Perspective for the Safe and High-Efficiency Storage of Liquid Hydrogen: Thermal Behaviors and Insulation
Abstract
:1. Introduction
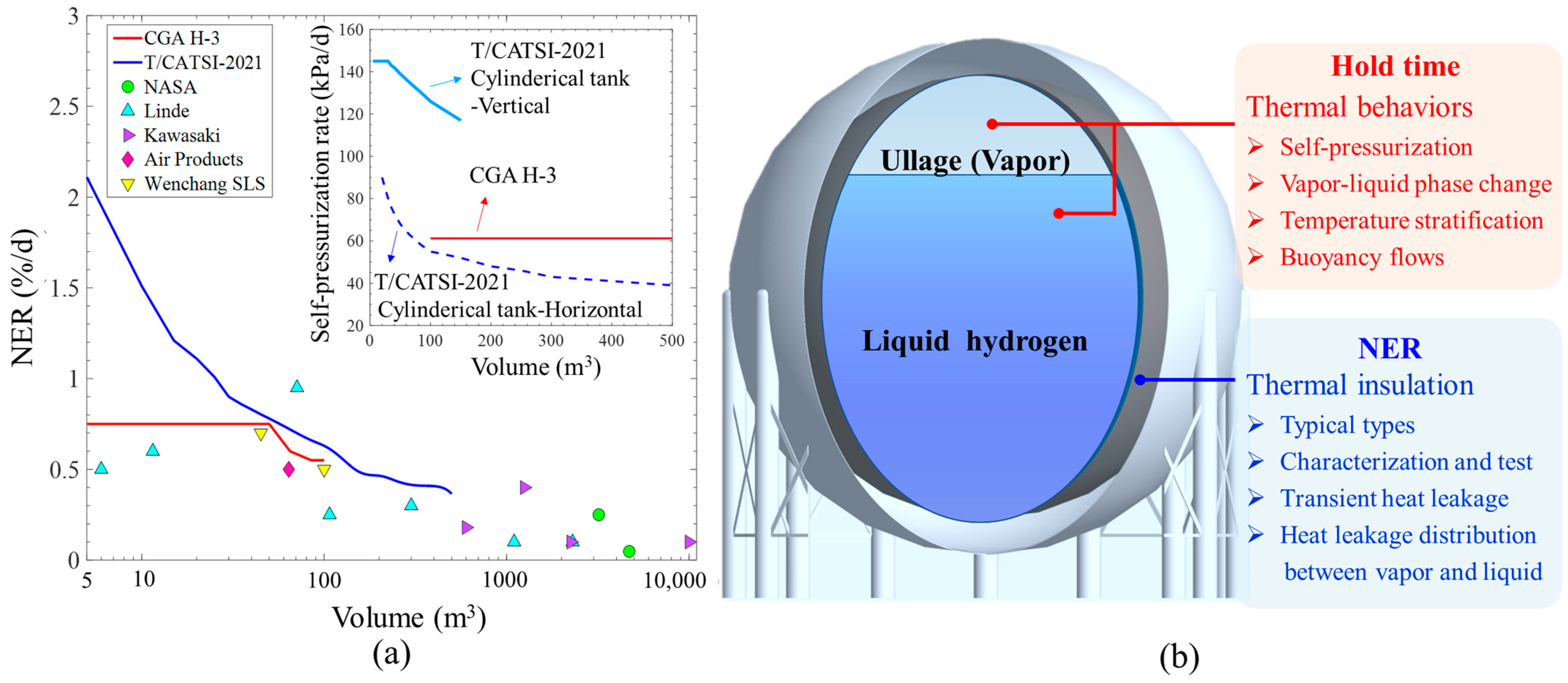
2. Thermal Behaviors during the No-Vented Storage of Liquid Hydrogen
2.1. Overview of Experimental Investigation
| Exp. | Tank Shape | Volume (m3) | Time (s, h) | Liquid Fill Ratio (%)/Heat Flux (W/m2) (a) | Measurement Content | Accuracies |
|---|---|---|---|---|---|---|
| [39] | Spherical | 208 | 38 h | 54.2%, 84.7%/1.9 |
| ±1.38 kPa ±0.83 K |
| [33,40] | Spherical | 0.00637; 0.09195 | 222–2720 s | 31.6–79.8%/53–202 |
| ±13.8 kPa ±0.5 K |
| [34,41] | Spherical | 4.95 | 12–20 h | 29–83%/0.35–3.5 |
| ±0.01 kPa ±0.3 K |
| [42,43] | Cylindrical | 18.09 | 6.9–18.37 h | 25–90%/0.526–1.514 (b) |
| ±0.13 kPa |
| [44,45] | Cylindrical | 0.02 | 1.75 h | 14%/14.8 |
| − |
| [46] | Cylindrical | 31.1 | 0.53–2.28 h | 25–70%/63.52–164.76 (c) |
| ±0.69 kPa ±1 K |
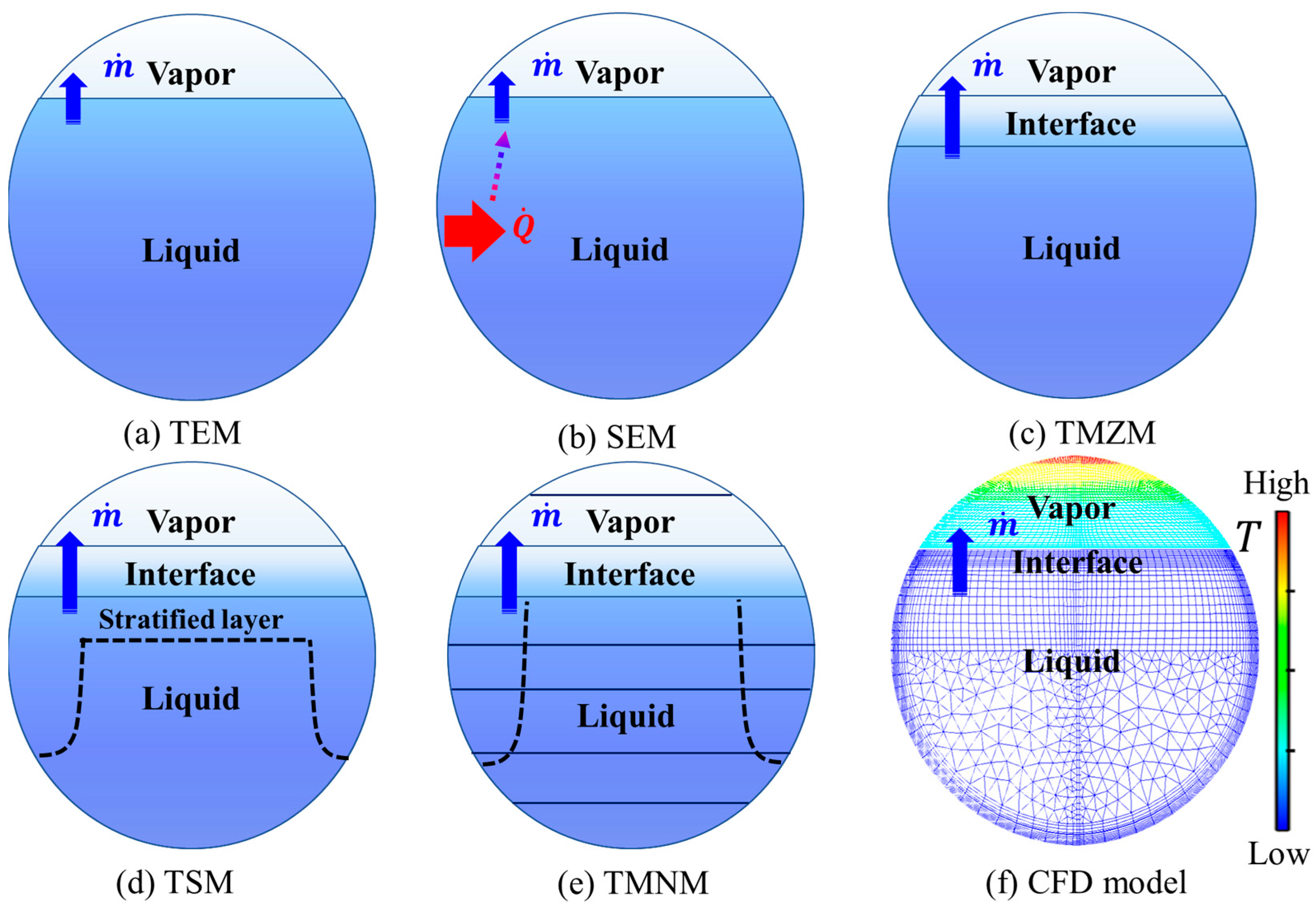
2.2. Status of Theoretical Investigation
| Model | Classification | Node (Cell) Number | Advantages | Limitations |
|---|---|---|---|---|
| TEM | - | 1 | A clear description of the no-vented storage process in thermodynamics | Assuming no temperature difference existed between vapor and liquid phases |
| NTEM | SEM [48] | 2 | Estimation of the maximum self-pressurization rate | The temperature of the liquid phase is regarded as a constant |
| TMZM [27,49,50,51] | 3 | Incorporating the temperature difference between vapor and liquid phases and interfacial mass transfer | Lack of consideration of temperature stratification in the vapor and liquid | |
| TSM [52,53] | 3~5 | Incorporating the boundary layer zone and thermal stratification in the liquid | Lack of consideration of temperature stratification in the vapor | |
| TMNM [54,55] | ≥4 | Discretization of vapor, liquid, and boundary layer zone with more nodes | Simplification of modeling flow fields | |
| CFD model [56,57] | ≥5000 | Multi-scale and multi-dimensional description of thermal behaviors for no-vented storage of LH2 | Time-consuming for thermal design and prediction; The selection of some sub-models remains a disagreement | |
2.3. Perspectives and Recommendations for Thermal Behaviors
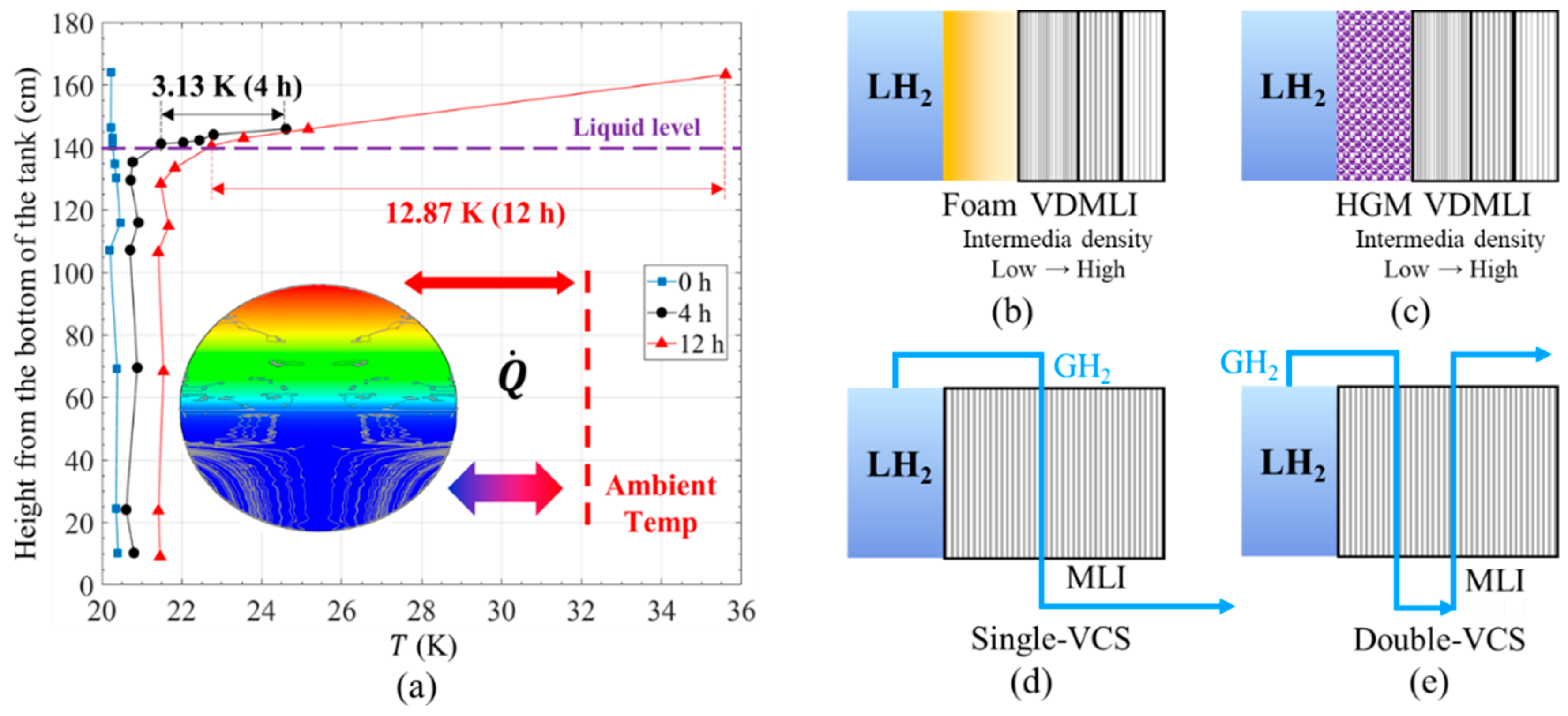
2.3.1. Long-Term Tests to Observe Transient Thermal Behaviors
2.3.2. Mechanism of Vapor–Liquid Phase Change in Liquid Hydrogen Tanks
2.3.3. Effect of Temperature Stratification on Self-Pressurization Rate
2.3.4. Effect of Free Convection Flows Driven by Buoyancy Force on Self-Pressurization Rate
3. Thermal Insulation for the No-Vented Liquid Hydrogen Storage
3.1. Typical Thermal Insulation Forms and Testing Method for LH2 Storage
| Insulation Forms | Advantages | Disadvantages | Performance |
|---|---|---|---|
| Foam-outside |
|
| >0.01 W/(m·K) |
| Foam-inside |
|
| - |
| Aerogel |
|
| 2 × 10−3~1.4 × 10−2 W/(m·K) at 185 K [69] |
| Perlite |
|
| 1 × 10−3~5 × 10−2 W/(m·K) |
| Glass bubbles/Hollow glass microspheres |
|
| 2 × 10−4~1 × 10−3 W/(m·K) |
| Multi-layer insulation |
|
| 1 × 10−5~5 × 10−4 W/(m·K) [70] |
3.2. Perspectives and Recommendations for Thermal Insulations
3.2.1. Alternative Testing Methods for Thermal Insulations at Liquid Hydrogen Temperatures
3.2.2. Measurement of Transient Heat Leak at Liquid Hydrogen Temperatures
3.2.3. Development of High-Performance Thermal Insulation for Protecting LH2 Tanks
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- IEA. Global Hydrogen Review 2023, 2023, IEA. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 15 June 2024).
- Gür, T.M. Review of electrical energy storage technologies, material and systems: Challenges and prospects for large-scale grid storage. Energy Environ. Sci. 2018, 11, 2696–2767. [Google Scholar] [CrossRef]
- Parkinson, B.; Balcombe, P.; Speirs, J.F.; Hawkes, A.D.; Hellgardt, K. Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ. Sci. 2019, 12, 19–40. [Google Scholar] [CrossRef]
- Alsaba, W.; Al-Sobhi, S.A.; Qyyum, M.A. Recent advancements in the hydrogen value chain: Opportunities, challenges, and the way Forward-Middle East perspectives. Int. J. Hydrogen Energy 2023, 48, 26408–26435. [Google Scholar] [CrossRef]
- Bartela, L. A hybrid energy storage system using compressed air and hydrogen as the energy carrier. Energy 2020, 196, 117088. [Google Scholar] [CrossRef]
- Xie, X.B.; Hou, C.X.; Chen, C.G.; Sun, X.Q.; Pang, Y.; Zhang, Y.P.; Yu, R.H.; Wang, B.; Du, W. First-principles studies in Mg-based hydrogen storage materials: A review. Energy 2020, 211, 118959. [Google Scholar] [CrossRef]
- Andersson, J.; Grönkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Fesmire, J.; Swanger, A.; Jacobson, J.; Notardonto, W. Energy efficient large-scale storage of liquid hydrogen. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1240, 012088. [Google Scholar] [CrossRef]
- Liquefied Hydrogen Carrier -SUISO FRONTIER- Receives Classification from Nippon Kaiji Kyokai. Available online: https://global.kawasaki.com/en/corp/newsroom/news/detail/?f=20211203_9557 (accessed on 18 June 2024).
- Decker, L. Liquid Hydrogen Distribution Technology. Available online: https://www.sintef.no/globalassets/project/hyper/presentations-day-2/day2_1105_decker_liquid-hydrogen-distribution-technology_linde.pdf/ (accessed on 18 June 2024).
- LH2 Transport Trailers. Available online: https://files.chartindustries.com/21746492_LH2Trailer.pdf (accessed on 18 June 2024).
- IRENA. Global hydrogen trade to meet the 1.5 °C climate goal. In Part II: Technology Review of Hydrogen Carriers; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]
- Berstad, D.; Gardarsdottir, S.; Roussanaly, S.; Voldsund, M.; Ishimoto, Y.; Nekså, P. Liquid hydrogen as prospective energy carrier: A brief review and discussion of underlying assumptions applied in value chain analysis. Renew. Sustain. Energy Rev. 2022, 154, 111772. [Google Scholar] [CrossRef]
- Wang, H.R.; Wang, B.; Li, R.Z.; Shen, X.; Wu, Y.Z.; Pan, Q.W.; He, Y.X.; Zhou, W.M.; Gan, Z.H. Thermodynamic analysis of the effect of initial ortho-hydrogen concentration on thermal behaviors for liquid hydrogen tanks. Int. J. Hydrogen Energy 2024, 55, 243–260. [Google Scholar] [CrossRef]
- Cursu, S.; Sherif, S.A.; Veziroglu, T.N.; Sheffield, J.W. Analysis and optimization of thermal stratification and self-pressurization effects in liquid hydrogen storage systems-Part 1: Model development. J. Energy Resour. Technol. 1993, 115, 221–227. [Google Scholar]
- Mao, Z.Q. Hydrogen Safety; Chemical Industry Press Co., Ltd.: Beijing, China, 2020. (In Chinese) [Google Scholar]
- Wang, H.R.; Wang, B.; Sun, J.C.; Pan, Q.W.; Luo, G.Q.; Tao, X.; He, Y.X.; Pfotenhauer, J.; Jin, T.; Gan, Z.H. Experimental and computational fluid dynamic investigation on thermal behaviors of liquid hydrogen during the no-vented storage process: A literature review. Int. J. Hydrogen Energy 2024, 57, 822–843. [Google Scholar] [CrossRef]
- The Compressed Gas Association. Cryogenic Hydrogen Storage, 2nd ed.; CGA H-3-2013; The Compressed Gas Association, Inc.: McLean, VA, USA.
- T/CATSI 05006-2021; China Association for Technical Supervision Information. Special Technical Requirements for Static Vacuum-Insulated Liquid Hydrogen Pressure Vessels. Standards Press of China: Beijing, China, 2021. (In Chinese)
- T/CATSI 05007-2023; China Association for Technical Supervision Information. Special Technical Requirements for Transportable Vacuum-Insulated Liquid Hydrogen Pressure Vessels. Standards Press of China: Beijing, China, 2023. (In Chinese)
- Ishimoto, Y.; Voldsund, M.; Neksa, P.; Roussanaly, S.; Berstad, D.; Gardarsdottir, S.O. Large-scale production and transport of hydrogen from Norway to Europe and Japan: Value chain analysis and comparison of liquid hydrogen and ammonia as energy carriers. Int. J. Hydrogen Energy 2020, 45, 32865–32883. [Google Scholar] [CrossRef]
- Vanessa, T.; Sebastian, L.; Detlef, S. Hydrogen Science and Engineering: Material, Processes, Systems and Technology; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2016; pp. 659–690. [Google Scholar]
- Kawasaki Completes Basic Design for World’s Largest Class (11200-Cubic-Meter) Spherical Liquefied Hydrogen Storage Tank. Kawasaki. 2020. Available online: https://global.kawasaki.com/en/corp/newsroom/news/detail/?f=20201224_8018 (accessed on 15 June 2024).
- Al Ghafri, S.Z.S.; Munro, S.; Cardella, U.; Funke, T.; Notardonato, W.; Trusler, J.P.M.; Leachman, J.; Span, R.; Kamiya, S.; Pearce, G.; et al. Hydrogen liquefication: A review of the fundamental physics, engineering practice and future opportunities. Energy Environ. Sci. 2022, 15, 2690–2731. [Google Scholar] [CrossRef]
- Air Products. Safegram 9: Liquid Hydrogen; Air Products and Chemicals, Inc.: Lehigh Valley, PA, USA, 2014; Available online: https://www.airproducts.com.cn/en/company/sustainability/safetygrams (accessed on 18 June 2024).
- Chen, H.; Gao, X.; Xing, K.W.; Li, J.J. Influence of enthalpy on static evaporation rate of liquid hydrogen tank. Cryogenics 2015, 208, 62–66. (In Chinese) [Google Scholar]
- Matveev, K.I.; Leachman, J.W. The effect of liquid hydrogen tank size on self-pressurization and constant-pressure venting. Hydrogen 2023, 4, 444–455. [Google Scholar] [CrossRef]
- Matveev, K.I.; Leachman, J.W. Modeling of liquid hydrogen tank cooled with para-orthohydrogen conversion. Hydrogen 2023, 4, 146–153. [Google Scholar] [CrossRef]
- Wang, H.R.; Wang, B.; Xu, T.C.; Shen, X.; He, Y.X.; Zhou, W.M.; Pfotenhauer, J.; Jin, T.; Gan, Z.H. Thermal models for self-pressurization prediction of liquid hydrogen tanks: Formulation, validation, assessment, and prospects. Fuel 2024, 365, 131247. [Google Scholar] [CrossRef]
- Zuo, Z.Q.; Zhu, W.X.; Huang, Y.H.; Wang, L.; Tong, L.G. A review of cryogenic quasi-steady liquid-vapor phase change: Theories, models, and state-of-the-art applications. Int. J. Heat Mass Transf. 2023, 205, 123916. [Google Scholar] [CrossRef]
- Aasadnia, M.; Mehrpooya, M. Large-scale liquid hydrogen production methods and approaches: A review. Appl. Energy 2018, 212, 57–83. [Google Scholar] [CrossRef]
- Clark, J.A. A review of pressurization, stratification, and interfacial phenomena. Adv. Cryog. Eng. 1964, 10, 259–283. [Google Scholar]
- Aydelott, J.C. Effect of Size on Normal-Gravity Self-Pressurization of Spherical Liquid Hydrogen Tankage; NASA TN D-5196; NASA: Washington, DC, USA, 1969; pp. 1–44. [Google Scholar]
- Van, D.; Lin, C.S. Self-Pressurization of a Flight Weight Liquid Hydrogen Tank: Effects of Fill Level at Low Wall Heat Flux; NASA TM-105411; NASA: Washington, DC, USA, 1992; pp. 2–7. [Google Scholar]
- Zhang, C.G.; Li, C.J.; Jia, W.L.; Pang, Y. Thermodynamic study on thermal insulation schemes for liquid helium storage tank. Appl. Therm. Eng. 2021, 195, 117185. [Google Scholar] [CrossRef]
- Seo, M.; Jeong, S. Analysis of self-pressurization phenomenon of cryogenic fluid storage tank with thermal diffusion model. Cryogenics 2010, 50, 549–555. [Google Scholar] [CrossRef]
- Zuo, Z.Q.; Jiang, W.B.; Qin, X.J.; Huang, Y.H. A numerical model for liquid-vapor transition in self-pressurized cryogenic containers. Appl. Therm. Eng. 2021, 193, 117005. [Google Scholar] [CrossRef]
- Yang, Y.L.; Jiang, W.B.; Huang, Y.H. Experiment on transient thermodynamic behavior of a cryogenic storage tank protected by a composite insulation structure. Energy 2023, 270, 126929. [Google Scholar] [CrossRef]
- Liebenberg, D.H.; Edeskuty, F.J. Pressurization analysis of a large-scale liquid hydrogen dewar. Int. Adv. Cryog. Eng. 1965, 10, 284–289. [Google Scholar]
- Aydelott, J.C. Normal Gravity Self-Pressurization of 9-Inch-Diameter Spherical Liquid Hydrogen Tankage; NASA TN D-4171; NASA: Washington, DC, USA, 1967; pp. 1–10. [Google Scholar]
- Hasan, M.; Lin, C.S. Self-pressurization of a Flight Weight Liquid Hydrogen Storage Tank Subjected to Low Heat Flux; NASA TM-103804; NASA: Washington, DC, USA, 1991; pp. 2–5. [Google Scholar]
- Hastings, L.J.; Flachbart, R.H.; Martin, J.J.; Hedayat, A.; Fazah, M.; Lak, T.; Nguyen, H.; Bailey, J.W. Spray Bar Zero-Gravity Vent System for On-Orbit Liquid Hydrogen Storage; NASA TM-12926; NASA: Washington, DC, USA, 2003; pp. 43–133. [Google Scholar]
- Martin, J.J.; Hastings, L. Large-Scale Liquid Hydrogen Testing of a Variable Density Multilayer Insulation with a Foam Substrate; NASA TM-211089; NASA: Washington, DC, USA, 2001; pp. 25–35. [Google Scholar]
- Maekawa, K.; Takeda, M.; Hamaura, T.; Suzuki, K.; Miyake, Y.; Matsuno, Y.; Fujikawa, S.; Kumakura, H. First experiment on liquid hydrogen transportation by ship inside Osaka bay. IOP Conf. Ser. Mater. Sci. Eng. 2017, 278, 012066. [Google Scholar] [CrossRef]
- Maekawa, K.; Takeda, M.; Miyake, Y.; Kumakura, H. Sloshing measurements inside a liquid hydrogen tank with external-heating-type MgB2 level sensors during marine transportation by the training ship Fukae-Maru. Sensors 2018, 18, 3694. [Google Scholar] [CrossRef]
- Johnson, W.L.; Balasubramaniam, R.; Hibbs, R.G.; Zimmerli, G.A.; Asipaukas, M.; Bittinger, S.A.; Dardano, C.; Koci, F.D. Demonstration of Multilayer Insulation, Vapor Cooling of Structure, and Mass Gauging for Large-Scale Upper Stages: Structural Heat Intercept, Insulation, and Vibration Evaluation Rig (SHIIVER) Final Report; NASA TP-5008233; NASA: Washington, DC, USA, 2021. [Google Scholar]
- Rotenberg, Y. Numerical simulation of self-pressurization in a small cryogenic tank. Adv. Cryog. Eng. 1989, 29, 962–971. [Google Scholar]
- Schmidt, A.F.; Purcell, J.R.; Wilson, A.; Smith, R.V. An experimental study concerning the pressurization and stratification of liquid hydrogen. Adv. Cryog. Eng. 1960, 5, 487–497. [Google Scholar]
- Wang, H.R.; Wang, B.; Pan, Q.W.; Wu, Y.Z.; Jiang, L.; Wang, Z.H.; Gan, Z.H. Modeling and thermodynamic analysis of thermal performance in self-pressurized liquid hydrogen tanks. Int. J. Hydrogen Energy 2022, 47, 30530–30545. [Google Scholar] [CrossRef]
- Bailey, T.E.; Fearn, R.F. Analytical and experimental determination of liquid hydrogen temperature stratification. Adv. Cryog. Eng. 1963, 9, 254–264. [Google Scholar]
- Zilliac, G.; Karabeyoglu, M. Modeling of propellant tank pressurization. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Y.Z. Thermal physical performance in liquid hydrogen tank under constant wall temperature. Renew. Energy 2019, 130, 601–612. [Google Scholar] [CrossRef]
- Arnett, R.W.; Votb, R.O. A Computer Program for the Calculation of Thermal Stratification and Self-Pressurization in a Liquid Hydrogen Tank; NASA: Washington, DC, USA, 1972. [Google Scholar]
- Sakowski, B.; Hauser, D.M.; Kassemi, M. SINDA/FLUINT and thermal desktop multi-node settled and unsettled propellant tank modeling of zero boil off test. In Proceedings of the 55th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Indianapolis, IN, USA, 19–22 August 2019. [Google Scholar] [CrossRef]
- Lan, E.; Shi, S.B.; Ji, W.; Ishii, M. Modeling and simulation of cryogenic propellant tank pressurization in normal gravity. Appl. Therm. Eng. 2024, 236, 121628. [Google Scholar] [CrossRef]
- Kassemi, M.; Kartuzova, O. Effect of interfacial turbulence and accommodation coefficient on CFD predictions of pressurization and pressure control in cryogenic storage tank. Cryogenics 2016, 74, 138–153. [Google Scholar] [CrossRef]
- Kartuzova, O.; Kassemi, M.; Moder, J.P. Self-pressurization and spray cooling simulations of the Multipurpose Hydrogen Test Bed (MHTB) ground-based experiment. In Proceedings of the 50th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Cleveland, OH, USA, 28–30 July 2014. [Google Scholar] [CrossRef]
- Mattick, S.J.; Lee, C.P.; Hosangadi, A.; Ahuja, V. Progress in modeling pressurization in propellant tanks. In Proceedings of the 46th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Nashville, TN, USA, 25–28 July 2010. [Google Scholar] [CrossRef]
- Schrage, R.W. A Theoretical Study of Interphase Mass Transfer; Columbia University Press: New York, NY, USA, 1953. [Google Scholar]
- Tanasawa, I. Advances in condensation heat transfer. Adv. Heat Transf. 1991, 21, 55–139. [Google Scholar] [CrossRef]
- Lee, W.H. Pressure iteration scheme for two-phase flow modeling. In Multiphase Transport: Fundamentals, Reactor Safety, Applications; World Scientific: Singapore, 1980. [Google Scholar]
- Zuo, Z.Q.; Wu, J.Y.; Huang, Y.H. Validity evaluation of popular liquid-vapor phase change models for cryogenic self-pressurization process. Int. J. Heat Mass Transf. 2021, 181, 121879. [Google Scholar] [CrossRef]
- Bourgarel, M.H.; Segel, M.P.; Huffenus, J.P. Study of stratification similitude laws in liquid hydrogen. Adv. Cryog. Eng. 1967, 13, 103–111. [Google Scholar]
- Holman, J.P. Heat Transfer, 10th ed.; McGraw Hill Higher Education: New York, NY, USA, 2012. [Google Scholar]
- Kartuzova, O.; Kassemi, M. Modeling interfacial turbulent heat transfer during ventless pressurization of a large scale cryogenic storage tank in microgravity. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar] [CrossRef]
- Wan, C.C.; Zhu, S.L.; Shi, C.Y.; Bao, S.R.; Zhi, X.Q.; Qiu, L.M.; Wang, K. Numerical simulation on pressure evolution process of liquid hydrogen storage tank with active cryogenic cooling. Int. J. Refrig. 2023, 150, 47–58. [Google Scholar] [CrossRef]
- Yin, L.; Yang, H.N.; Ju, Y.L. Review on the key technologies and future development of insulation structure for liquid hydrogen storage tanks. Int. J. Hydrogen Energy 2024, 57, 1302–1315. [Google Scholar] [CrossRef]
- Ratnakar, R.R.; Sun, Z.; Balakotaiah, V. Effective thermal conductivity of insulation materials for cryogenic LH2 storage tanks: A review. Int. J. Hydrogen Energy 2023, 48, 7770–7793. [Google Scholar] [CrossRef]
- Sakatain, N.; Ogawa, K.; Iijima, Y.I.; Arakawa, M.; Honda, R.; Tanaka, S. Thermal conductivity model for powered materials under vacuum based on experimental studies. AIP Adv. 2017, 7, 015310. [Google Scholar] [CrossRef]
- Gao, Y.F.; Wang, B.; Wang, H.R.; Sun, X.; Li, R.Z.; Xu, X.; Wang, Z.H.; Gan, Z.H. Progress of vacuum multilayer insulation materials at liquid hydrogen temperatures. Cryogenics 2021, 6, 12–21. (In Chinese) [Google Scholar]
- Fesmire, J.E.; Johnson, W.L. Cylindrical cryogenic calorimeter testing of six types of multilayer insulation systems. Cryogenics 2018, 89, 58–75. [Google Scholar] [CrossRef]
- Ohmori, T.; Kodama, K.; Tomaru, T.; Kimura, N.; Suzuki, T. Test apparatus utilizing a Gifford-Mcmahon cryocooler to measure the thermal performance of multilayer insulation. Phys. Procedia 2015, 67, 999–1004. [Google Scholar] [CrossRef]
- Hurd, J.A. Multilayer Insulation Testing at Variable Boundary Temperatures; Florida State University: Tallahassee, FL, USA, 2013. [Google Scholar]
- Funke, T.; Haberstroh, C. Performance measurements of multilayer insulation at variable cold temperature. Adv. Cryog. Eng. 2012, 1434, 1279–1284. [Google Scholar] [CrossRef]
- Funke, T.; Haberstroh, C. A calorimeter for measurements of multilayer insulation at variable cold temperature. Phys. Procedia 2015, 67, 1062–1067. [Google Scholar] [CrossRef]
- Funke, T.; Haberstroh, C. New measurements of multilayer insulation at variable cold temperature and elevated residual gas pressure. IOP Conf. Ser. Mater. Sci. Eng. 2015, 101, 012058. [Google Scholar] [CrossRef]
- Wang, H.R.; Wang, B.; Li, R.Z.; Shen, X.; Wu, Y.Z.; Pan, Q.W.; He, Y.X.; Zhou, W.M.; Gan, Z.H. Theoretical investigation on heat leakage distribution between vapor and liquid in liquid hydrogen tanks. Int. J. Hydrogen Energy 2023, 48, 17187–17201. [Google Scholar] [CrossRef]
- Zheng, J.P.; Chen, L.B.; Wang, J.; Xi, X.T.; Zhu, H.L.; Zhou, Y.; Wang, J.J. Thermodynamic analysis and comparison of four insulation schemes for liquid hydrogen storage tank. Energy Convers. Manag. 2019, 186, 526–534. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wang, B.; Zhou, S.H.; Wu, J.Y.; Lei, G.; Li, P.; Sun, P.J. Modeling and experimental study on combination of foam and variable density multilayer insulation for cryogen storage. Energy 2017, 123, 487–498. [Google Scholar] [CrossRef]
- Wang, P.; Ji, L.; Yuan, J.; An, Z.G.; Yan, K.Q.; Zhang, J.J. Modeling and optimization of composite thermal insulation system with HGMs and VDMLI for liquid hydrogen on orbit storage. Int. J. Hydrogen Energy 2020, 45, 7088–7097. [Google Scholar] [CrossRef]
- Meng, C.J.; Qin, X.J.; Jiang, W.B.; Pu, L.M.; Liu, W.; Huang, Y.H. Cooling effect analysis on para-ortho hydrogen conversion coupled in vapor-cooled shield. Int. J. Hydrogen Energy 2023, 48, 15600–15611. [Google Scholar] [CrossRef]
- Xu, Z.L.; Tan, H.B.; Wu, H. Performance comparison of multilayer insulation coupled with vapor cooled shield and different para-ortho hydrogen conversion types. Appl. Therm. Eng. 2023, 234, 121250. [Google Scholar] [CrossRef]
- Shi, C.Y.; Zhu, S.L.; Wan, C.C.; Bao, S.R.; Zhi, X.Q.; Qiu, L.M.; Wang, K. Performance analysis of vapor-cooled shield insulation integrated with para-ortho hydrogen conversion for liquid hydrogen tanks. Int. J. Hydrogen Energy 2023, 48, 3078–3090. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Gao, Y.; Wang, B.; Pan, Q.; Gan, Z. Perspective for the Safe and High-Efficiency Storage of Liquid Hydrogen: Thermal Behaviors and Insulation. Hydrogen 2024, 5, 559-573. https://doi.org/10.3390/hydrogen5030031
Wang H, Gao Y, Wang B, Pan Q, Gan Z. Perspective for the Safe and High-Efficiency Storage of Liquid Hydrogen: Thermal Behaviors and Insulation. Hydrogen. 2024; 5(3):559-573. https://doi.org/10.3390/hydrogen5030031
Chicago/Turabian StyleWang, Haoren, Yunfei Gao, Bo Wang, Quanwen Pan, and Zhihua Gan. 2024. "Perspective for the Safe and High-Efficiency Storage of Liquid Hydrogen: Thermal Behaviors and Insulation" Hydrogen 5, no. 3: 559-573. https://doi.org/10.3390/hydrogen5030031





