Biomass-to-Green Hydrogen: A Review of Techno-Economic-Enviro Assessment of Various Production Methods
Abstract
:1. Introduction
2. Methodology Framework
- What kinds of H2-production methods are there?
- Is thermochemical conversion a feasible choice from an economic standpoint?
- Is it economically feasible to convert using biochemistry?
- Does producing H2 cost less when using water-splitting methods?
- What are the various technological and financial obstacles preventing the commercialization of the H2-production process?
- Is it possible to perform sensitivity analysis using capital and feedstock costs for various H2-production techniques?
- What distinct opportunities do these papers present?
- Which feedstocks are utilized in varying amounts to lower the cost of producing H2?
- Is it possible to reduce the total cost of producing H2 using diverse methods?
3. A Cost Assessment of Various H2-Production Methods
3.1. Thermochemical Conversion (TC)
3.1.1. Pyrolysis
3.1.2. Gasification
3.1.3. Steam Reforming of NG
3.2. Water Electrolysis
3.3. Renewable Liquid Reforming
3.4. Biochemical Conversion (BC)
3.4.1. Dark Fermentation (DF)
3.4.2. Photobiological Hydrogen Production
4. Comparison of the Economics and Technical Aspects of Several Ways of Producing Hydrogen
4.1. Comparison of the H2 Production Cost
4.2. Sensitivity Analysis
4.2.1. Capital Cost Sensitivity
4.2.2. Sensitivity to IRR
4.3. Technology Readiness Level (TRL), Efficiency, and Scalability Comparison of Several Hydrogen-Production Methods
| Method | Efficiency (%) | TRL | Scale |
|---|---|---|---|
| Pyrolysis | 65 (using HDPE) [63] | 7 [64] | Bench scale [8] |
| Gasification | 35–50 [11] | 4–7 [65] | Laboratory and bench scale [8] |
| Biogas Reforming | 46.2–51.7 (SR) | 9 (SMR) | Large-scale (SR) [66] |
| 24.5–27.8 (ATR) | 8 (ATR), for natural gas | plant model [67] | |
| Dark fermentation | 60–80 [11] | 5 [3] | Laboratory scale [42] |
| Photo fermentation | Light conversion efficiency 1–5 | 4 [3] | Laboratory scale [42] |
| Water Electrolysis | 51–60 (AEL) 46–60 (PEMEL) 76–81 (SOEL) [68] | 9 (AEL) 8 (PEM) [69] | Laboratory and industrial scale [70] |
4.4. Comparison of the CO2 Emissions of Several Ways of Producing Hydrogen
4.5. Commercialization Obstacle
4.5.1. Technical Obstacles
4.5.2. Financial Obstacles
5. Assessment and Potential Future Approaches
6. Conclusions
- Thermochemical technologies are the most commonly utilized methods. Numerous scholars and policymakers are intrigued by the process of steam reforming natural gas to create hydrogen due to its ability to generate hydrogen with a high efficiency of 70–85% and low operational and production expenses of 0.3 dollars per kilogram of hydrogen. More research is needed to reduce both carbon dioxide emissions and manufacturing costs during the steam reforming process. Economic obstacles to steam reforming include process and catalyst costs. Increased efficiency and extended durability of the valuable metal catalyst need to compensate for the elevated individual catalyst expense in order to address these issues. Furthermore, expanding the plant’s scale will result in higher CAPEX but far lower H2 production costs. The technical obstacles to gasification include product normalization, catalyst deactivation, corrosion, clogging, and a lack of commercial use. Economic hurdles to gasification can include high operating and investment costs since high temperatures are needed. Membrane reactors can be used to incorporate hydrogen generation processes, thereby increasing the thermochemical process’s efficacy and mitigating these problems.
- Furthermore, research has employed microbes to facilitate the biological conversion of biomass, mainly using dark fermentation techniques. The main benefits are found in moderate-use circumstances. Progress is impeded by the sluggish conversion rate and low production quantities of these technologies, which are their primary limits. The capital costs of these systems are increased by the need for costly bioreactors and separation processes. Pre-treatments are also required for biomass that is resistant to treatment since this results in the synthesis of inhibitors and lower operating costs and capital costs. The creation of novel bacterial strains, more effective bioreactors, and small-scale local production facilities should be the main goals of future advancements. Economic constraints to photofermentation include increased yields at high energy costs. To overcome these obstacles, metabolic engineering can make up for the notable advancements in the biohydrogen process. Nutrient limitation and substrate usage effects were studied in order to identify genes in microalgae that promote increased hydrogen generation. The creation of photobioreactors must have an optimal design. The inhibitory chemicals during the pretreatment generate a substantial impediment, which is one of the technical key limitations of combined dark and photofermentation. The substrate inhibits one or both of the processes. The high cost of the feedstock, the processing costs resulting from the wastewater treatment effluents’ toxicity, the sequential reactor’s operation and maintenance, and the operating costs during the pretreatment of dark fermentation effluent are additional economic barriers to this method.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DF | Dark Fermentation |
| SR | Steam Reforming |
| TC | Thermochemical |
| BC | Biochemical |
| PF | Photobiological Fermentation |
| NG | Natural Gas |
| IRR | Internal Rate of Return |
| PB | Payback period |
| TRL | Technology Readiness Level |
| CBF | Carbon-Based Fuels |
| HC | Hydrocarbon |
| SMR | Steam Methane Reforming |
| SRNG | Steam Reforming of Natural Gas |
| WGS | Water Gas Shift |
| CPV | Concentrated Photovoltaic |
| ROI | Return on Investment |
| FCI | Fixed Capital Investment |
| AF | Annual Profit |
| TCC | Total Capital Cost |
| NPV | Net Present Value |
| DR | Discount Rate |
| ATR | Autothermal reforming |
| CCS | Carbon Capture Systems |
| PSA | Pressure Swing Absorption |
References
- Sridevi, V.; Surya, D.V.; Reddy, B.R.; Shah, M.; Gautam, R.; Kumar, T.H.; Puppala, H.; Pritam, K.S.; Basak, T. Challenges and opportunities in the production of sustainable hydrogen from lignocellulosic biomass using microwave-assisted pyrolysis: A review. Int. J. Hydrogen Energy 2024, 52, 507–531. [Google Scholar] [CrossRef]
- Sharafi, L.S.; Zeinali, M.; Mahmoudi, S.M.S.; Soltani, S.; Rosen, M.A. Biomass co-fired combined cycle with hydrogen production via proton exchange membrane electrolysis and waste heat recovery: Thermodynamic assessment. Int. J. Hydrogen Energy 2023, 48, 33795–33809. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-hydrogen: A review of main routes production, processes evaluation and techno-economical assessment. Biomass-Bioenerg. 2021, 144, 105920. [Google Scholar] [CrossRef]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Bhosale, R.R.; Kumar, G. Industrial wastewater to biohydrogen: Possibilities towards successful biorefinery route. Bioresour. Technol. 2020, 298, 122378. [Google Scholar] [CrossRef] [PubMed]
- Dou, B.; Zhang, H.; Song, Y.; Zhao, L.; Jiang, B.; He, M.; Ruan, C.; Chen, H.; Xu, Y. Hydrogen production from the thermochemical conversion of biomass: Issues and challenges. Sustain. Energy Fuels 2019, 3, 314–342. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S. Economic assessment of selected hydrogen production methods: A review. Energ Source Part B 2017, 12, 1022–1029. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of thermochemical routes for hydrogen production from biomass: A review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Yukesh, K.R.; Kavitha, S.; Preethi; Parthiba Karthikeyan, O.; Kumar, G.; Dai-Viet, N.V.; Rajesh Banu, J. Techno-economic assessment of various hydrogen production methods—A review. Bioresour. Technol. 2021, 319, 124175. [Google Scholar] [CrossRef]
- Rezaei, P.S.; Shafaghat, H.; Daud, W.M.A.W. Production of green aromatics and olefins by catalytic cracking of oxygenate compounds derived from biomass pyrolysis: A review. Applied Catalysis A General. 2014, 469, 490–511. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Bi, P.; Wang, J.; Zhang, Y.; Jiang, P.; Wu, X.; Liu, J.; Xue, H.; Wang, T.; Li, Q. From lignin to cycloparaffins and aromatics: Directional synthesis of jet and diesel fuel range biofuels using biomass. Bioresour. Technol. 2015, 183, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, Y.; Li, S.; Yuan, Y.; Zhang, D.; Wu, Y.; Xie, H.; Brindhadevi, K.; Pugazhendhi, A.; Xia, C. A review of biomass pyrolysis gas: Forming mechanisms, influencing parameters, and product application upgrades. Fuel 2023, 347, 128461. [Google Scholar] [CrossRef]
- Holladay, D.J.; Hu, J.; King, D.L.; Wang, Y.A. overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state-of-the-art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A comprehensive review of primary strategies for tar removal in biomass gasification. Energy Convers. Manag. 2023, 276, 116496. [Google Scholar] [CrossRef]
- Hannula, I.; Kurkela, E. A semi-empirical model for pressurised air-blown fluidised-bed gasification of biomass. Bioresour. Technol. 2010, 101, 4608–4615. [Google Scholar] [CrossRef]
- Xu, C.; Chen, S.; Soomro, A.; Sun, Z.; Xiang, W. Hydrogen rich syngas production from biomass gasification using synthesized Fe/CaO active catalysts. J. Energy Inst. 2018, 91, 805–816. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Gondal, I.A.; Masood, S.A.; Khan, R. Green hydrogen production potential for developing a hydrogen economy in Pakistan. Int. J. Hydrogen Energy 2018, 43, 6011–6039. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chinese J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Ferrero, D.; Santarelli, M. Investigation of a novel concept for hydrogen production by PEM water electrolysis integrated with multi-junction solar cells. Energy Convers. Manag. 2017, 148, 16–29. [Google Scholar] [CrossRef]
- Boudries, R. Techno-economic Assessment of Solar Hydrogen Production Using CPV-electrolysis Systems. Energy Proc. 2016, 93, 96–101. [Google Scholar] [CrossRef]
- Lima da Silva, A.; Müller, I.L. Hydrogen production by sorption enhanced steam reforming of oxygenated hydrocarbons (ethanol, glycerol, n-butanol and methanol): Thermodynamic modelling. Int. J. Hydrogen Energy 2011, 36, 2057–2075. [Google Scholar] [CrossRef]
- Braga, A.H.; Sodre, E.R.; Santos, J.B.O.; de Paula Marques, C.M.; Bueno, J.M.C. Steam reforming of acetone over Ni- and Co-based catalysts: Effect of the composition of reactants and catalysts on reaction pathways. Appl. Catal. B Environ. 2016, 195, 16–28. [Google Scholar] [CrossRef]
- Nabgan, W.; Abdullah, T.A.T.; Mat, R.; Nabgan, B.; Jalil, A.A.; Firmansyah, L.; Triwahyono, S. Production of hydrogen via steam reforming of acetic acid over Ni and Co supported on La2O3 catalyst. Int. J. Hydrogen Energy 2017, 42, 8975–8985. [Google Scholar] [CrossRef]
- Carrero, A.; Vizcaíno, A.J.; Calles, J.A.; García-Moreno, L. Hydrogen production through glycerol steam reforming using Co catalysts supported on SBA-15 doped with Zr, Ce and La. J. Energy Chem. 2017, 26, 42–48. [Google Scholar] [CrossRef]
- Basagiannis, A.C.; Verykios, X.E. Catalytic steam reforming of acetic acid for hydrogen production. Int. J. Hydrogen Energy 2007, 32, 3343–3355. [Google Scholar] [CrossRef]
- Cai, W.; de la Piscina, P.R.; Gabrowska, K.; Homs, N. Hydrogen production from oxidative steam reforming of bio-butanol over CoIr-based catalysts: Effect of the support. Bioresour. Technol. 2013, 128, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.S.; Kim, J.; Kang, M. Hydrogen production from ethanol steam reforming over core–shell structured NixOy–, FexOy–, and CoxOy–Pd catalysts. Int. J. Hydrogen Energy 2010, 35, 11829–11843. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Chen, S.; Liu, Y. Co–Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen. Int. J. Hydrogen Energy 2014, 39, 5644–5652. [Google Scholar] [CrossRef]
- Moraes, T.S.; Cozendey da Silva, H.N.; Zotes, L.P.; Mattos, L.V.; Pizarro Borges, L.E.; Farrauto, R.; Noronha, F.B. A techno-economic evaluation of the hydrogen production for energy generation using an ethanol fuel processor. Int. J. Hydrogen Energy 2019, 44, 21205–21219. [Google Scholar] [CrossRef]
- Song, H.; Ozkan, U.S. Economic analysis of hydrogen production through a bio-ethanol steam reforming process: Sensitivity analyses and cost estimations. Int. J. Hydrogen Energy 2010, 35, 127–134. [Google Scholar] [CrossRef]
- Bahzad, H.; Shah, N.; Dowell, N.M.; Boot-Handford, M.; Soltani, S.M.; Ho, M.; Fennell, P.S. Development and techno-economic analyses of a novel hydrogen production process via chemical looping. Int. J. Hydrogen Energy 2019, 44, 21251–21263. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A comprehensive review on biological hydrogen production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Sivashanmugham, P.; Kumar, G.; Nguyen, D.D.; Chang, S.W.; Banu, J.R. Biohydrogen production from rice straw: Effect of combinative pretreatment, modelling assessment and energy balance consideration. Int. J. Hydrogen Energy 2019, 44, 2203–2215. [Google Scholar] [CrossRef]
- Kavitha, S.; Kannah, R.Y.; Gunasekaran, M.; Kumar, G.; Banu, J.R. Rhamnolipid induced deagglomeration of anaerobic granular biosolids for energetically feasible ultrasonic homogenization and profitable biohydrogen. Int. J. Hydrogen Energy 2020, 45, 5890–5899. [Google Scholar] [CrossRef]
- Kumar, M.D.; Kannah, R.Y.; Kumar, G.; Sivashanmugam, P.; Banu, J.R. A novel energetically efficient combinative microwave pretreatment for achieving profitable hydrogen production from marine macro algae (Ulva reticulate). Bioresour. Technol. 2020, 301, 122759. [Google Scholar] [CrossRef]
- Li, Y.C.; Liu, Y.F.; Chu, C.Y.; Chang, P.L.; Hsu, C.W.; Lin, P.J.; Wu, S.Y. Techno-economic evaluation of biohydrogen production from wastewater and agricultural waste. Int. J. Hydrogen Energy 2012, 37, 15704–15710. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in biological hydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H.; Sumathy, K. An overview of hydrogen production from biomass. Fuel Process. Technol. 2006, 87, 461–472. [Google Scholar] [CrossRef]
- Sara, H.R.; Enrico, B.; Mauro, V.; Andrea, D.C.; Vincenzo, N. Techno-economic Analysis of Hydrogen Production Using Biomass Gasification -A Small Scale Power Plant Study. Energy Proc. 2016, 101, 806–813. [Google Scholar] [CrossRef]
- Mohammed, M.A.A.; Salmiaton, A.; Wan Azlina, W.A.K.G.; Mohammad Amran, M.S.; Fakhru’l-Razi, A. Air gasification of empty fruit bunch for hydrogen-rich gas production in a fluidized-bed reactor. Energy Convers. Manag. 2011, 52, 1555–1561. [Google Scholar] [CrossRef]
- Lv, P.; Wu, C.; Ma, L.; Yuan, Z. A study on the economic efficiency of hydrogen production from biomass residues in China. Renew. Energy 2008, 33, 1874–1879. [Google Scholar] [CrossRef]
- Lee, D.-H.; Lee, D.-J. Hydrogen economy in Taiwan and biohydrogen. Int. J. Hydrogen Energy 2008, 33, 1607–1618. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.M.; Correas, L.C. Techno-economic modelling of water electrolysers in the range of several MW to provide grid services while generating hydrogen for different applications: A case study in Spain applied to mobility with FCEVs. Int. J. Hydrogen Energy 2019, 44, 17431–17442. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-economic evaluation of a combined bioprocess for fermentative hydrogen production from food waste. Bioresour. Technol. 2016, 202, 107–111. [Google Scholar] [CrossRef]
- Han, W.; Hu, Y.Y.; Li, S.Y.; Li, F.F.; Tang, J.H. Biohydrogen production from waste bread in a continuous stirred tank reactor: A techno-economic analysis. Bioresour. Technol. 2016, 221, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Liu, Z.; Fang, J.; Huang, J.; Zhao, H.; Li, Y. Techno-economic analysis of dark fermentative hydrogen production from molasses in a continuous mixed immobilized sludge reactor. J. Clean. Prod. 2016, 127, 567–572. [Google Scholar] [CrossRef]
- Han, W.; Yan, Y.; Gu, J.; Shi, Y.; Tang, J.; Li, Y. Techno-economic analysis of a novel bioprocess combining solid state fermentation and dark fermentation for H2 production from food waste. Int. J. Hydrogen Energy 2016, 41, 22619–22625. [Google Scholar] [CrossRef]
- Zhang, Y.; Brown, T.R.; Hu, G.; Brown, R.C. Comparative techno-economic analysis of biohydrogen production via bio-oil gasification and bio-oil reforming. Biomass Bioenergy 2013, 51, 99–108. [Google Scholar] [CrossRef]
- Porcu, A.; Sollai, S.; Marotto, D.; Mureddu, M.; Ferrara, F.; Pettinau, A. Techno-Economic Analysis of a Small-Scale Biomass-to-Energy BFB Gasification-Based System. Energies 2019, 12, 494. [Google Scholar] [CrossRef]
- Stenberg, V.; Rydén, M.; Mattisson, T.; Lyngfelt, A. Exploring novel hydrogen production processes by integration of steam methane reforming with chemical-looping combustion (CLC-SMR) and oxygen carrier aided combustion (OCAC-SMR). Int. J. Greenh. Gas. Control 2018, 74, 28–39. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.; Xiong, X.; Tsang, D.C.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef] [PubMed]
- Ditzig, J.; Liu, H.; Logan, B.E. Production of hydrogen from domestic wastewater using a bioelectrochemically assisted microbial reactor (BEAMR). Int. J. Hydrogen Energy 2007, 32, 2296–2304. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Nguyen-Thi, T.X.; Nguyen, P.Q.P.; Tran, V.D.; Agbulut, Ü.; Nguyen, L.H.; Balasubramanian, D.; Tarelko, W.; Bandh, S.A.; Pham, N.D.K. Recent advances in hydrogen production from biomass waste with a focus on pyrolysis and gasification. Int. J. Hydrogen Energy 2024, 54, 127–160. [Google Scholar] [CrossRef]
- Hajjaji, N.; Martinez, S.; Trably, E.; Steyer, J.-P.; Helias, A. Life cycle assessment of hydrogen production from biogas reforming. Int. J. Hydrogen Energy 2016, 41, 6064–6075. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of hydrogen production from biogas reforming: Technological advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar] [CrossRef]
- Antonini, C.; Treyer, K.; Streb, A.; van der Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage—A techno-environmental analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar] [CrossRef]
- Kumar, G.; Saratale, R.G.; Kadier, A.; Sivagurunathan, P.; Zhen, G.; Kim, S.-H.; Saratale, G.D. A review on bio-electrochemical systems (BESs) for the syngas and value added biochemicals production. Chemosphere 2017, 177, 84–92. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.; Esposito, G. A review on dark fermentative biohydrogen production from organic biomass: Process parameters and use of by-products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Johan, M.A. Renewable Hydrogen Production from Biomass. Available online: https://www.etipbioenergy.eu/images/Renewable_Hydrogen_Production_from_Biomass.pdf (accessed on 26 April 2024).
- Wang, M.; Wang, G.; Sun, Z.; Zhang, Y.; Xu, D. Review of renewable energy-based hydrogen production processes for sustainable energy innovation. Glob. Energy Interconnect. 2019, 2, 436–443. [Google Scholar] [CrossRef]
- Rasul, M.; Hazrat, M.; Sattar, M.; Jahirul, M.; Shearer, M. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Madeira, J.G.F.; Delgado, A.R.S.; Boloy, R.A.M.; Coutinho, E.R.; Loures, C.C.A. Exergetic and economic evaluation of incorporation of hydrogen production in a cassava wastewater plant. Appl. Therm. Eng. 2017, 123, 1072–1078. [Google Scholar] [CrossRef]
- Lachén, J.; Durán, P.; Menéndez, M.; Peña, J.; Herguido, J. Biogas to high purity hydrogen by methane dry reforming in TZFBR+MB and exhaustion by Steam-Iron Process. Techno–economic assessment. Int. J. Hydrogen Energy 2018, 43, 11663–11675. [Google Scholar] [CrossRef]
- Panigrahy, B.; Narayan, K.; Rao, B.R. Green hydrogen production by water electrolysis: A renewable energy perspective. Mater. Today Proc. 2022, 67 Pt 8, 1310–1314. [Google Scholar] [CrossRef]
- Hassan, N.; Jalil, A.; Rajendran, S.; Khusnun, N.; Bahari, M.; Johari, A.; Kamaruddin, M.; Ismail, M. Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrogen Energy 2024, 52, 420–441. [Google Scholar] [CrossRef]
- Grigoriev, S.; Fateev, V.; Bessarabov, D.; Millet, P. Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 2020, 45, 26036–26058. [Google Scholar] [CrossRef]
- Rosa, L.; Mazzotti, M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew. Sustain. Energy Rev. 2022, 157, 112123. [Google Scholar] [CrossRef]
- Parkinson, B.; Balcombe, P.; Speirs, J.F.; Hawkes, A.D.; Hellgardt, K. Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ. Sci. 2019, 12, 19–40. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from different biomass gasification processes. Int. J. Hydrogen Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-economic analysis and life cycle assessment of hydrogen production from natural gas using current and emerging technologies. Int. J. Hydrogen Energy 2017, 42, 18894–18909. [Google Scholar] [CrossRef]
- Ren, N.-Q.; Zhao, L.; Chen, C.; Guo, W.-Q.; Cao, G.-L. A review on bioconversion of lignocellulosic biomass to H2: Key challenges and new insights. Bioresour. Technol. 2016, 215, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-W.; Lin, C.-Y. Using social network analysis to examine the technological evolution of fermentative hydrogen production from biomass. Int. J. Hydrogen Energy 2016, 41, 21573–21582. [Google Scholar] [CrossRef]
- Bundhoo, M.A.Z.; Mohee, R. Inhibition of dark fermentative bio-hydrogen production: A review. Int. J. Hydrogen Energy 2016, 41, 6713–6733. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Alnazha, A.A.; Ahmed, U.; El-Adawy, M.; Habib, M.A. Review on techno-economics of hydrogen production using current and emerging processes: Status and perspectives. Results Eng. 2024, 21, 101890. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, R.; Man, Y.; Ren, J. Recent developments of hydrogen production from sewage sludge by biological and thermochemical process. Int. J. Hydrogen Energy 2019, 44, 19676–19697. [Google Scholar] [CrossRef]
- Kumar, M.; Oyedun, A.O.; Kumar, A. A comparative analysis of hydrogen production from the thermochemical conversion of algal biomass. Int. J. Hydrogen Energy 2019, 44, 10384–10397. [Google Scholar] [CrossRef]
- Nguyen, T.; Abdin, Z.; Holm, T.; Merida, W. Grid-connected hydrogen production via large-scale water electrolysis. Energy Convers. Manag. 2019, 200, 112108. [Google Scholar] [CrossRef]
- Esposito, D.V. Membraneless Electrolyzers for Low-Cost Hydrogen Production in a Renewable Energy Future. Joule 2017, 1, 651–658. [Google Scholar] [CrossRef]
- Gonzalez, J.F.; Roman, S.; Bragado, D.; Calderon, M. Investigation on the reactions influencing biomass air and air/steam gasification for hydrogen production. Fuel Process. Technol. 2008, 89, 764–772. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Venkata Mohan, S. Bio-electrohydrolysis as a pretreatment strategy to catabolize complex food waste in closed circuitry: Function of electron flux to enhance acidogenic biohydrogen production. Int. J. Hydrogen Energy 2014, 39, 11411–11422. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, R.; Yasin, M.; Khan, A.L.; Shahid, M.K.; Hossain, S.; Khan, Z.; Jamil, F.; Rafiq, S.; Bilad, M.R.; et al. Anaerobic membrane bioreactors for biohydrogen production: Recent developments, challenges and perspectives. Bioresour. Technol. 2018, 269, 452–464. [Google Scholar] [CrossRef]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lin, C.Y.; Chang, J.S. Biohydrogen production: Current perspectives and the way forward. Int. J. Hydrogen Energy 2012, 37, 15616–15631. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark fermentative biohydrogen production from lignocellulosic biomass: Technological challenges and future prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Zhang, X.; Guo, J.; Chang, S.W.; Nguyen, D.D.; Wang, J. Biohydrogen production from anaerobic digestion and its potential as renewable energy. Renew. Energy 2018, 129, 754–768. [Google Scholar] [CrossRef]
- Kumar, G.; Shobana, S.; Nagarajan, D.; Lee, D.-J.; Lee, K.-S.; Lin, C.-Y.; Chen, C.-Y.; Chang, J.-S. Biomass based hydrogen production by dark fermentation—Recent trends and opportunities for greener processes. Curr. Opin. Biotechnol. 2018, 50, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.K.; Singh, S.P. Integrated dark- and photo-fermentation: Recent advances and provisions for improvement. Int. J. Hydrogen Energy 2016, 41, 19957–19971. [Google Scholar] [CrossRef]
- Rashid, N.; Rehman, M.S.U.; Memon, S.; Ur Rahman, Z.; Lee, K.; Han, J.I. Current status, barriers and developments in biohydrogen production by microalgae Renew. Sustain. Energy Rev. 2013, 22, 571–579. [Google Scholar] [CrossRef]

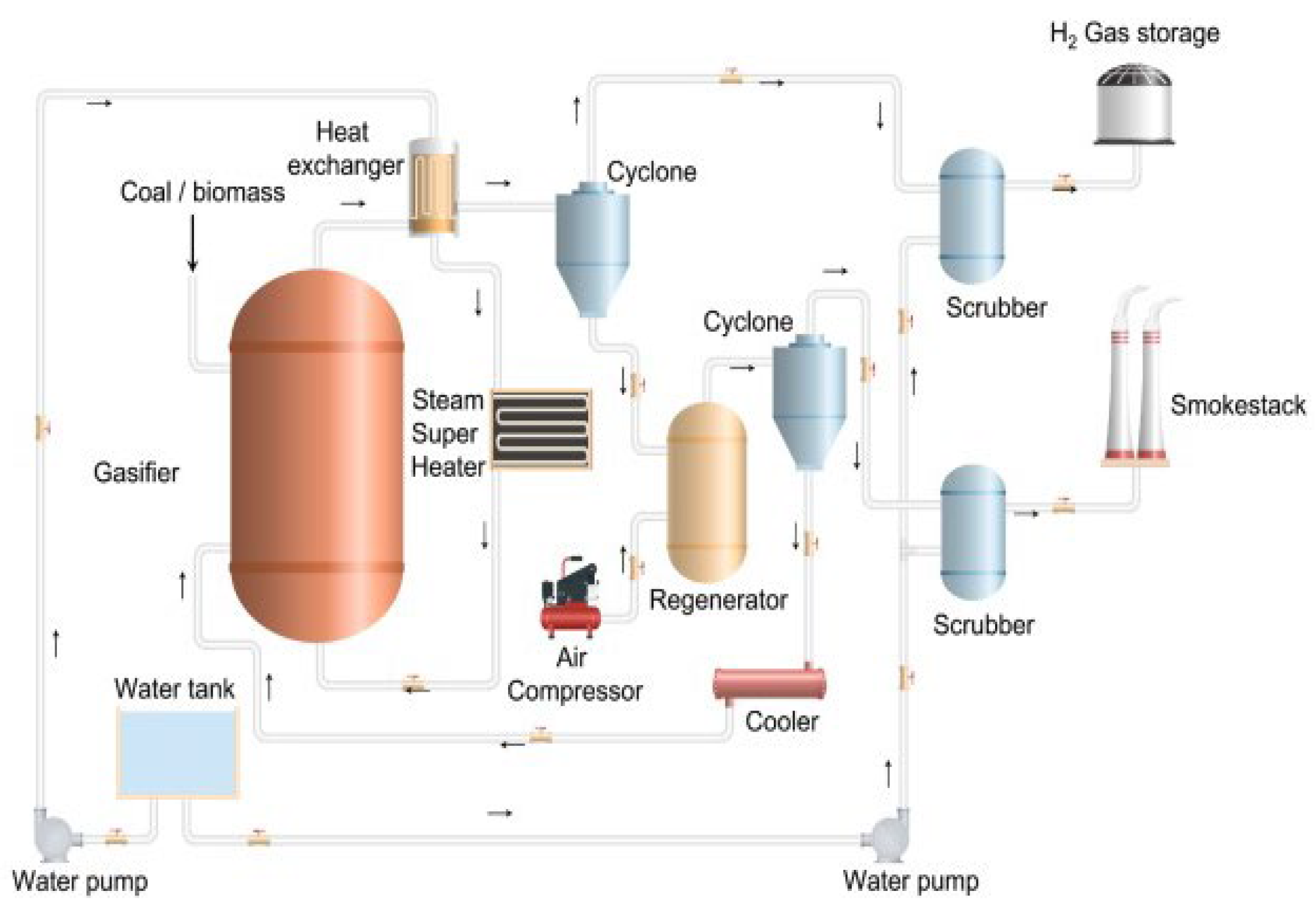

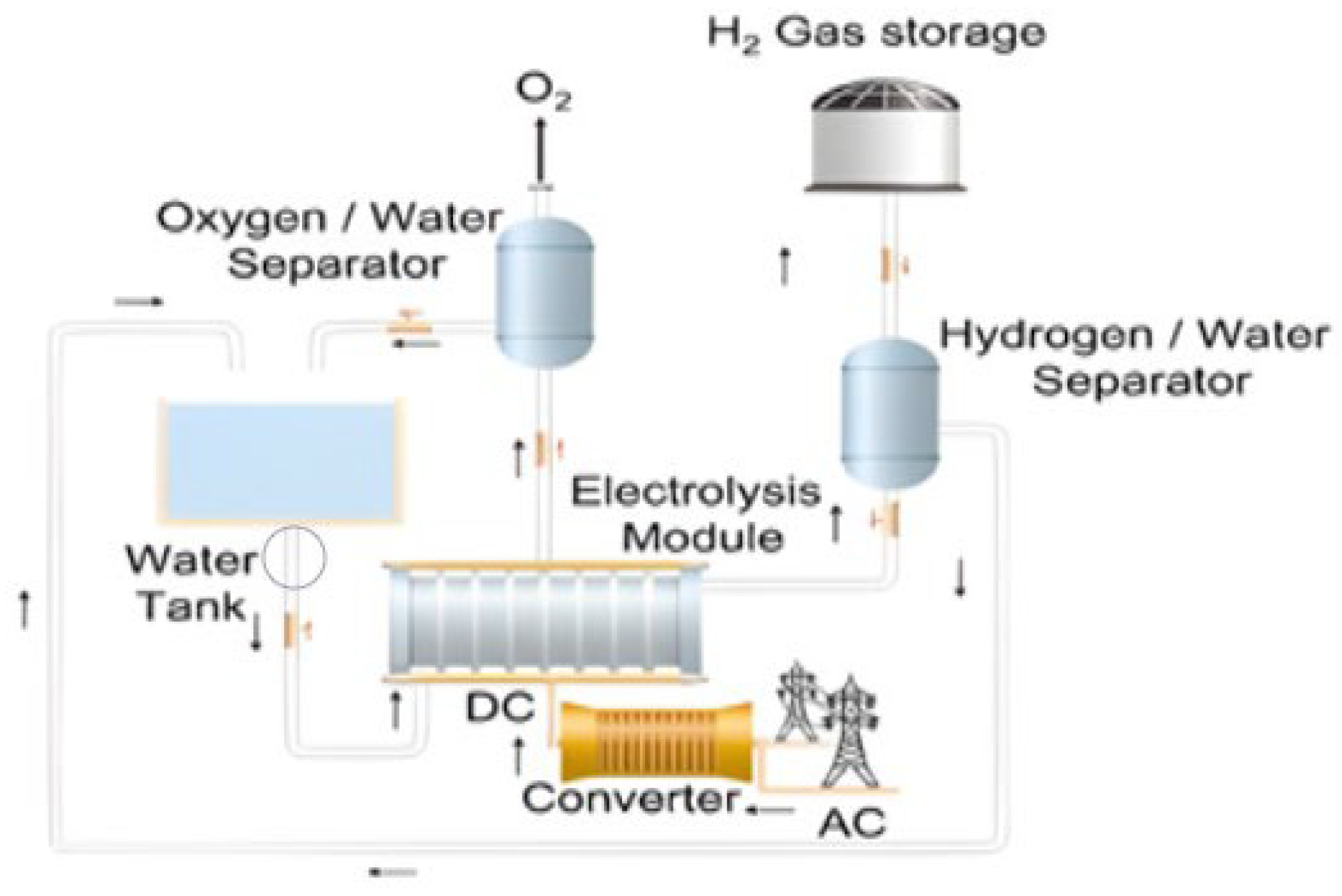

| Method | Feedstock | Production Cost | Ref. |
|---|---|---|---|
| Pyrolysis | CH4 | 1.25 to 2.20 $/kg | [11] |
| Pyrolysis | Biomass | 1.77 to 2.05 $/kg | [11] |
| Pyrolysis | Biomass | 1.25 to 2.20 $/kg | [42] |
| Gasification | Biomass | 12.75 €/kg | [43] |
| Gasification | Fruit bunches | 2.11 $/kg | [44] |
| Gasification | Agricultural waste | 1.69 $/kg | [45] |
| Steam reforming | Natural gas | 2.33 to 4.00 $/kg | [46] |
| Steam reforming (CO2 capture and storage) | Natural gas | 2.27 $/kg | [11] |
| Water electrolysis | AWE | 6 €/kg | [47] |
| Water electrolysis | PEM-based | 7 €/kg | [47] |
| DF | Wastewater | 2.7 $/m3 | [40] |
| DF | Agricultural waste | 2.7 $/m3 | [40] |
| DF | Food waste | 1.02 $/m3 | [48] |
| DF | Food waste | 1.34 $/m3 | [49] |
| DF | Molasses | 2.7 $/m3 | [50] |
| DF | Food waste | 2.29 $/m3 | [51] |
| DF | Organic biomass | 2.57 $/kg | [11] |
| PF | Organic biomass | 2.83 $/kg | [11] |
| Process | Capital Cost | Feedstock Cost | IRR | PB | Ref. |
|---|---|---|---|---|---|
| Reforming | $333 × 106 | 83 $/ton | 18.6 | NA | [52] |
| Gasification | $435 × 106 | 83 $/ton | 8.4 | NA | [52] |
| DF | $583,092 | 7408.4 $/y | 24.07 | 5 | [48] |
| DF | $931,020 | 19,120 $/y | 21.77 | 4.8 | [49] |
| DF | $478,200 | 10,480 $/y | 9.25 | 6.9 | [50] |
| DF | $707,850 | 118,750 $/y | 20.2 | 5 | [51] |
| Gasification | 12,597.5 k€ | 367.5 k€/y | 17.1 | NA | [53] |
| Process | TRL | Emission (kg CO2/kg H2) |
|---|---|---|
| Coal gasification | 9 [72] | 14.72–30.90 [72] |
| Coal gasification and CCS | 6–7 [72] | 2.11–10.35 [72] |
| Fossil methane SMR | 9 [69] | 11 [73] 10.09–17.21 [69] |
| Fossil methane SMR and CCS | 7–8 [72] | 2.7 [74] 2.97–9.16 [69] |
| Biomass gasification | 4–7 | 0.31–8.63 [72] |
| Biomass gasification and CCS | 3–5 [72] | (−)17.50–(−)11.66 [72] |
| Thermochemical Conversion | Technical Obstacles | Financial Obstacles | Potential Strategies for Overcoming These Barriers | Ref. |
|---|---|---|---|---|
| Gasification | Energy consumption | Cost of CO2 | [43] | |
| Steam reforming | The enhanced performance and extended lifespan of the valuable metal catalyst offset the increased cost per unit of catalyst incurred in the catalytic process. | [78] | ||
| Gasification | Issues such as corrosion, fouling, and catalyst deactivation as well as the lack of widespread industrial adoption and standardization of the product can hinder the success of catalyst applications. | The necessity for elevated temperatures results in significant capital and operational expenses when implementing certain processes. | By incorporating various hydrogen-production techniques, membrane reactors can enhance the efficiency of thermochemical processes. | [79] |
| Supercritical water gasification | The feasibility of a project is contingent upon the financial implications associated with the procurement of algal biomass and the resulting yield. | Optimization is crucial in research to enhance fuel production.If a payment is made from a dioxide carbon producer to an algal conversion facility, the cost of hydrogen decreases. | [80] | |
| Water electrolysis | Combining energy systems with business operations is a significant obstacle to the widespread implementation of this technology. | Electricity expenses make up 40–57% of the total cost of hydrogen production.Various geographical areas and intelligent operational tactics can be taken into account in order to lower expenses. | Achieving minimal CO2 emissions is possible by taking into account the carbon footprint of the source of electricity. | [81] |
| Membrane less electrolyser | Reduced efficiency at high current densities is caused by increased solution resistance losses as well as concerns related to product purity and safety during the process of scaling up. | Electrolyzers paired with sporadic renewable energy sources need to have extremely affordable upfront expenses. It will also be crucial to lower the capital expenditure of membraneless electrolyzers by creating affordable electrocatalysts that are abundant in the earth. | [82] |
| Biochemical Conversion | Technical Obstacles | Financial Obstacles | Potential Strategies for Overcoming These Barriers | Ref. |
|---|---|---|---|---|
| Dark fermentation | Creation, building, running, and controlling an appropriate bioreactor. | The main issue affecting the cost of biohydrogen is substrate cost. | The feedback inhibition is reduced when dark and photo fermentation are combined. | [75] |
| Dark fermentation | Since pretreatment techniques vary depending on the feedstock, pretreatment prior to fermentation presents a significant problem. | Costly procedure. | Large-scale, advanced research gets over the financial and technological obstacles. | [87] |
| Anaerobic digestion | Hydrogen yield variations are due to varying biomass, process inhibition, bacteria that consume hydrogen, elevated concentrations of heavy metal ions, optimization problems, and hydrogen storage. | Price of storing hydrogen in liquid form. | The efficiency of H2 generation can be increased by adding chemical additives. | [88] |
| Dark fermentation | There are thermodynamic restrictions on the amount of hydrogen produced by microbial fermentation as well as by the design and management of functioning bioreactors. The main technological barrier to dark fermentation’s use in the field is its limited hydrogen output of 4 mol H2/mol of glucose. | High cost linked to the feedstock. | The recovery of energy from the substrate is improved when DF is integrated with other energy-generating systems. | [89] |
| The process of integrated dark and photo fermentation involves the combination of both dark fermentation and photo fermentation techniques. | One of the main obstacles during the pretreatment is the inhibitory chemicals. The substrate inhibits one or both of the processes. | Because wastewater treatment effluents are harmful, processing costs rise as a result. The cost of the process is increased in a sequential reactor by reactor operation and maintenance. The processing of dark fermentation effluent results in an increase in operational costs. | By choosing the right hydrogen producers, the use of genetic or metabolic engineering in the combined dark and light fermentation process improves the efficiency of hydrogen generation. | [90] |
| Photo fermentation | Greater output at a higher cost of energy | The significant advancement in the biohydrogen process can be offset by metabolic engineering. By studying the impacts of nutrient limitation and substrate utilization, researchers identified the chromosomal genes in microalgae responsible for enhancing hydrogen production. Developments in photobioreactor design must be conducted with optimal efficiency. | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, A.; Nikafshan Rad, H.; Akrami, M. Biomass-to-Green Hydrogen: A Review of Techno-Economic-Enviro Assessment of Various Production Methods. Hydrogen 2024, 5, 474-493. https://doi.org/10.3390/hydrogen5030027
Ghasemi A, Nikafshan Rad H, Akrami M. Biomass-to-Green Hydrogen: A Review of Techno-Economic-Enviro Assessment of Various Production Methods. Hydrogen. 2024; 5(3):474-493. https://doi.org/10.3390/hydrogen5030027
Chicago/Turabian StyleGhasemi, Amir, Hima Nikafshan Rad, and Mohammad Akrami. 2024. "Biomass-to-Green Hydrogen: A Review of Techno-Economic-Enviro Assessment of Various Production Methods" Hydrogen 5, no. 3: 474-493. https://doi.org/10.3390/hydrogen5030027
APA StyleGhasemi, A., Nikafshan Rad, H., & Akrami, M. (2024). Biomass-to-Green Hydrogen: A Review of Techno-Economic-Enviro Assessment of Various Production Methods. Hydrogen, 5(3), 474-493. https://doi.org/10.3390/hydrogen5030027








