G. Metallic and carbon nanotube-catalyzed coupling of hydrogenation in magnesium. J. Am. Chem. Soc. 2007, 129, 15650–15654. [Google Scholar] [CrossRef]
- Pan, Y.-C.; Zou, J.-X.; Zeng, X.-Q.; Ding, W.-J. Hydrogen storage properties of Mg–TiO2 composite powder prepared by arc plasma method. Trans. Nonferrous Met. Soc. China 2014, 24, 3834–3839. [Google Scholar] [CrossRef]
- Liu, H.; Sun, P.; Bowman, R.C.; Fang, Z.Z.; Liu, Y.; Zhou, C. Effect of air exposure on hydrogen storage properties of catalyzed magnesium hydride. J. Power Sources 2020, 454, 227936. [Google Scholar] [CrossRef]
- Ma, T.; Isobe, S.; Wang, Y.; Hashimoto, N.; Ohnuki, S. Nb-Gateway for Hydrogen Desorption in Nb2O5 Catalyzed MgH2 Nanocomposite. J. Phys. Chem. C 2013, 117, 10302–10307. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Ma, Y.; Li, F.; Zhu, H.; Zeng, X.; Ding, W.; Wu, J.; Deng, T.; Zou, J. Visualization of fast “hydrogen pump” in core-shell nanostructured Mg@Pt through hydrogen stabilized Mg3Pt. J. Mater. Chem. A 2019, 24, 14629–14637. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Sun, P. An experimental survey of additives for improving dehydrogenation properties of magnesium hydride. J. Power Sources 2015, 278, 38–42. [Google Scholar] [CrossRef] [Green Version]
- Rizo-Acosta, P.; Cuevas, F.; Latroche, M. Hydrides of early transition metals as catalysts and grain growth inhibitors for enhanced reversible hydrogen storage in nanostructured magnesium. J. Mater. Chem. A 2019, 7, 23064–23075. [Google Scholar] [CrossRef]
- Charbonnier, J.; de Rango, P.; Fruchart, D.; Miraglia, S.; Pontonnier, L.; Rivoirard, S.; Skryabina, N.; Vulliet, P. Hydrogenation of transition metal additives (Ti, V) during ball milling of Magnesium Hydride. J. Alloys Compds 2004, 383, 205–208. [Google Scholar] [CrossRef]
- Lu, H.B.; Poh, C.K.; Zhang, L.C.; Guo, Z.P.; Yu, X.B.; Liu, H.K. Dehydrogenation characteristics of Ti- and Ni/Ti-catalyzed Mg hydrides. J. Alloys Compd. 2009, 481, 152–155. [Google Scholar] [CrossRef]
- Shahi Rohit, R.; Tiwari Anand, P.; Shaz, M.A.; Srivastava, O.N. Studies on de/rehydrogenation characteristics of nanocrystalline MgH2 co-catalyzed with Ti, Fe and Ni. Int. J. Hydrog. Energy 2013, 38, 2778–2784. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Q.; Wang, Y.; Jiao, L.; Yuan, H. Catalytic effects of different Ti-based materials on dehydrogenation performances of MgH2. J. Alloys Compd. 2015, 645, S509–S512. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lu, J.; Sohn, H.Y.; Fang, Z.Z. Hydrogen storage properties of the Mg-Ti-H system prepared by high-energy-high-pressure reactive milling. J. Power Sources 2008, 180, 491–497. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jun, L.; Hong, Y.; Zhigang, Z.F.; Ewa, R. Effect of Milling Parameters on the Dehydrogenation Properties of the Mg-Ti-H System. J. Phys. Chem. C 2009, 130, 19344–19350. [Google Scholar] [CrossRef]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Ronnebro, E. Hydrogen Storage Properties of Nanosized MgH2-0.1TiH2 Prepared by Ultrahigh-Energy-High-Pressure Milling. J. Am. Chem. Soc. 2009, 131, 15843–15852. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Choi, Y.J.; Fang, Z.Z.; Sohn, H.Y.; Roennebro, E. Hydrogenation of Nanocrystalline Mg at Room Temperature in the Presence of TiH2. J. Am. Chem. Soc. 2010, 132, 6616–6617. [Google Scholar] [CrossRef] [PubMed]
- Patelli, N.; Migliori, A.; Pasquini, L. Reversible metal-hydride transformation in Mg-Ti-H nanoparticles at remarkably low temperatures. ChemPhysChem 2019, 20, 1325–1333. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.; Song, M.Y. Hydriding and dehydriding features of a titanium-added magnesium hydride composite. Mat. Sci. 2020, 26, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Lotoskyy, M.; Denys, R.; Yartys, V.A.; Eriksen, J.; Goh, J.; Nyamsi, S.N.; Sita, C.; Cummings, F. An outstanding effect of graphite in nano-MgH2-TiH2 on hydrogen storage performance dagger. J. Mater. Chem. A 2018, 6, 10740–10754. [Google Scholar] [CrossRef]
- Cuevas, F.; Korablov, D.; Latroche, M. Synthesis, structural and hydrogenation properties of Mg-rich MgH2–TiH2 nanocomposites prepared by reactive ball milling under hydrogen gas. Phys. Chem. Chem. Phys. 2012, 14, 1200–1211. [Google Scholar] [CrossRef]
- Shao, H.; Felderhoff, M.; Schuth, F. Hydrogen storage properties of nanostructured MgH2/TiH2composite prepared by ball milling under high hydrogen pressure. Int. J. Hydrog. Energy 2011, 36, 10828–10838. [Google Scholar] [CrossRef]
- Pasquini, L.; Callini, E.; Brighi, M.; Boscherini, F.; Montone, A.; Jensen, T.R.; Maurizio, C.; Antisari, M.V.; Bonetti, E. Magnesium nanoparticles with transition metal decoration for hydrogen storage. J. Nanopart. Res. 2011, 13, 5727–5737. [Google Scholar] [CrossRef]
- Vincent, S.; Lang, J.; Huot, J. Addition of catalysts to magnesium hydride by means of cold rolling. J. Alloys Compd. 2012, 512, 290–295. [Google Scholar] [CrossRef]
- Ma, L.-P.; Wang, P.; Kang, X.-D.; Cheng, H.-M. Preliminary investigation on the catalytic mechanism of TiF3 additive in MgH2–TiF3 H-storage system. J. Mater. Res. 2011, 22, 1779–1786. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti Intermetallic catalysts on hydrogen storage properties of magnesium hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Choi, Y.J.; Choi, J.W.; Sohn, H.Y.; Ryu, T.; Hwang, K.S.; Fang, Z.Z. Chemical vapor synthesis of Mg–Ti nanopowder mixture as a hydrogen storage material. Int. J. Hydrog. Energy 2009, 34, 7700–7706. [Google Scholar] [CrossRef]
- Cui, J.; Wang, H.; Liu, J.; Ouyang, L.; Zhang, Q.; Sun, D.; Yao, X.; Zhu, M. Remarkable enhancement in dehydrogenation of MgH2 by a nano-coating of multi-valence Ti-based catalysts. J. Mater. Chem. A 2013, 492, 251–258. [Google Scholar] [CrossRef]
- Calizzi, M.; Venturi, F.; Ponthieu, M.; Cuevas, F.; Morandi, V.; Perkisas, T.; Bals, S.; Pasquini, L. Gas-phase synthesis of Mg–Ti nanoparticles for solid-state hydrogen storage. Phys. Chem. Chem. Phys. 2016, 18, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Zou, J.; Shi, X.; Zeng, X.; Ding, W. Synthesis and hydrogen storage properties of core–shell structured binary Ti and ternary Ni composites. Int. J. Hydrog. Energy 2017, 42, 2239–2247. [Google Scholar] [CrossRef]
- Liu, T.; Chen, C.; Wang, F.; Li, X. Enhanced hydrogen storage properties of magnesium by the synergic catalytic effect of TiH1.971 and TiH1.5 nanoparticles at room temperature. J. Power Sources 2014, 267, 69–77. [Google Scholar] [CrossRef]
- Manivasagam, T.G.; Magusin, P.C.M.M.; Iliksu, M.; Notten, P.H.L. Influence of Nickel and Silicon Addition on the Deuterium Siting and Mobility in fcc Mg–Ti Hydride Studied with 2H MAS NMR. J. Phys. Chem. C 2014, 118, 10606–10615. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Zhang, S.; Zhao, H.; Will, G.; Liu, P. An efficient and lowcost TiO2 compact layer for performance improvement of dye-sensitized solar cells. Electrochim. Acta 2009, 54, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Polanski, M.; Bystrzycki, J. Comparative studies of the influence of different nano-sized metal oxides on the hydrogen sorption properties of magnesium hydride. J. Alloys Compd. 2009, 486, 697–701. [Google Scholar] [CrossRef]
- Pramoch, R.; Pattaraporn, S.; Boonyarach, K.; Santi, K. Effects of TiCl3, TiO2, ZrCl4, and ZrO2 on Hydrogen Desorption of MgH2 and Its Reversibility. Chem. Eng. Trans. 2014, 39, 1201–1206. [Google Scholar]
- Zhang, M.; Xiao, X.; Luo, B.; Liu, M.; Chen, M.; Chen, L. Superior de/hydrogenation performances of MgH2 catalyzed by 3D flower-like TiO2@C nanostructures. J. Energy Chem. 2020, 46, 191–198. [Google Scholar] [CrossRef]
- Pandey, S.K.; Bhatnagar, A.; Shahi, R.R.; Hudson, M.S.L.; Singh, M.K.; Srivastava, O.N. Effect of TiO2 nanoparticles on the hydrogen sorption characteristics of magnesium hydride. J. Nanosci. Nanotechnol. 2013, 13, 5493–5499. [Google Scholar] [CrossRef]
- Daryani, M.; Simchi, A.; Sadati, M.; Mdaah Hosseini, H.; Targholizadeh, H.; Khakbiz, M. Effects of Ti-based catalysts on hydrogen desorption kinetics of nanostructured magnesium Hydride. Int. J. Hydrog. Energy. 2014, 39, 21007–21014. [Google Scholar] [CrossRef]
- Mirabile Gattia, D.; Di Girolamo, G.; Montone, A. Microstructure and kinetics evolution in MgH2–TiO2 pellets after hydrogen cycling. J. Alloys Compd. 2014, 615, S689–S692. [Google Scholar] [CrossRef]
- Jung, K.S.; Kim, D.H.; Lee, E.Y.; Lee, K.S. Hydrogen sorption of magnesium hydride doped with nano-sized TiO2. Catal. Today 2007, 120, 270–275. [Google Scholar] [CrossRef]
- Vujasin, R.; Mrakovic, A.; Kurko, S.; Novakovic, N.; Matovic, L.; Novakovic, J.G.; Milosevic, S. Catalytic activity of titania polymorphs towards desorption reaction of MgH2. Int. J. Hydrog. Energy. 2016, 41, 4703–4711. [Google Scholar] [CrossRef]
- Zhang, X.; Leng, Z.; Gao, M.; Hu, J.; Fang, D.; Yao, J.; Pan, H.; Liu, Y. Enhanced hydrogen storage properties of MgH2 catalyzed with carbon-supported nanocrystalline TiO2. J. Power Sources 2018, 398, 183–192. [Google Scholar] [CrossRef]
- Berezovets, V.; Denys, R.; Zavaliy, I.; Kosarchyn, Y. Effect of Ti-based nanosized additives on the hydrogen storage properties of MgH2. Int. J. Hydrog. Energy 2022, 47, 2789–7298. [Google Scholar] [CrossRef]
- Oelerich, W.; Klassen, T.; Bormann, R. Mg-based hydrogen storage materials with improved hydrogen sorption. Mater. Trans 2001, 42, 1588–1592. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Wang, A.; Zhang, H.; Ding, B.; Hu, Z. Hydrogenation characteristics of Mg–TiO (rutile) composite. J. Alloys Compd. 2000, 313, 218–223. [Google Scholar] [CrossRef]
- Chen, M.; Xiao, X.; Zhang, M.; Liu, M.; Huang, X.; Zheng, J.; Zhang, Y.; Jiang, L.; Chen, L. Excellent synergistic catalytic mechanism of in-situ formed nanosized Mg2Ni and multiple valence titanium for improved hydrogen desorption properties of magnesium hydride. Int. J. Hydrog. Energy. 2019, 44, 1750–1759. [Google Scholar] [CrossRef]
- Jangir, M.; Mirabile Gattia, D.; Peter, A.; Jain, I.P. Effect of Ti-Additives on Hydrogenation/Dehydrogenation Properties of MgH2. In Proceedings of the AIP Conference Proceeding, Rome, Italy, 11–14 September 2019; Volume 2145. [Google Scholar]
- Liu, G.; Wang, L.; Hu, Y.; Sun, C.; Leng, H.; Li, Q.; Wu, C. Enhanced catalytic effect of TiO2@rGO synthesized by one-pot ethylene glycol-assisted solvothermal method for MgH2. J. Alloys Compd. 2021, 881, 160644. [Google Scholar] [CrossRef]
- Jin, S.-A.; Ahn, J.-P.; Shim, J.-H.; Cho, Y.W.; Yi, K. Dehydrogenation and hydrogenation characteristics of MgH2 with transition metal fluorides. J. Power Sources 2007, 172, 859–862. [Google Scholar] [CrossRef]
- Deledda, S.; Borissova, A.; Poinsignon, C.; Botta, W.; Dornheim, J.; Klassen, M. H-sorption in MgH2 nanocomposites containing Fe or Ni with fluorine. J. Alloys Compd. 2005, 404, 409–412. [Google Scholar] [CrossRef]
- de Castro, J.F.R.; Yavari, A.R.; LeMoulec, A.; Ishikawa, T.T.; Botta, W.J. Improving H-sorption in MgH2 powders by addition of nanoparticles of transition metal fluoride catalysts and mechanical alloying. J. Alloys Compd. 2005, 389, 270–274. [Google Scholar] [CrossRef]
- Yavari, A.R.; LeMoulec, A.; de Castro, J.F.R.; Deledda, S.; Friedrichs, O.; Botta, W.J. Improvement in H- sorption kinetics of MgH2 powders by using Fe nanoparticles generated by reactive FeF3 addition. Scr. Mater. 2005, 52, 719–724. [Google Scholar] [CrossRef]
- Kim, J.W.; Ahn, J.-P.; Jin, S.-A.; Lee, S.H.; Chung, H.-S.; Shim, J.-H. Microstructural evolution of NbF5-doped MgH2 exhibiting fast hydrogen sorption kinetics. J. Power Sources 2008, 178, 373–378. [Google Scholar] [CrossRef]
- Ma, L.-P.; Kang, X.-D.; Dai, H.-B.; Liang, Y.; Fang, Z.-Z.; Wang, P.J. Superior catalytic effect of TiF3 over TiCl3 in improving the hydrogen sorption kinetics of MgH2: Catalytic role of fluorine anion. Acta Mater. 2009, 57, 2250–2258. [Google Scholar] [CrossRef]
- Malka, I.E.; Czujko, T.; Bystrzycki, J. Catalytic effect of halide additives ball milled with Magnesium hydride. Int. J. Hydrog. Energy. 2010, 35, 1706–1712. [Google Scholar] [CrossRef]
- Peng, S.-K.; Xiao, X.-Z.; Xu, R.-J.; Li, L.; Wu, F.; Li, S.-Q.; Wang, Q.-D.; Chen, L.-X. Hydrogen storage behaviours and microstructure of MF3 (M = Ti, Fe)-doped magnesium hydride. Trans. Nonferrous Met. Soc. 2010, 20, 1879–1884. [Google Scholar] [CrossRef]
- Jangir, M.; Jain, A.; Yamaguchi, S.; Ichikawa, T.; Lal, C.; Jain, I.P. Catalytic effect of TiF4 in improving hydrogen storage properties of MgH2. Int. J. Hydrog. Energy. 2016, 41, 14178–14183. [Google Scholar] [CrossRef]
- Ma, L.-P.; Wang, P.; Cheng, H.-M. Improving hydrogen sorption kinetics of MgH2 by mechanical milling with TiF3. J. Alloys Compd. 2007, 432, L1–L4. [Google Scholar] [CrossRef]
- Danaie, M.; Mitlin, D. TEM analysis of the microstructure in TiF3-catalyzed and pure MgH2 during the hydrogen storage cycling. Acta Mater. 2012, 60, 6441–6456. [Google Scholar] [CrossRef]
- Mustafa, N.S.; Ismai, M. Influence of K2TiF6 additive on the hydrogen sorption properties of MgH2. Int. J. Hydrog. Energy 2014, 39, 15563–15569. [Google Scholar] [CrossRef]
- Jain, A.; Agarwal, S.; Kumar, S.; Yamaguchi, S.; Miyaoka, H.; Kojima, Y.; Ichikawa, T. How does TiF4 affect the decomposition of MgH2 and its complex variants?—An XPS investigation. J. Mater. Chem. A 2017, 5, 15543–15551. [Google Scholar] [CrossRef]
- Guoxian, L.; Erde, W.; Shoushi, F. Hydrogen absorption and desorption characteristics of mechanically milled Mg-35 wt% FeTi1.2 powders. J. Alloys Compd. 1995, 223, 111–114. [Google Scholar] [CrossRef]
- Meena, P.; Jangir, M.; Singh, R.; Sharma, V.K.; Jain, I.P. Hydrogen kinetics studies of MgH2-FeTi composites. AIP Conf. Proc. 2018, 1, 030010. [Google Scholar]
- Lal, C.; Singh, V.; Jangir, M.; Jain, R.K.; Jain, I.P. Evolution of microstructure of Mg + FeTi nanocomposite prepared by mechanical alloying. AIP Conf. Proc. 2013, 1536, 883–884. [Google Scholar]
- Reule, H.; Hirscher, M.; Weißhardt, A.; Kronmüller, H. Hydrogen desorption properties of mechanically alloyed MgH2 composite materials. J. Alloys Compd. 2000, 305, 246–252. [Google Scholar] [CrossRef]
- Cui, N.; Luan, B.; Zhao, H.; Liu, H.K.; Dou, S.X. Synthesis and electrode characteristics of the new composite alloys Mg2Ni-xwt%Ti2Ni. J. Alloys Compd. 1996, 240, 229–234. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, H.; Wang, A.; Ding, B.; Hu, Z. Preparation and hydriding/dehydriding properties of mechanically milled Mg–30 wt% TiMn1.5 composite. J. Alloys Compd. 2003, 354, 296–302. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E.; Aldakheel, F.; Alkandary, A.; Behbehani, M.; Al-Saidi, M. Synthetic nanocomposite MgH2/5 wt.% TiMn2 powders for solid-hydrogen storage tank integrated with PEM fuel cell. Sci. Rep. 2017, 7, 13296. [Google Scholar] [CrossRef] [Green Version]
- El-Eskandarany, M.S.; Al-Ajmi, F.; Banyan, M.; Al-Duweesh, A. Synergetic effect of reactive ball milling and cold pressing on enhancing the hydrogen storage behavior of nanocomposite MgH2/10 wt% TiMn2 binary system. Int. J. Hydrog. Energy 2019, 44, 26428–26443. [Google Scholar] [CrossRef]
- Dai, J.H.; Jiang, X.W.; Song, Y. Stability and hydrogen adsorption properties of Mg/TiMn2 interface by first principles calculation. Surf. Sci. 2016, 653, 22–26. [Google Scholar] [CrossRef]
- Kalisvaart, W.; Harrower, C.; Haagsma, J.; Zahiri, B.; Luber, E.; Ophus, C.; Poirier, E.; Fritzsche, H.; Mitlin, D. Hydrogen storage in binary and ternary Mg-based alloys: A comprehensive experimental study. Int. J. Hydrog. Energy 2010, 35, 2091–2103. [Google Scholar] [CrossRef] [Green Version]
- Zahiri, B.; Amirkhiz, B.S.; Mitlin, D. Hydrogen storage cycling of MgH2 thin film nanocomposites catalyzed by bimetallic Cr Ti. Appl. Phys. Lett. 2010, 97, 083106. [Google Scholar] [CrossRef]
- Wu, Z.; Fang, J.; Liu, N.; Wu, J.; Kong, L. The Improvement in Hydrogen Storage Performance of MgH2 Enabled by Multilayer Ti3C2. Micromachines 2021, 12, 1190. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Du, Y.; Liao, W. Catalytic effect of Ti2C MXene on the dehydrogenation of MgH2. Int. J. Hydrog. Energy 2019, 44, 6787–6794. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, H.; Zhu, Y.; Li, S.; Zhang, J.; Li, L. Excellent catalytic activity of a two-dimensional Nb4C3Tx (MXene) on hydrogen storage of MgH2. Appl. Surf. Sci. 2019, 493, 431–440. [Google Scholar] [CrossRef]
- Zhu, W.; Panda, S.; Lu, C.; Ma, Z.; Khan, D.; Dong, D.; Sun, F.; Xu, H.; Zhang, Q.; Zou, J. Using a Self-Assembled Two-Dimensional MXene-Based Catalyst (2D-Ni@Ti3C2) to Enhance Hydrogen Storage Properties of MgH2. ACS Appl Mater Interfaces 2020, 12, 50333–50343. [Google Scholar] [CrossRef]
- Huang, T.; Huang, X.; Hu, C.; Wang, J.; Liu, H.; Xu, H.; Sun, F.; Ma, Z.; Zou, J.; Ding, W. MOF-derived Ni nanoparticles dispersed on monolayer MXene as catalyst for improved hydrogen storage kinetics of MgH2. Chem. Eng. J. 2021, 421, 127851. [Google Scholar] [CrossRef]
- Zhu, W.; Ren, L.; Lu, C.; Xu, H.; Sun, F.; Ma, Z.; Zou, J. Nanoconfined and in Situ Catalyzed MgH2 Self-Assembled on 3D Ti3C2 MXene Folded Nanosheets with Enhanced Hydrogen Sorption Performances. ACS Nano 2021, 15, 18494–18504. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, H.; Yuan, Z.; Liu, J.; Li, L.; Fan, Y.; Fan, G.; Liu, B. Hamamelis-like K2Ti6O13 Synthesized by Alkali Treatment of Ti3C2 MXene: Catalysis for Hydrogen Storage in MgH2. ACS Sustain. Chem. Eng. 2020, 8, 4755–4763. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Zhang, D.; Fan, Y.; Liu, B. Striking enhanced effect of PrF3 particles on Ti3C2 MXene for hydrogen storage properties of MgH2. J. Alloys Compd. 2022, 914, 165291. [Google Scholar] [CrossRef]
- Shen, Z.; Wang, Z.; Zhang, M.; Gao, M.; Hu, J.; Du, F.; Liu, Y.; Pan, H. A novel solid-solution MXene (Ti0.5V0.5)3C2 with high catalytic activity for hydrogen storage in MgH2. Materialia 2018, 1, 114–120. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Y.; Zhu, Y.; Zhang, J.; Li, L. Catalytic effect of sandwich-like Ti3C2/TiO2(A)-C on hydrogen storage performance of MgH2. Nanotechnology 2020, 31, 115404. [Google Scholar] [CrossRef]
- Liu, H.Z.; Lu, C.L.; Wang, X.C.; Xu, L.; Huang, X.T.; Wang, X.H.; Ning, H.; Lan, Z.Q.; Guo, J. Combinations of V2C and Ti3C2 MXenes for Boosting the Hydrogen Storage Performances of MgH2. ACS Appl. Mater. Interfaces 2021, 13, 13235–13247. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Cheng, H.; Zhu, Y.; Li, L.; Lin, H. Effects of two-dimension MXene Ti3C2 on hydrogen storage performances of MgH2-LiAlH4 composite. Chem. Phys. 2019, 522, 178–187. [Google Scholar] [CrossRef]
- Huang, X.; Lu, C.; Li, Y.; Tang, H.; Duan, X.; Wang, K.; Liu, H. Hydrogen Release and Uptake of MgH2 Modified by Ti3CN MXene. Inorganics 2023, 11, 243. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Shaban, E. Contamination Effects on Improving the Hydrogenation/Dehydrogenation Kinetics of Binary Magnesium Hydride/Titanium Carbide Systems Prepared by Reactive Ball Milling. Materials 2015, 8, 6880–6892. [Google Scholar] [CrossRef] [Green Version]
- Khalil, R.M.A.; Hussain, F.; Imran, M.; Rasheed, U.; Rana, A.M.; Murtaza, G. An ab initio study of spectroscopic and thermodynamic characteristics of MgH2 and TiC systems. Int. J. Hydrog. Energy 2019, 44, 6756–6762. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Z.; Yao, P.; Xia, C.; Yang, T.; Li, Q. Hydrogen storage behaviors of magnesium hydride catalyzed by transition metal carbides. Int. J. Hydrog. Energy 2021, 46, 40203–40216. [Google Scholar] [CrossRef]
- Sazelee, N.; Md Din, M.F.; Ismail, M.; Rather, S.-U.; Bamufleh, H.S.; Alhumade, H.; Taimoor, A.A.; Saeed, U. Effect of LaCoO3 Synthesized via Solid-State Method on the Hydrogen Storage Properties of MgH2. Materials 2023, 16, 2449. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, J.; Hou, Q.; Guo, X. Regulation of Kinetic Properties of Chemical Hydrogen Absorption and Desorption by Cubic K2MoO4 on Magnesium Hydride. Nanomaterials 2022, 12, 2468. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Al-Ajmi, F.; Banyan, M. Mechanically-Induced Catalyzation of MgH2 Powders with Zr2Ni-Ball Milling Media. Catalysts 2019, 9, 382. [Google Scholar] [CrossRef] [Green Version]
- An, C.; Deng, Q. Improvement of Hydrogen Desorption Characteristics of MgH2 with Core-shell Ni@C Composites. Molecules 2018, 23, 3113. [Google Scholar] [CrossRef] [Green Version]
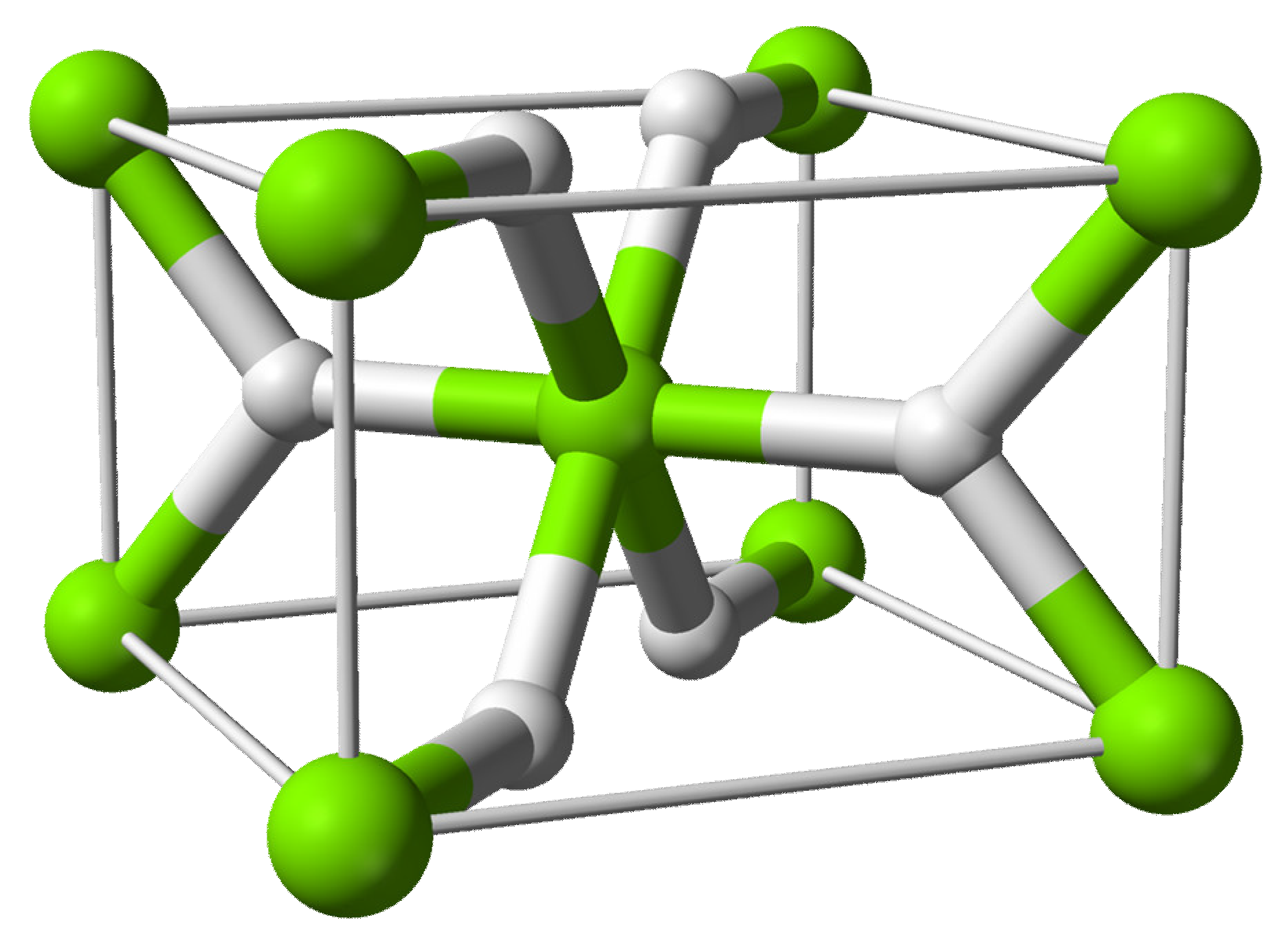


| Structure Type | Unit Cell (Å) | Positional Parameters | BO (GPa) | Bo | ||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| β-MgH2 | 4.5176 | 4.5176 | 3.0206 | Mg(2a), 0, 0, 0 | 45.00 ± 2 | 3.35 ± 0.3 |
| γ-MgH2 | 4.6655 | 4.6655 | 4.6655 | Mg(4a), 0, 0, 0 | 47.41 ± 4 | 3.39 ± 0.4 |
| α-MgH2 | 4.5248 | 5.442 | 4.9285 | Mg(4c), 0, 0, 0 | 44.03 ± 2 | 3.17 ± 0.4 |
| δ-MgH2 | 8.8069 | 4.6838 | 4.3699 | Mg(4c), 0.8823, 0.271, 0.2790 | 49.83 ± 5 | 3.49 ± 0.6 |
| Materials | Synthetic Method | Tdes | Hydrogen Desorption Capacity (wt%) | Activation Energy (kJ/mol H2) | Reference |
|---|---|---|---|---|---|
| 7MgH2/TiH2 | Ball milling | 126 °C | 5.5 | 79 | [53] |
| 10MgH2/TiH2 | Ball milling | 101 °C | 5 | 71 | [53] |
| MgH2-1 at%Ti | Ball milling | 278 °C | 4.9 | 208 | [49] |
| MgH2-5 at%Ti | Ball milling | 274 °C | 4.5 | 156 | [49] |
| MgH2-Ni/Ti | Ball milling | 256 °C | 2.9 | 81 | [49] |
| MgH2-Ti5Fe5Ni5 | Ball milling | 270 °C | 5.3 | 45.63 | [50] |
| MgH2-Ti2 | Ball milling | 257 °C | 6.18 | 103.9 | [51] |
| MgH2-0.1TiH2 | Ultrahigh-energy−high pressure (UHEHP) ball milling | 290 °C | 6.20 | 58.4 | [54] |
| 0.7MgH2–0.3TiH2 | Reactive ball milling | 573 K less than 100 s | - | [59] | |
| MgH2/0.1TiH2 | High pressure ball milling | 269–301 | - | 77.4 | [60] |
| Mg-2% Ti | Inert gas condensation | 320 °C | 4.50 | [61] | |
| MgH2 + 2 at%Ti | Cold rolling (5 times, air) | 623 K | 6.00 | [62] | |
| MgH2-4 mol%Ti | Ball Milling | 573 K | 1.10 | [63] | |
| MgH2-5 at%Ti | Ball Milling | 235.6 °C | 70.11 | [64] | |
| MgH2-5 at%Ti | Ball Milling | 523 K | 5.50 | 71.1 | [64] |
| MgH2-5 at%Ti | 573 K | 5.20 | [64] | ||
| Mg-5%Ti | Chemical Vapor Synthesis | 104 | [65] | ||
| Mg-14 at%Ti | Gas phase condensation | 35 | [56] | ||
| MgH2-15%Ti | Ball Milling | 573 K | 0.12 | [57] | |
| Mg0.9Ti0.1 | Ball Milling | 76 | [58] | ||
| Mg0.75Ti0.25 | Ball Milling | 88 | [58] | ||
| Mg0.5Ti0.5 | Ball Milling | 91 | [58] | ||
| MgH2-20%Ti | Ball Milling | 72 ± 3 | [66] | ||
| MgH2-coated Ti | Ball Milling | 250 °C | 5.00 | [66] | |
| Mg83.5Ti16.5 | Ball Milling | 300 °C | 2.50 | [67] | |
| 15Mg-Ti | Chemical Method | [68] | |||
| MgH2-5 at%Ti | Chemical Method | 270 °C | 5.80 | 67.24 | [63] |
| 4MgH2-TiH2 | Ball Milling | 68 | [52] | ||
| MgH2 + 10 mol%TiH2 | Ball Milling | 16.24 | [55] | ||
| Mg-9.2% TiH1.971-3.7%TiH1.5 | Ball Milling | 573 K | 4.10 | 46.2 | [69] |
| Mg0.65Ti0.35D1.2 | Ball Milling | 17 | [70] |
| Materials | Synthetic Method | Tdes. | Hydrogen Desorption Capacity (wt%) | Eact(kJ/mol H2) | Reference |
|---|---|---|---|---|---|
| Mg-TiO2 | Arc evaporation | 300 °C | 6.34 | 77.2 | [42] |
| MgH2-5 mol%TiO2 (rutile) | Ball milling | 300 °C | 4.40 | [78] | |
| MgH2-5 mol%TiO2 (anatase) | Ball milling | 300 °C | 1.95 | 52.7 | [78] |
| MgH2-10% TiO2 | Ball Milling | 300 °C | 6.00 | [82] | |
| MgH2-20% TiO2 | Ball Milling | 350 °C | 4.40 | [83] | |
| MgH2 +5 wt%TiO2(np) (>50 nm) | Ball Milling | 335 °C | [75] | ||
| MgH2 + 5 wt%TiO2 (np) (<50 nm) | Ball milling | 310 °C | 57 | [75] | |
| MgH2 + 6% TiO2 | Ball Milling | 145.8 | [84] | ||
| MgH2 +10% TiO2 | Ball Milling | 200 °C | 75.50 | [85] | |
| MgH2-x wt%TiO2@C | Ball Milling | 195 °C | 6.2 | 106 | [79] |
| MgH2-TiO2@rGO-EA | Ball Milling | 265 °C | 4.2 | 86.7 ± 8.0 | [86] |
| MgH2-40TiO2@rGO-EG | Ball Milling | 261 °C | 5.9 | [86] |
| Materials | Synthetic Method | Tdes. | Hydrogen Desorption Capacity (wt%) | Eact (kJ/mol H2) | Reference |
|---|---|---|---|---|---|
| MgH2 + 4 mol%TiF3 | Ball milling | 173 °C | 6.14 | [51] | |
| MgH2 + 4 mol%TiF3 | Ball milling | 300 °C | 5 | [96] | |
| MgH2 + 5 wt%TiF3 | Ball milling | 300 °C | 5.5 | [97] | |
| MgH2 + 10 wt%K2TiF6 | Ball Milling | 245 °C | 6.5 | [98] | |
| MgH2 + 7 wt%TiCl3 | Ball Milling | 225 °C | 79 | [93] | |
| MgH2-10% TiF4 | Ball Milling | 216 °C | 6.6 | 71 | [99] |
| MgH2-10% TiF4 | Ball milling | 154 °C | 70 | [95] | |
| MgH2 + 4 mol%TiF3 | Ball Milling | 573 °C | 4.5 | [63] | |
| MgH2 + 4 mol%TiCl3 | Ball Milling | 573 °C | 3.70 | [63] |
| Materials | Synthetic Method | Tdes. | Hydrogen Desorption Capacity (wt%) | Eact (kJ/mol H2) | Reference |
|---|---|---|---|---|---|
| MgH2-5 at%TiAl | Ball milling | 270 °C | 4.90 | 65.08 | [64] |
| MgH2-5 at%Ti3Al | Ball milling | 232.3 | 70.61 | [64] | |
| Mg85Al7.5Ti7.5 | DC-Magnetron Co-Sputtering | 200 °C | 5.30 | [109] | |
| Mg0.63Ti0.27Si0.10D1.1 | Ball Milling | 27 | [70] | ||
| MgH2-5 at%TiNi | Ball Milling | 242.4 °C | 73.09 | [64] | |
| 15Mg-Ti-0.75Ni | Chemical method | 63 | [68] | ||
| MgH2-5 at%TiNb | Ball Milling | 27 °C | 5.90 | 71.72 | [64] |
| MgH2-5 at%Cr-5 at%Ti | Film | 200 °C | 6.00 | 3.70 | [110] |
| MgH2-7 at%Cr-13 at%Ti | Film | 200 °C | 5.00 | [110] | |
| MgH2-5 at%TiFe | Ball Milling | 270 °C | 5.20 | 72.63 | [64] |
| MgH2-5 at%TiMn2 | Ball Milling | 270 °C | 4.60 | 74.22 | [64] |
| Mg87.5Ti9.6V2.9 | Hydrogen plasma metal reaction | 300 °C | 4.00 | 73.08 | [69] |
| MgH2-5 at%TiVMn | Ball Milling | 270 °C | 5.70 | 85.20 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jangir, M.; Jain, I.P.; Mirabile Gattia, D. Effect of Ti-Based Additives on the Hydrogen Storage Properties of MgH2: A Review. Hydrogen 2023, 4, 523-541. https://doi.org/10.3390/hydrogen4030034
Jangir M, Jain IP, Mirabile Gattia D. Effect of Ti-Based Additives on the Hydrogen Storage Properties of MgH2: A Review. Hydrogen. 2023; 4(3):523-541. https://doi.org/10.3390/hydrogen4030034
Chicago/Turabian StyleJangir, Mukesh, Indra Prabh Jain, and Daniele Mirabile Gattia. 2023. "Effect of Ti-Based Additives on the Hydrogen Storage Properties of MgH2: A Review" Hydrogen 4, no. 3: 523-541. https://doi.org/10.3390/hydrogen4030034
APA StyleJangir, M., Jain, I. P., & Mirabile Gattia, D. (2023). Effect of Ti-Based Additives on the Hydrogen Storage Properties of MgH2: A Review. Hydrogen, 4(3), 523-541. https://doi.org/10.3390/hydrogen4030034






