Advancing Hydrogen Gas Utilization in Industrial Boilers: Impacts on Critical Boiler Components, Mitigation Measures, and Future Perspectives
Abstract
:1. Introduction
- To provide a fundamental understanding of the HE and HTHA phenomena and their potential occurrences in hydrogen-fuel boilers.
- To highlight mitigation measures, hydrogen-compatible materials, and barrier coatings that are feasible under in-service hydrogen boiler operations.
- To discuss advanced computational and data-driven approaches for the simulation and prediction of hydrogen-assisted damages in critical boiler components under varying operational conditions.
- To provide future research directions to guide the advancement of H2 use in industrial boiler operations.
2. Adverse Impacts of the Industrial Use of H2 in Boiler Operation
2.1. Hydrogen Damage and Simultaneous Embrittlement
2.1.1. Theoretical Modeling of the HE Phenomenon
HPT Model
HESIV Model
HEDE Model
HELP Model
AIDE Model
Potential Gaps in Theoretical Modeling of HE
2.2. HTHA under Higher-Temperature H2 Conditions
2.2.1. Factors Influencing HTHA Damages
Temperature
Pressure
Exposure Time
Stress
Material Composition
3. General Mitigation Measures for Hydrogen-Induced Damages
3.1. Mitigation Measures to Improve the HE Resistance of Materials
3.2. Mitigation Measures to Improve the HTHA Resistance of Materials
3.3. The Importance of Controlling HE and HTHA under Different Operational Contexts
4. Material Selection of Hydrogen-Compatible Materials
4.1. Technical Databases, Standards, and Charts for Selecting Hydrogen-Compatible Materials
4.2. General Material Selection of Hydrogen-Compatible Materials
The Emergence of High-Entropy Alloys
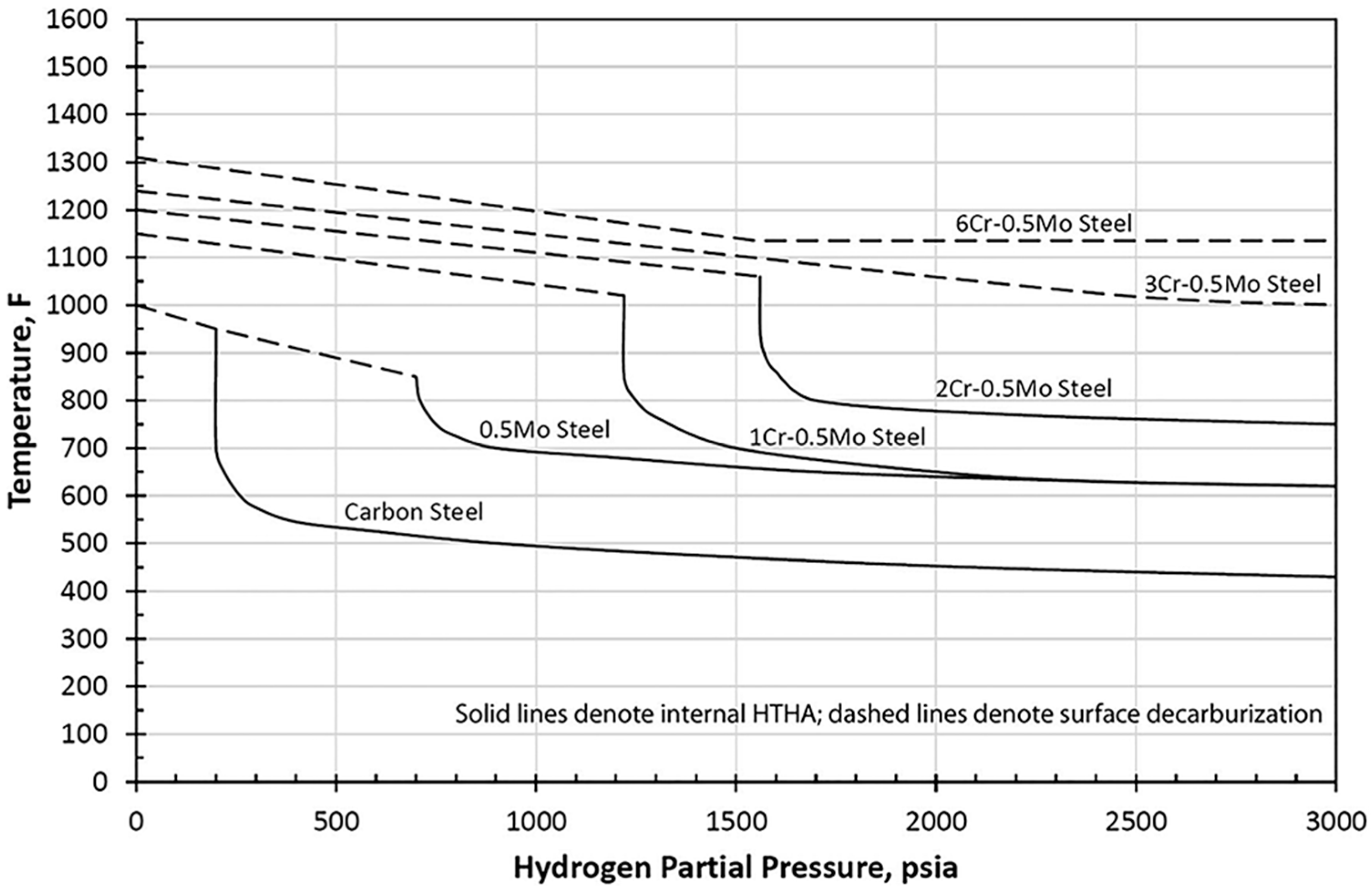
4.3. Nelson Curves for Material Selection
Enhancement of Nelson Curves to Mitigate HTHA Damages
4.4. Defining the HEE Index for Evaluating Material Susceptibility to Hydrogen-Induced Damages
4.5. Defining a Hydrogen Safety Factor in Material Selection Studies
4.6. Hydrogen Permeation Barriers
4.7. Implementation of General Mitigation Measures in an Industrial Context
5. Experimental and Computational Modeling of Hydrogen-Induced Damages
5.1. Experimental Investigation of Hydrogen-Induced Damages
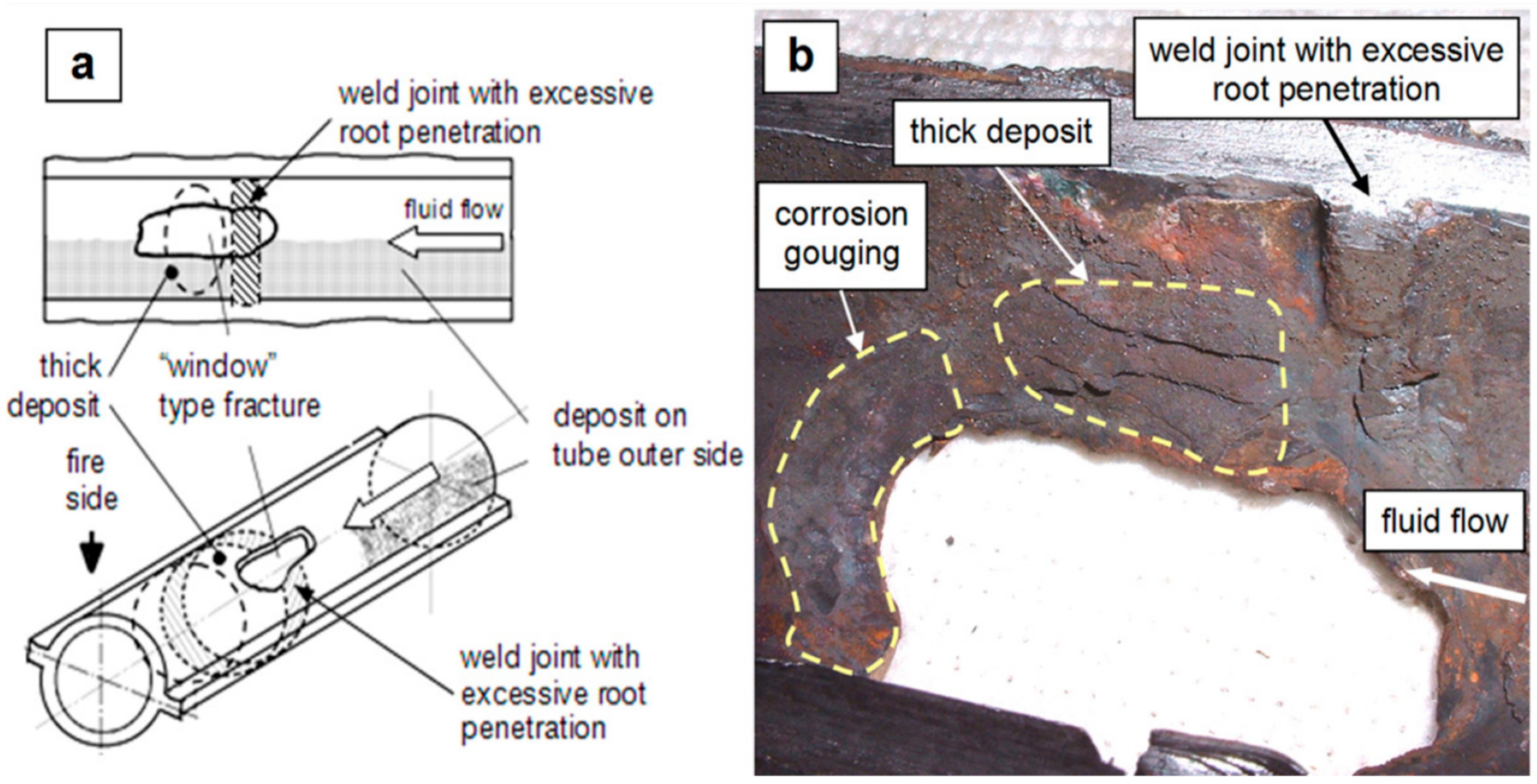
5.1.1. Experimental Investigation of Hydrogen Damage in Plain Carbon Steels
5.1.2. Challenges Associated with Experimental Modeling of Hydrogen-Induced Damages
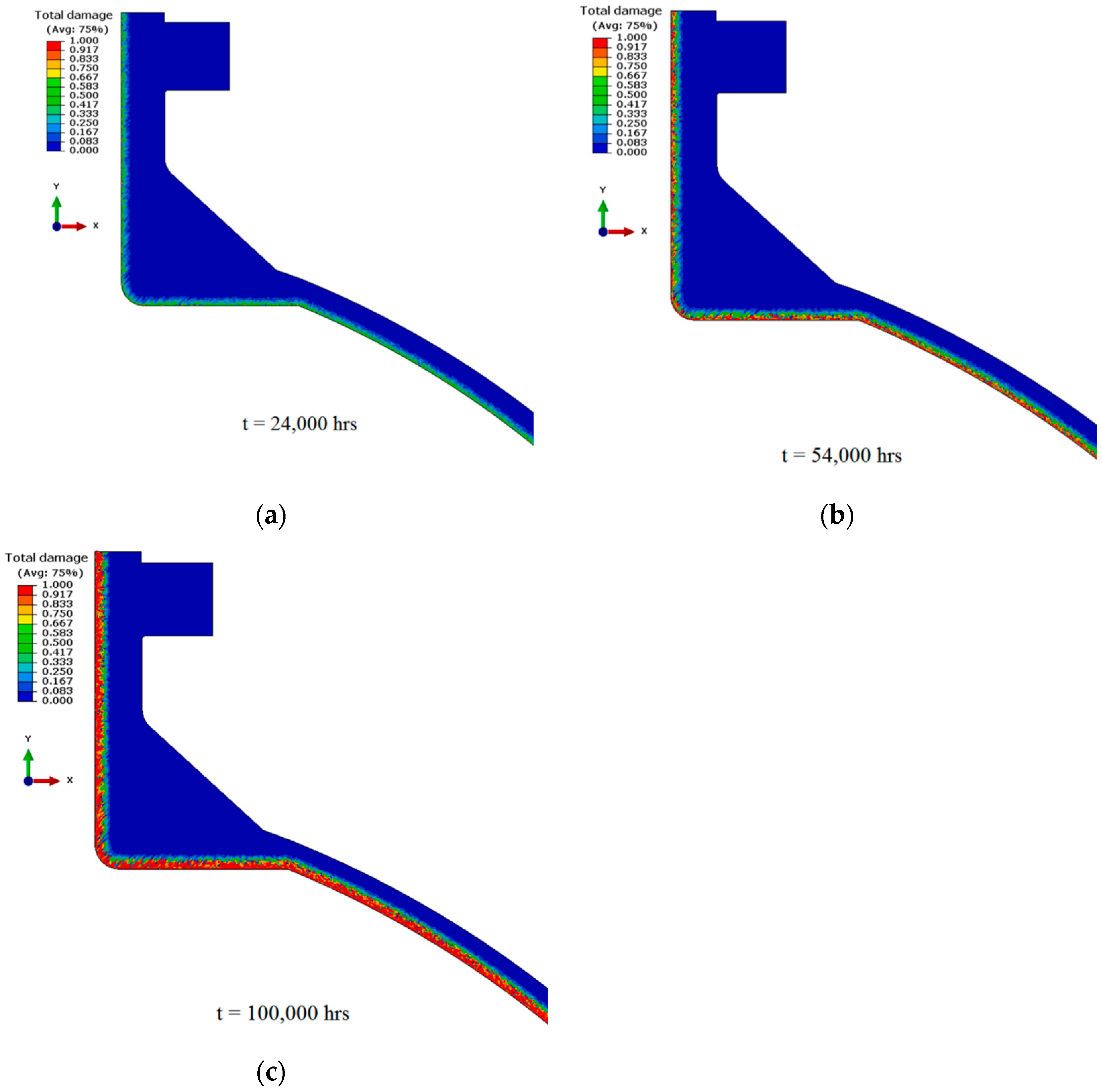
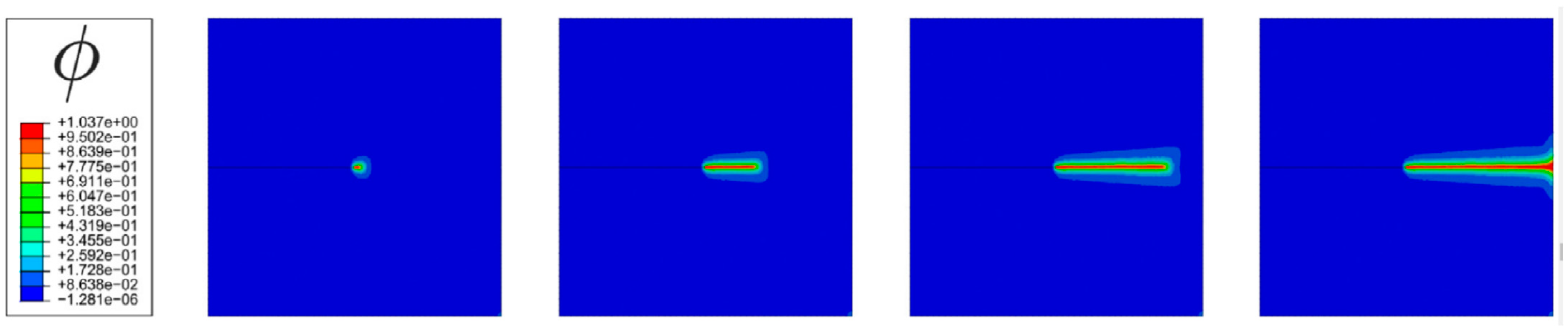
5.2. Emerging Computational Models for Simulating Hydrogen-Induced Damages
5.3. Data-Driven Approaches for the Prediction of Material Susceptibility and Compatibility
Limitations of Data-Driven Approaches
6. Conclusions and Future Studies
6.1. Conclusions
- Over the years, the HE and HTHA phenomena have been complex damage mechanisms to understand and daunting research hurdles for material designers and engineers. Nonetheless, significant experimental, theoretical, and computational advancements have been made to substantially bridge these gaps. These advancements have yielded satisfactory accuracy in characterizing hydrogen diffusion into materials and assessing the subsequent effects of hydrogen-assisted damage. Nevertheless, significant challenges remain as the acceptance of the most accurate theoretical model for driving the HE mechanism in materials continues to be a major point of debate among experts. Establishing a standard theoretical model for defining HE manifestations could enhance the precision of numerical modeling and simulations of hydrogen-assisted fractures, thereby supporting the development of novel hydrogen-specific materials and components.
- To reduce the occurrence of HE and HTHA in boiler components, general mitigation measures that could be explored include proper thermomechanical treatments, the use of HPBs, the addition of inhibitors to reduce H2 purity, proper weld procedures and post-heat treatments, the selection of proper operating temperatures and pressures, the introduction and reduction of alloying elements, and the proper selection of materials. In an industrial context, visual and advanced inspection procedures using sophisticated inspection techniques such as LPT, PAUT, SWUT, and MPT can also be utilized for rapid failure detection to inform decisions to prevent potential equipment failure. However, when exploring mitigation measures, it is important to consider the specific operational contexts of the critical components and the potential hydrogen-induced damage mechanisms (HE or HTHA) that may affect them.
- Numerous studies and technical references have identified several prominent metals and metallic alloys, such as aluminum, austenitic stainless steel alloys, and superalloys, as promising candidates for H2 boiler applications. In particular, superalloys such as single-crystal PWA 1480E, Inconel 625, and Hastelloy X exhibit promising HE-resistant capabilities under high-pressure, high-temperature H2 conditions. However, the material selection for both traditional and emerging alloys, such as BCC HEAs, must be validated through rigorous fracture mechanics analysis to establish hydrogen safety factors and confirm their suitability as hydrogen-compatible materials for elevated hydrogen boiler operations.
- Metallurgically bonded austenitic stainless steel liners and dielectrics, such as oxides, carbides, and nitrides, have been considered promising candidates for HPB coating applications. Notably, the TiAlN HPBs exhibit the most promising HPB coating characteristics, with permeabilities on the order of 10−18 mols−1m−1()−1. Therefore, TiAlN coatings can be further explored for use in coating boiler components. However, extensive investigation of these coatings beyond laboratory-scale conditions would be valuable for validating their effectiveness in industrial hydrogen boiler operations.
- Emerging numerical and computational models, such as MD and phase-field models, have proven to be efficient tools for modeling and predicting HE, HTHA, and complex crack morphologies in materials subjected to H2 conditions. In particular, phase-field modeling of hydrogen-assisted fracture or hydrogen-assisted fatigue crack growth can allow sophisticated virtual testing of hydrogen-specific critical boiler components. In the future, computational modeling of hydrogen-assisted damage in hydrogen boiler materials can assist in: (1) confident validation of material selection studies; (2) informing major material development decisions of hydrogen-specific engineering components; and (3) mapping out safe regimes of operations for materials working under elevated H2 boiler conditions to ensure good performance.
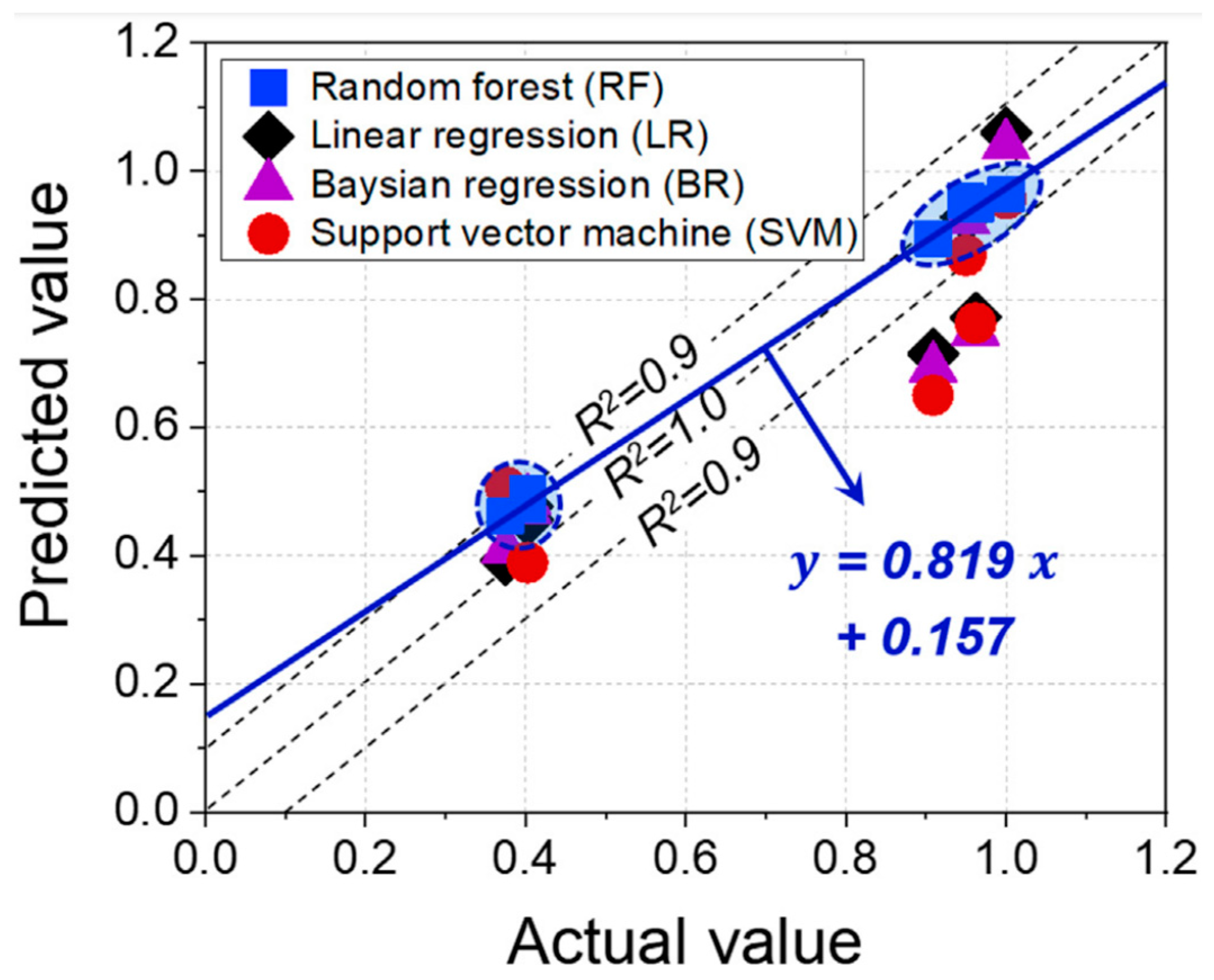
- Data-driven machine learning (ML) models, such as DTC, have shown significant potential for the rapid prediction of material susceptibility to hydrogen-induced damage and their compatibility with H2 under specific loading conditions. These models were validated against experimental and theoretical studies and demonstrated remarkable agreement with the observed data. However, limitations such as small datasets and a lack of consistent, standardized databases can significantly impact the prediction accuracy and validation of these models in a practical context. In the future, the development of more sophisticated ML algorithms and the creation of exhaustive material testing databases using advanced computational modeling can offer valuable insights into the design of novel, advanced hydrogen-compatible materials and infrastructure for H2 boiler operation.
6.2. Future Research Roadmap
- Implementation of hydrogen-specific standards and codes for rigorous fracture mechanics investigations into the feasibility of traditional alloys, superalloys, and novel materials, such as BCC HEAs, for the design of hydrogen-specific boiler components.
- Extensive investigations into the viability of HPBs and liners on critical boiler components under actual H2 conditions beyond laboratory-scale testing.
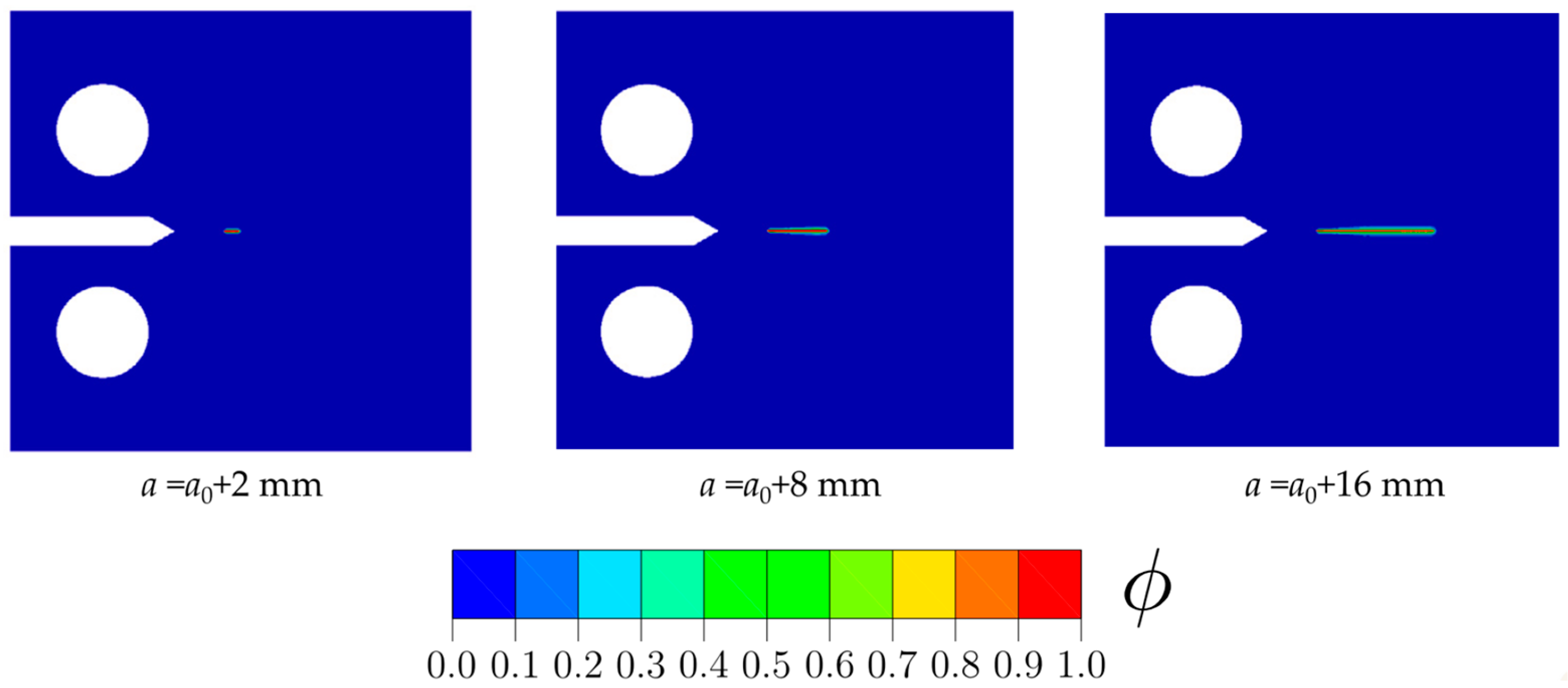
- Employing phase-field modeling to conduct rigorous virtual testing of hydrogen-specific components to assess their suitability for specific H2 conditions and industrial-scale applications. Additionally, these models should be employed to design safe operational regimes for conventional components and old boiler infrastructures.
- Development of robust ML algorithms and standardized, extensive mechanical testing databases to ensure remarkable prediction accuracy of a material’s susceptibility to hydrogen-induced damages and its compatibility with H2. As previously mentioned, phase-field modeling of hydrogen-assisted fracture and fatigue crack growth enables efficient and cost-effective numerical testing, extending beyond laboratory-scale conditions. Thus, phase-field modeling can be instrumental in developing a comprehensive, standardized ML database. This approach can guide industrial decision-making in selecting materials and designing components specifically for hydrogen-fuel boilers.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature, Subscripts, Superscripts, Acronyms, and Abbreviations
| CO2 | Carbon dioxide |
| H2 | Hydrogen gas |
| HENG | Hydrogen-enriched natural gas |
| CH4 | Methane |
| kg | Kilogram |
| MJ | Megajoules |
| kWh | Kilowatt-hours |
| LPG | Liquified petroleum gas |
| RFG | Reformulated gasoline |
| HE | Hydrogen embrittlement |
| HTHA | High-temperature hydrogen attack |
| AHSS | Advanced high-strength steel |
| HIC | Hydrogen-induced cracking |
| HPT | Hydrogen pressure theory |
| HEDE | Hydrogen-enhanced decohesion |
| HELP | Hydrogen-enhanced localized plasticity |
| HESIV | Hydrogen-enhanced strain-induced vacancies |
| AIDE | Absorption-induced dislocation emission theory |
| HRE | Hydrogen reaction embrittlement |
| N2O | Nitrous oxide |
| O2 | Oxygen |
| CO | Carbon monoxide |
| PWHT’ed | Post-weld heat treated |
| PWHT | Post-weld heat treatment |
| API RP | American Petroleum Institute Recommended Practice |
| HAT | Hydrogen attack tendency |
| HAZ | Heat-affected zone |
| NDE | Non-destructive examination |
| NASA | National Aeronautics and Space Administration |
| ASME | American Society of Mechanical Engineers |
| BPVC | Boiler and pressure vessel codes |
| HEAs | High-entropy alloys |
| BCC | Body-centered cubic |
| FCC | Face-centered cubic |
| HEE | Hydrogen environment embrittlement |
| NTS | Notched tensile strength |
| RA | Reduction in area |
| EL | Elongation |
| RNTS | Notched tensile strength ratio |
| RRA | Reduction in area ratio |
| ANSI | American National Standard Institute |
| CHMCI | Compressed Hydrogen Materials Compatibility |
| SBCs | Surface barrier coatings |
| TBCs | Thermal barrier coatings |
| HPBs | Hydrogen permeation barriers |
| Al2O3 | Aluminum oxide |
| Cr2O3 | Chromium oxide |
| Er2O3 | Erbium oxide |
| PRF | Permeation reduction factor |
| BN | Boron nitride |
| TiN | Titanium nitride |
| SiN | Silicon nitride |
| TiC | Titanium carbide |
| SiC | Silicon carbide |
| Ds | Substrate diameter |
| Df | Film diameter |
| Ps | Substrate permeability value |
| Pf | Film permeability value |
| HIAD | Hydrogen Incidents and Accidents Database |
| LPT | Liquid penetrant testing |
| PAUT | Phased array ultrasonic testing |
| SWUT | Shear wave ultrasonic testing |
| MPT | Magnetic particle testing |
| TDS | Thermal desorption spectroscopy |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive X-ray spectroscopy |
| AAS | Atomic absorption spectroscopy |
| Z–R | Zimmermann–Reinhardt |
| AHSS | Advanced high-strength steel |
| TWIP | Twinning-induced plasticity |
| TRIP | Transformation-induced plasticity |
| DFT | Density functional theory |
| MD | Molecular dynamic |
| FEA | Finite element analysis |
| UEL | User element |
| UMAT | User material |
| Phase-field parameter | |
| C0 | Initial hydrogen concentration |
| C | Hydrogen concentration |
| a | Crack size |
| a0 | Initial crack size |
| K | Stress intensity factor |
| N | Fatigue cycle |
| R | Loading ratio |
| f | Loading frequency |
| Fe3C | Iron carbide |
| APT | Atomic probe tomography |
| ML | Machine learning |
| SVM | Support vector machine |
| PAG | Prior austenite grain |
| XGBoost | Extreme gradient boosting |
| ANN | Artificial neural network |
| GB | Gradient boosting |
| DTC | Decision tree classifier |
| RF | Random Forest |
| LR | Linear regression |
| BR | Bayesian regression |
| PCC | Pearson’s correlation coefficient |
| MIC | Maximum information coefficient |
| R2 | Coefficient of determination |
| AdaBoost | Adaptive Boosting |
| CatBoost | Categorical Boosting |
| S | Solubility |
| p(H2) | Hydrogen partial pressure |
| AI | Artificial intelligence |
References
- Jayamaha, D.L. Energy-Efficient Building Systems; Mcgraw-Hill Publishing Company: New York, NY, USA, 2007. [Google Scholar]
- Total Energy Annual Data—U.S. Energy Information Administration (EIA). Available online: https://www.eia.gov/totalenergy/data/annual/index.php (accessed on 30 December 2023).
- Barma, M.C.; Saidur, R.; Rahman, S.M.A.; Allouhi, A.; Akash, B.A.; Sait, S.M. A review on boilers energy use, energy savings, and emissions reductions. Renew. Sustain. Energy Rev. 2017, 79, 970–983. [Google Scholar] [CrossRef]
- Jorgenson, D.W.; Wilcoxen, P.J. Reducing U.S. carbon dioxide emissions: An assessment of different instruments. J. Policy Model. 1993, 15, 491–520. [Google Scholar] [CrossRef]
- Jorgenson, D.W.; Wilcoxen, P.J. Reducing US carbon emissions: An econometric general equilibrium assessment. Resour. Energy Econ. 1993, 15, 7–25. [Google Scholar] [CrossRef]
- Glaeser, E.L.; Kahn, M.E. The greenness of cities: Carbon dioxide emissions and urban development. J. Urban. Econ. 2010, 67, 404–418. [Google Scholar] [CrossRef]
- Qazi, A.; Hussain, F.; Rahim, N.A.; Hardaker, G.; Alghazzawi, D.; Shaban, K.; Haruna, K. Towards Sustainable Energy: A Systematic Review of Renewable Energy Sources, Technologies, and Public Opinions. IEEE Access 2019, 7, 63837–63851. [Google Scholar] [CrossRef]
- Uney, M.S.; Cetinkaya, N. Comparison of CO2 emissions fossil fuel based energy generation plants and plants with Renewable Energy Source. In Proceedings of the 2014 6th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Bucharest, Romania, 23–25 October 2014; IEEE: Piscataway, NJ, USA, 2014; pp. 29–34. [Google Scholar] [CrossRef]
- Schiro, F.; Stoppato, A.; Benato, A. Modelling and analyzing the impact of hydrogen enriched natural gas on domestic gas boilers in a decarbonization perspective. Carbon Resour. Convers. 2020, 3, 122–129. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Najjar, Y.S.H. Hydrogen safety: The road toward green technology. Int. J. Hydrogen Energy 2013, 38, 10716–10728. [Google Scholar] [CrossRef]
- Hodges, J.P.; Geary, W.; Graham, S.; Hooker, P.; Goff, R. Injecting Hydrogen into the Gas Network—A Literature Search. 1 January 2015. Available online: https://www.h2knowledgecentre.com/content/policypaper1193 (accessed on 19 March 2023).
- Hwang, H.T.; Varma, A. Hydrogen storage for fuel cell vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]
- Becherif, M.; Ramadan, H.S.; Cabaret, K.; Picard, F.; Simoncini, N.; Bethoux, O. Hydrogen Energy Storage: New Techno-Economic Emergence Solution Analysis. Energy Procedia 2015, 74, 371–380. [Google Scholar] [CrossRef]
- Colozza, A.J.; Kohout, L. Hydrogen Storage for Aircraft Applications Overview. E-13540. 1 September 2002. Available online: https://ntrs.nasa.gov/citations/20020085127 (accessed on 20 March 2023).
- de Jongh, P.E.; Adelhelm, P. Nanosizing and Nanoconfinement: New Strategies Towards Meeting Hydrogen Storage Goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Sherif, S.A.; Goswami, D.Y.; (Lee) Stefanakos, E.K.; Steinfeld, A. Handbook of Hydrogen Energy; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Sadhasivam, T.; Kim, H.-T.; Jung, S.; Roh, S.-H.; Park, J.-H.; Jung, H.-Y. Dimensional effects of nanostructured Mg/MgH2 for hydrogen storage applications: A review. Renew. Sustain. Energy Rev. 2017, 72, 523–534. [Google Scholar] [CrossRef]
- In-Depth Analysis in Support on the COM(2018) 773: A Clean Planet for All—A European Strategic Long-Term Vision for a Prosperous, Modern, Competitive and Climate Neutral Economy | Knowledge for Policy. Available online: https://knowledge4policy.ec.europa.eu/publication/depth-analysis-support-com2018-773-clean-planet-all-european-strategic-long-term-vision_en (accessed on 15 February 2024).
- Gersena, S.; Slima, B.; Zeijlmakera, R.; Van Essena, M.; Tichelaarb, R. The Development of a Natural Gas/Hydrogen Boiler System. In Proceedings of the International Gas Union Research Conference; Muscat, Oman, 24–26 February 2020, pp. 1–8. Available online: https://www.researchgate.net/publication/339899796_The_Development_of_a_Natural_GasHydrogen_Boiler_System (accessed on 13 March 2023).
- Djukic, M.B.; Zeravcic, V.S.; Bakic, G.M.; Sedmak, A.; Rajicic, B. Hydrogen damage of steels: A case study and hydrogen embrittlement model. Eng. Fail. Anal. 2015, 58, 485–498. [Google Scholar] [CrossRef]
- Lamioni, R.; Bronzoni, C.; Folli, M.; Tognotti, L.; Galletti, C. Impact of H2-enriched natural gas on pollutant emissions from domestic condensing boilers: Numerical simulations of the combustion chamber. Int. J. Hydrogen Energy 2023, 48, 19686–19699. [Google Scholar] [CrossRef]
- Skalska, K.; Miller, J.S.; Ledakowicz, S. Trends in NOx abatement: A review. Sci. Total Environ. 2010, 408, 3976–3989. [Google Scholar] [CrossRef]
- Gómez-García, M.A.; Pitchon, V.; Kiennemann, A. Pollution by nitrogen oxides: An approach to NOx abatement by using sorbing catalytic materials. Environ. Int. 2005, 31, 445–467. [Google Scholar] [CrossRef]
- Yu, B.; Lee, S.; Lee, C.-E. Study of NOx emission characteristics in CH4/air non-premixed flames with exhaust gas recirculation. Energy 2015, 91, 119–127. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Effect of hydrogen in advanced high strength steel materials. Int. J. Hydrogen Energy 2019, 44, 28007–28030. [Google Scholar] [CrossRef]
- Ichihara, T.; Koike, R.; Watanabe, Y.; Amano, Y.; Machida, M. Hydrogen damage in a power boiler: Correlations between damage distribution and thermal-hydraulic properties. Eng. Fail. Anal. 2023, 146, 107120. [Google Scholar] [CrossRef]
- Yan, F.; Xu, L.; Wang, Y. Application of hydrogen enriched natural gas in spark ignition IC engines: From fundamental fuel properties to engine performances and emissions. Renew. Sustain. Energy Rev. 2018, 82, 1457–1488. [Google Scholar] [CrossRef]
- Regulagadda, P.; Dincer, I.; Naterer, G.F. Exergy analysis of a thermal power plant with measured boiler and turbine losses. Appl. Therm. Eng. 2010, 30, 970–976. [Google Scholar] [CrossRef]
- Sánchez, A.L.; Williams, F.A. Recent advances in understanding of flammability characteristics of hydrogen. Prog. Energy Combust. Sci. 2014, 41, 1–55. [Google Scholar] [CrossRef]
- Campari, A.; Akel, A.J.N.; Ustolin, F.; Alvaro, A.; Ledda, A.; Agnello, P.; Moretto, P.; Patriarca, R.; Paltrinieri, N. Lessons learned from HIAD 2.0: Inspection and maintenance to avoid hydrogen-induced material failures. Comput. Chem. Eng. 2023, 173, 108199. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Vishwakarma, M. Hydrogen embrittlement in different materials: A review. Int. J. Hydrogen Energy 2018, 43, 21603–21616. [Google Scholar] [CrossRef]
- Lee, J.A.; Woods, S. Hydrogen Embrittlement. 2016. Available online: https://ntrs.nasa.gov/api/citations/20160005654/downloads/20160005654.pdf (accessed on 5 March 2023).
- Yang, G.; Gou, Y.; Liu, X.; Zhang, X.; Zhang, T. Failure Analysis of the Corroded Water Wall Tube in a 50MW Thermal Power Plant. High Temp. Mater. Process. 2018, 37, 995–999. [Google Scholar] [CrossRef]
- Poorhaydari, K. A Comprehensive Examination of High-Temperature Hydrogen Attack—A Review of over a Century of Investigations. J. Mater. Eng. Perform. 2021, 30, 7875–7908. [Google Scholar] [CrossRef]
- Subramanian, C. Reducing the Risk of High Temperature Creep Failures in Refinery Service Component and Equipment. J. Press. Vessel. Technol. 2019, 141, 064501. [Google Scholar] [CrossRef]
- Smiyan, O.D.; Grigorenko, G.M.; Vainman, A.B. Effect of hydrogen on corrosion damage of metal of the high-pressure energetic boiler drum. Int. J. Hydrogen Energy 2002, 27, 801–812. [Google Scholar] [CrossRef]
- Marchetti, L.; Herms, E.; Laghoutaris, P.; Chêne, J. Hydrogen embrittlement susceptibility of tempered 9%Cr–1%Mo steel. Int. J. Hydrogen Energy 2011, 36, 15880–15887. [Google Scholar] [CrossRef]
- Venezuela, J.; Zhou, Q.; Liu, Q.; Li, H.; Zhang, M.; Dargusch, M.S.; Atrens, A. The influence of microstructure on the hydrogen embrittlement susceptibility of martensitic advanced high strength steels. Mater. Today Commun. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Khare, A.; Vishwakarma, M.; Parashar, V. A review on failures of industrial components due to hydrogen embrittlement & techniques for damage prevention. Int. J. Appl. Eng. Res. 2017, 12, 1784–1792. [Google Scholar]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of hydrogen safety during storage, transmission, and applications processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Lynch, S.P. 2—Hydrogen embrittlement (HE) phenomena and mechanisms. In Stress Corrosion Cracking; Woodhead Publishing Series in Metals and Surface Engineering; Raja, V.S., Shoji, T., Eds.; Woodhead Publishing: Sawston, UK, 2011; pp. 90–130. [Google Scholar] [CrossRef]
- Thomas, S.; Ott, N.; Schaller, R.F.; Yuwono, J.A.; Volovitch, P.; Sundararajan, G.; Medhekar, N.V.; Ogle, K.; Scully, J.R.; Birbilis, N. The effect of absorbed hydrogen on the dissolution of steel. Heliyon 2016, 2, e00209. [Google Scholar] [CrossRef] [PubMed]
- Popov, B.N.; Lee, J.-W.; Djukic, M.B. Chapter 7—Hydrogen Permeation and Hydrogen-Induced Cracking. In Handbook of Environmental Degradation of Materials, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Amsterdam, The Netherlands, 2018; pp. 133–162. [Google Scholar] [CrossRef]
- Wróbel, I.; Sikora, K. Simulation of a System for Controlling Atmosphere in Furnace Used to Heating of Blanks. In Proceedings of the 14th International Scientific Conference: Computer Aided Engineering; Lecture Notes in Mechanical Engineering. Rusiński, E., Pietrusiak, D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 829–835. [Google Scholar] [CrossRef]
- Stahle, R.W.; Hochmann, J.; McCright, R.D.; Slater, J.E.; Shatynski, S.R. Stress Corrosion Cracking and Hydrogen Embrittlement of Iron Base Alloys. J. Electrochem. Soc. 1979, 126, 215C. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.S.; Jung, S.-P. Corrosion-induced hydrogen evolution, absorption, and cracking behaviors of ultra-high-strength galvanized and galvannealed steel sheets. NPJ Mater. Degrad. 2022, 6, 31. [Google Scholar] [CrossRef]
- Turnbull, A. 4—Hydrogen diffusion and trapping in metals. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead Publishing Series in Metals and Surface Engineering; Gangloff, R.P., Somerday, B.P., Eds.; Woodhead Publishing: Sawston, UK, 2012; Volume 1, pp. 89–128. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Lynch, S. Hydrogen embrittlement phenomena and mechanisms. Corros. Rev. 2012, 30, 105–123. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. Hydrogen embrittlement of industrial components: Prediction, prevention, and models. Corrosion 2016, 72, 943–961. [Google Scholar] [CrossRef]
- Liu, Q.; Atrens, A. A critical review of the influence of hydrogen on the mechanical properties of medium-strength steels. Corros. Rev. 2013, 31, 85–103. [Google Scholar] [CrossRef]
- Garrison, W.M.; Moody, N.R. 12—Hydrogen embrittlement of high strength steels. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead Publishing Series in Metals and Surface Engineering; Gangloff, R.P., Somerday, B.P., Eds.; Woodhead Publishing: Sawston, UK, 2012; Volume 2, pp. 421–492. [Google Scholar] [CrossRef]
- Sun, B.; Wang, D.; Lu, X.; Wan, D.; Ponge, D.; Zhang, X. Current Challenges and Opportunities Toward Understanding Hydrogen Embrittlement Mechanisms in Advanced High-Strength Steels: A Review. Acta Metall. Sin. Engl. Lett. 2021, 34, 741–754. [Google Scholar] [CrossRef]
- Murakami, Y.; Kanezaki, T.; Mine, Y. Hydrogen Effect against Hydrogen Embrittlement. Metall. Mater. Trans. A 2010, 41, 2548–2562. [Google Scholar] [CrossRef]
- Takakuwa, O.; Mano, Y.; Soyama, H. Increase in the local yield stress near surface of austenitic stainless steel due to invasion by hydrogen. Int. J. Hydrogen Energy 2014, 39, 6095–6103. [Google Scholar] [CrossRef]
- Yamabe, J.; Yoshikawa, M.; Matsunaga, H.; Matsuoka, S. Hydrogen trapping and fatigue crack growth property of low-carbon steel in hydrogen-gas environment. Int. J. Fatigue 2017, 102, 202–213. [Google Scholar] [CrossRef]
- Pradhan, A.; Vishwakarma, M.; Dwivedi, S.K. A review: The impact of hydrogen embrittlement on the fatigue strength of high strength steel. Mater. Today Proc. 2020, 26, 3015–3019. [Google Scholar] [CrossRef]
- Chen, T.C.; Chen, S.T.; Kai, W.; Tsay, L.W. The effect of phase transformation in the plastic zone on the hydrogen-assisted fatigue crack growth of 301 stainless steel. Mater. Charact. 2016, 112, 134–141. [Google Scholar] [CrossRef]
- Briottet, L.; Moro, I.; Escot, M.; Furtado, J.; Bortot, P.; Tamponi, G.; Solin, J.; Odemer, G.; Blanc, C.; Andrieu, E. Fatigue crack initiation and growth in a CrMo steel under hydrogen pressure. Int. J. Hydrogen Energy 2015, 40, 17021–17030. [Google Scholar] [CrossRef]
- Djukic, M.B.; Bakic, G.M.; Zeravcic, V.S.; Sedmak, A.; Rajicic, B. The synergistic action and interplay of hydrogen embrittlement mechanisms in steels and iron: Localized plasticity and decohesion. Eng. Fract. Mech. 2019, 216, 106528. [Google Scholar] [CrossRef]
- Al-Anezi, M.A.; Frankel, G.S.; Agrawal, A.K. Susceptibility of Conventional Pressure Vessel Steel to Hydrogen-Induced Cracking and Stress-Oriented Hydrogen-Induced Cracking in Hydrogen Sulfide-Containing Diglycolamine Solutions. Corrosion 1999, 55, 1101–1109. [Google Scholar] [CrossRef]
- Herring, D.H. Hydroqen embrittlement. Wire Form. Technol. Int. 2010, 13, 24–27. [Google Scholar]
- Martin, M.L.; Sofronis, P. Hydrogen-induced cracking and blistering in steels: A review. J. Nat. Gas Sci. Eng. 2022, 101, 104547. [Google Scholar] [CrossRef]
- McMahon, C.J. Hydrogen-induced intergranular fracture of steels. Eng. Fract. Mech. 2001, 68, 773–788. [Google Scholar] [CrossRef]
- Song, J.; Curtin, W.A. Atomic mechanism and prediction of hydrogen embrittlement in iron. Nat. Mater. 2013, 12, 145–151. [Google Scholar] [CrossRef]
- Khare, A.; Dwivedi, S.K.; Vishwakarma, M.; Ahmed, S. Experimental Investigation of Hydrogen Embrittlement during Coating Process and Effect on Mechanical Properties of High Strength Steel used for Fasteners. Mater. Today Proc. 2018, 5, 18707–18715. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, J.; Akiyama, E.; Wang, Y.; Song, X. Review of hydrogen embrittlement in metals: Hydrogen diffusion, hydrogen characterization, hydrogen embrittlement mechanism and prevention. Acta Metall. Sin. Engl. Lett. 2020, 33, 759–773. [Google Scholar] [CrossRef]
- Nagumo, M. Manifestations of Hydrogen Embrittlement. In Fundamentals of Hydrogen Embrittlement; Nagumo, M., Ed.; Springer: Singapore, 2016; pp. 103–135. [Google Scholar] [CrossRef]
- Nagumo, M. Fundamentals of Hydrogen Embrittlement; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Ren, X.; Chu, W.; Su, Y.; Li, J.; Qiao, L. Effects of atomic hydrogen and flaking on mechanical properties of wheel steel. ACTA Metall. Sin.-Chin. Ed. 2006, 42, 153. [Google Scholar]
- Zappfe, C.A.; Sims, C.E. Hydrogen embrittlement, internal stress and defects in steel. Trans. Metall. Soc. AIME 1941, 145, 225–271. [Google Scholar]
- Nagumo, M. Hydrogen related failure of steels–a new aspect. Mater. Sci. Technol. 2004, 20, 940–950. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Fu, Q.; Song, X.; Shen, S.; Li, Q. A comparative study of hydrogen embrittlement of 20SiMn2CrNiMo, PSB1080 and PH13-8Mo high strength steels. Mater. Sci. Eng. A 2018, 724, 518–528. [Google Scholar] [CrossRef]
- Martin, M.L.; Somerday, B.P.; Ritchie, R.O.; Sofronis, P.; Robertson, I.M. Hydrogen-induced intergranular failure in nickel revisited. Acta Mater. 2012, 60, 2739–2745. [Google Scholar] [CrossRef]
- Kappes, M.; Iannuzzi, M.; Carranza, R.M. Hydrogen Embrittlement of Magnesium and Magnesium Alloys: A Review. J. Electrochem. Soc. 2013, 160, C168. [Google Scholar] [CrossRef]
- Sofronis, P.; Birnbaum, H.K. Mechanics of the hydrogendashdislocationdashimpurity interactions—I. Increasing shear modulus. J. Mech. Phys. Solids 1995, 43, 49–90. [Google Scholar] [CrossRef]
- Robertson, I.M.; Sofronis, P.; Nagao, A.; Martin, M.L.; Wang, S.; Gross, D.W.; Nygren, K.E. Hydrogen Embrittlement Understood. Metall. Mater. Trans. A 2015, 46, 2323–2341. [Google Scholar] [CrossRef]
- Lynch, S.P. Mechanisms of hydrogen assisted cracking—A review. In Hydrogen Effects on Material Behaviour and Corrosion Deformation Interactions; The Minerals, Metals & Materials Society: Pittsburgh, PA, USA, 2003; pp. 449–466. [Google Scholar]
- Nibur, K.A.; Bahr, D.F.; Somerday, B.P. Hydrogen effects on dislocation activity in austenitic stainless steel. Acta Mater. 2006, 54, 2677–2684. [Google Scholar] [CrossRef]
- Connor, M.B. Molecular Dynamics of High Temperature Hydrogen Attack. Ph.D Thesis, Mississippi State University, Starkville, MS, USA, 2022. Available online: https://scholarsjunction.msstate.edu/td/5702 (accessed on 7 February 2024).
- Sabine, M.; Der Giessen, V. Micromechanics of high temperature hydrogen attack. Int. J. Numer. Methods Eng. 2001, 52, 559–567. [Google Scholar]
- Shewmon, P.G. Hydrogen attack of carbon steel. Metall. Trans. A 1976, 7, 279–286. [Google Scholar] [CrossRef]
- Shewmon, P.G. Hydrogen attack of pressure-vessel steels. Mater. Sci. Technol. 1985, 1, 2–11. [Google Scholar] [CrossRef]
- Eliezer, D. High-temperature hydrogen attack of carbon steel. J. Mater. Sci. 1981, 16, 2962–2966. [Google Scholar] [CrossRef]
- Benac, D.J.; McAndrew, P. Reducing the risk of high temperature hydrogen attack (HTHA) failures. J. Fail. Anal. Prev. 2012, 12, 624–627. [Google Scholar] [CrossRef]
- Martin, M.L.; Dadfarnia, M.; Orwig, S.; Moore, D.; Sofronis, P. A microstructure-based mechanism of cracking in high temperature hydrogen attack. Acta Mater. 2017, 140, 300–304. [Google Scholar] [CrossRef]
- Becker, W.T. ASM Handbook: Failure Analysis and Prevention; ASM International: Almere, The Netherlands, 2002. [Google Scholar]
- Hadzihafizovic, D. API 571 Damage Mechanisms. 15 April 2023. Available online: https://zenodo.org/records/10391499 (accessed on 29 January 2024).
- Allen, R.E.; Jansen, R.J.; Rosenthal, P.C.; Vitovec, F.H. Analysis of probable mechanisms of high-temperature hydrogen attack of steel. Proc. API 1962, 42, 452–462. [Google Scholar]
- Krynicki, J.; Bagnoli, K.; McLaughlin, J.E. Probabilistic Risk Based Approach for Performing an Onstream High Temperature Hydrogen Attack Inspection. In NACE CORROSION; NACE, OnePetro: Richardson, TX, USA, 2006; p. NACE-06580. Available online: https://onepetro.org/NACECORR/proceedings-abstract/CORR06/All-CORR06/121255 (accessed on 25 January 2024).
- Sagüés, A.A.; Okray Hall, B.; Wiedersich, H. On the mechanisms of hydrogen attack. Scr. Metall. 1978, 12, 319–326. [Google Scholar] [CrossRef]
- American Petroleum Institute, Division of Refining. Steels for Hydrogen Service at Elevated Temperatures and Pressures in Petroleum Refineries and Petrochemical Plants; American Petroleum Institute: Washington, DC, USA, 1970. [Google Scholar]
- Ustolin, F.; Paltrinieri, N.; Berto, F. Loss of integrity of hydrogen technologies: A critical review. Int. J. Hydrogen Energy 2020, 45, 23809–23840. [Google Scholar] [CrossRef]
- Fletcher, E.E.; Elsea, A.R. The Effects of High Pressure, High Temperature Hydrogen on Steel; Defense Metals Information Center, Battelle Memorial Institute, Columbus, Ohio, USA 1964; Volume 202. Available online: https://books.google.com/books?hl=en&lr=&id=TvAQh9s6tV4C&oi=fnd&pg=PA1&dq=+E.E.+Fletcher+and+A.R.+Elsea,+The+Effects+of+High-Pressure+HighTemperature+Hydrogen+on+Steel,+Defense+Metals+Information+Center,+Columbus,+Ohio,+1964&ots=_X6RWkGHQq&sig=7MODWSSfa3jhY5hy90VvBUI5vdg (accessed on 25 January 2024).
- Prager, M. A Worldwide Look at Hot Hydrogen Attack; ASME-Publ.-PVP: New York, NY, USA, 2000; Volume 411, pp. 65–76. [Google Scholar]
- Nelson, G.A. Use curves to predict steel life. Hydrocarb. Process. 1965, 44, 185–188. [Google Scholar]
- Parthasarathy, T.A. Mechanisms of Hydrogen Attack of carbon and 214Cr-1Mo steels. Acta Metall. 1985, 33, 1673–1681. [Google Scholar] [CrossRef]
- Thygeson, J.R.; Molstad, M.C. High Pressure Hydrogen Attack of Steel. J. Chem. Eng. Data 1964, 9, 309–315. [Google Scholar] [CrossRef]
- Looney, L.; Hurst, R.C.; Taylor, D. The effect of high pressure hydrogen on the creep fracture of notched ferritic-steel components. J. Mater. Process. Technol. 1998, 77, 25–31. [Google Scholar] [CrossRef]
- Naumann, F.K. Der Einfluß von Legierungszusätzen auf die Beständigkeit von Stahl Gegen Wasserstoff unter Hohem Druck. No Title. 1938. Available online: https://cir.nii.ac.jp/crid/1130000795045189760 (accessed on 29 January 2024).
- Hattori, K.; Aikawa, S. Scheduling and planning inspection of C-1/2Mo equipment using the new hydrogen attack tendency chart. Am. Soc. Mech. Eng. Press. Vessel. Pip. Div. 1992, 239, 121–128. [Google Scholar]
- Woods, C.M.; Scott, T.E. Hydrogen Attack of Bainitic 2 1/4 Cr-1 Mo Steel; Mound Facility: Miamisburg, OH, USA, 1982. Available online: https://www.osti.gov/biblio/5228194 (accessed on 29 January 2024).
- Sakai, T.; Asami, K. Effect of Applied Stress on Hydrogen Attack of 2 1/4 Cr-1Mo Steel. Tetsu Hagane 1987, 73, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Allevato, C. Utilizing acoustic emission testing to detect high-temperature hydrogen attack (HTHA) in Cr-Mo reformer reactors and piping during thermal gradients. Procedia Eng. 2011, 10, 3552–3560. [Google Scholar] [CrossRef]
- Chiba, R. Effect of Carbon on Hydrogen Attack of 21/4Cr-1Mo Steels. Tetsu Hagane 1985, 71, 1639–1646. [Google Scholar] [CrossRef]
- Yokogawa, K.; Fukuyama, S.; Kudo, K.; Shewmon, P.G. Effect of hydrogen attack on tensile and creep properties of low carbon steel. Int. J. Press. Vessels Pip. 1989, 37, 365–385. [Google Scholar] [CrossRef]
- Ruoff, S.; Stone, D.; Li, C.-Y. Hydrogen attack in a 3Cr-1.5 Mo steel at elevated temperatures. Mater. Sci. Eng. 1987, 93, 217–225. [Google Scholar]
- der Burg, M.V.; der Giessen, E.V.; Brouwer, R.C. Investigation of hydrogen attack in 2.25 Cr-1Mo steels with a high-triaxiality void growth model. Acta Mater. 1996; 44, 505–518. [Google Scholar]
- Imanaka, T.; Shimomura, J.I. Temper Embrittlement and Hydrogen Attack on 2 1/4 Cr-1Mo Steels in High Pressure and High Temperature Hydrogen Atmosphers. In Proceedings of the 5th International Conference on Pressure Vessel Technology, San Francisco, CA, USA, 9–14 September 1984. [Google Scholar]
- Imanaka, T.; Shimomura, J.I.; Nakano, S.; Yasuda, K. Hydrogen Attack in Cr–Mo Steels and Disbonding of Austenitic Stainless Weld Overlay. Kawasaki Steel Tech. Rep. 1985, 13, 109–119. [Google Scholar]
- Sakai, T.; Kaji, H. Effects of P, Sn, As, Sb, Si, and Cu on the Formation of Bubbles by Hydrogen Attack in 2.25 Cr-1Mo Steel. Tetsu Hagané 1980, 66, 1133–1141. [Google Scholar]
- Shimomura, J.; Imanaka, T. Decomposition of carbides in 2 1/4Cr 1Mo steels during hydrogen attack. Scr. Metall. 1985, 19, 1507–1511. [Google Scholar] [CrossRef]
- Imanaka, T.; Sato, S.; Aso, K.; Shimomura, J.; Ueda, S.; Ejima, A.; Ohashi, N. Improvement in elevated temperature strength, hydrogen attack resistivity and stress relief cracking susceptibility of Cr-Mo steels. Nucl. Eng. Des. 1986, 96, 195–207. [Google Scholar] [CrossRef]
- Tiejian, S.U.; Xinghong, L.U.O.; Cungan, F.A.N.; Yiyi, L.I.; Xiao, C.; Aimin, G.U.O. Tempering Precipitates of Steel 1.25 Cr-0.5 Mo and Their Effects on Its Hydrogen Attack Resistance. Acta Met. Sin. 1998, 34, 393–399. [Google Scholar]
- Chiba, R.; Ohnishi, K.; Ishii, K.; Maeda, K. Effect of Heat Treatment on Hydrogen Attack Resistance of C-0.5 Mo Steels for Pressure Vessels, Heat Exchangers, and Piping. Corrosion 1985, 41, 415–426. [Google Scholar]
- Chiba, R.; Ohnishi, K.; Ishii, K.; Maeda, K. Effect of Postweld Heat Treatment on Critical Temperature in Hydrogen Attack of 1/2 Mo Steel Welds. Tetsu Hagane 1987, 73, 175–182. [Google Scholar] [CrossRef]
- Prager, M. Hydrogen Attack Susceptibility of Chrome-Moly Steels and Weldments. In American Society of Mechanical Engineers, Pressure Vessels and Piping Division (Publication) PVP; American Society of Mechanical Engineers: New York, NY, USA, 1992; Volume 239. [Google Scholar]
- Soleimani, M.; Mirzadeh, H.; Dehghanian, C. Effects of spheroidization heat treatment and intercritical annealing on mechanical properties and corrosion resistance of medium carbon dual phase steel. Mater. Chem. Phys. 2021, 257, 123721. [Google Scholar] [CrossRef]
- López, H.F. Effect of deoxidation practice and heat treatment on the hydrogen attack of carbon steels. Metall. Trans. A 1987, 18, 1971–1977. [Google Scholar] [CrossRef]
- Dong, C.F.; Li, X.G.; Shen, Z.S.; Chu, W.Y. Study on crack healing of hydrogen attack in carbon steel by heat treatment. Corrosion 2003, 59, 401–406. [Google Scholar] [CrossRef]
- Marchi, C.S.; Somerday, B.P.; Nibur, K.A. Development of methods for evaluating hydrogen compatibility and suitability. Int. J. Hydrogen Energy 2014, 39, 20434–20439. [Google Scholar] [CrossRef]
- Nelson, G.A.; Effinger, R.T. Blistering and Embrittlement of Pressure Vessel Steels by Hydrogen; Weld. J (NY); U.S. Department of Energy, Office of Scientific and Technical Information: Washington, DC, USA, 1955; Volume 34. Available online: https://www.osti.gov/biblio/4401716 (accessed on 17 February 2024).
- API RP 941; Steels for Hydrogen Service at Elevated Temperatures and Pressures in Petroleum Refineries and Petrochemical Plants. 8th ed. American Petroleum Institute: Washington, DC, USA, 2016.
- Hayden, L.E.; Stalheim, D. ASME B31.12 Hydrogen Piping and Pipeline Code Design Rules and Their Interaction With Pipeline Materials Concerns, Issues and Research. In Proceedings of the ASME 2009 Pressure Vessels and Piping Conference, Prague, Czech Republic, 26–30 July 2009; American Society of Mechanical Engineers Digital Collection; ASME: New York, NY, USA, 2010; pp. 355–361. [Google Scholar] [CrossRef]
- ASME BPVC Sec VIII-3 Alt Rules Construction High Pressure Vessels—ASME. Available online: https://www.asme.org/codes-standards/find-codes-standards/bpvc-viii-3-bpvc-section-viii-rules-construction-pressure-vessels-division-3-alternative-rules-construction-high-pressure-vessels/2021/print-book (accessed on 18 February 2024).
- Rana, M.D.; Rawls, G.B.; Sims, J.R.; Upitis, E. Technical Basis and Application of New Rules on Fracture Control of high Pressure Hydrogen Vessel in ASME Section VIII, Division 3 Code. 2008. Available online: https://asmedigitalcollection.asme.org/pressurevesseltech/article-abstract/130/2/021101/442772 (accessed on 18 February 2024).
- Rivkin, C.; Blake, C.; Burgess, R.; Buttner, W.J.; Post, M.B. A national set of hydrogen codes and standards for the United States. Int. J. Hydrogen Energy 2011, 36, 2736–2741. [Google Scholar] [CrossRef]
- Marchi, C.S.; Somerday, B. Technical Reference for Hydrogen Compatibility of Materials; SAND2012-7321; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2012. [Google Scholar] [CrossRef]
- Safety Standard for Hydrogen and Hydrogen Systems: Guidelines for Hydrogen System Design, Materials Selection, Operations, Storage and Transportation. NASA-TM-112540. 1 January 1997. Available online: https://ntrs.nasa.gov/citations/19970033338 (accessed on 3 May 2023).
- NASA: SAFETY Standard for Hydrogen and Hydrogen Systems. Available online: https://www.energy.gov/sites/prod/files/2014/03/f11/871916.pdf (accessed on 5 February 2024).
- Nelson, G.A. Operating limits and incubation times for steels in hydrogen Service. Am. Pet. Inst. Proc. Sect. 111 Refin. 1965, 45, 190–195. [Google Scholar]
- Sundararajan, G.; Shewmon, P.G. The hydrogen attack of HSLA steels. Metall. Trans. A 1980, 11, 509–516. [Google Scholar] [CrossRef]
- Shahi, R.R.; Gupta, A.K.; Kumari, P. Perspectives of high entropy alloys as hydrogen storage materials. Int. J. Hydrogen Energy 2023, 48, 21412–21428. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Marques, F.; Balcerzak, M.; Winkelmann, F.; Zepon, G.; Felderhoff, M. Review and outlook on high-entropy alloys for hydrogen storage. Energy Environ. Sci. 2021, 14, 5191–5227. [Google Scholar] [CrossRef]
- Xin, Y.; Li, S.; Qian, Y.; Zhu, W.; Yuan, H.; Jiang, P.; Guo, R.; Wang, L. High-Entropy Alloys as a Platform for Catalysis: Progress, Challenges, and Opportunities. ACS Catal. 2020, 10, 11280–11306. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Fu, Y. High-entropy alloys: Emerging materials for advanced functional applications. J. Mater. Chem. A 2021, 9, 663–701. [Google Scholar] [CrossRef]
- Brear, J.M.; Church, J.M. TECHNICAL BASIS FOR API PUBLICATION 941 (NELSON CURVES). 1996. Available online: https://www.researchgate.net/profile/John-Brear/publication/303919409_Technical_basis_for_API_Publication_RP941_Nelson_Curves/links/575db5ba08aed88462166d52/Technical-basis-for-API-Publication-RP941-Nelson-Curves.pdf (accessed on 10 February 2024).
- Nelson, G.A. Hydrogenation plant steels. Proc. API M 1949, 29, 163. [Google Scholar]
- Decker, S.; Hynes, T.; Buchheim, G. Safe Operation of a High Temperature Hydrogen Attack Affected DHT Reactor. In NACE CORROSION; NACE, OnePetro: Richardson, Texas, USA, 2009; p. NACE-09339. Available online: https://onepetro.org/NACECORR/proceedings-abstract/CORR09/All-CORR09/128742 (accessed on 21 February 2024).
- Nelson, G.A. Metals for high-pressure hydrogenation plants. Trans. Am. Soc. Mech. Eng. 1951, 73, 205–211. [Google Scholar] [CrossRef]
- Cramer, S.D.; Covino, B.S., Jr. ASM Handbook; ASM International Materials Park: Novelty, OH, USA, 2003; Volume 13, Available online: https://www.asminternational.org/wp-content/uploads/files/06494G/06494G-toc.pdf (accessed on 6 February 2024).
- Vijayvargia, K.; Dadfarnia, M.; Sofronis, P.; Kubota, M.; Staykov, A.; Wada, K.; Nguyen, T.; Pugh, J.; Eason, T. A Combined Micromechanics/Materials Science Approach to Understanding High Temperature Hydrogen Attack. 2023. Available online: https://dc.engconfintl.org/cgi/viewcontent.cgi?filename=0&article=1099&context=hydrogen&type=additional (accessed on 5 February 2024).
- McLaughlin, J.; Krynicki, J.; Bruno, T. Cracking of Non-PWHT’d Carbon Steel Operating at Conditions Immediately Below the Nelson Curve. In Proceedings of the Pressure Vessels and Piping Conference, Bellevu, WA, USA, 18–22 July 2010; pp. 919–930. Available online: https://asmedigitalcollection.asme.org/PVP/proceedings-abstract/PVP2010/919/342781 (accessed on 13 February 2024).
- De Marco, M.; Mannello, G. A Novel Approach for Risk Ranking, NDT Priorities and Damage Mitigation of TIP Units Piping Systems in High Temperature Hydrogen Service. In NACE CORROSION; NACE, OnePetro: Richardson, TX, USA, 2013; p. NACE-2013. Available online: https://onepetro.org/NACECORR/proceedings-abstract/CORR13/All-CORR13/121568 (accessed on 13 February 2024).
- Cantwell, J.E. High-Temperature Hydrogen Attack. Mater. Perform. 1994, 33, 7052013. Available online: https://www.osti.gov/biblio/7052013 (accessed on 25 January 2024).
- Rothwell, J. Maintaining the Integrity of Process Plant Susceptible to High Temperature Hydrogen Attack. Part 2: Factors Affecting Carbon Steel; Health and Safety Executive (HSE): London, UK, 2018. [Google Scholar]
- Van Der Burg, M.W.D.; Van der Giessen, E.; Tvergaard, V. A continuum damage analysis of hydrogen attack in a 2.25 Cr–1Mo pressure vessel. Mater. Sci. Eng. A 1998, 241, 1–13. [Google Scholar]
- Catastrophic Rupture of Heat Exchanger: Seven Fatalities; Investigation Report, no. Report no. 2010-08-I-WA; Chemical Safety and Hazard Investigation Board: Washington, DC, USA, 2010.
- Hattori, K. An Advanced Hydrogen Attack Tendency Chart as a Life Assessment Strategy for C-0.5 Mo Equipment. ASME-Publ.-PVP: New York, NY, USA, 1997; Volume 359, pp. 333–338. [Google Scholar]
- High Temperature Hydrogen Attack Method Using Time-Based Nelson Curves. Available online: https://inspectioneering.com/journal/2020-04-30/9160/practical-htha-experience-and-time-based-nelson-curves-for-improved-equipment-li (accessed on 13 February 2024).
- HTHA Time Dependent Nelson Curves and Inspection Planning Enhancements. Available online: https://inspectioneering.com/journal/2022-02-24/10019/breakthroughs-in-predictive-htha-life-assessment-time-dependent-nelson-curves-a (accessed on 13 February 2024).
- Harris, J.A.; Van Wanderham, M.C. Properties of Materials in High Pressure Hydrogen at Cryogenic, Room, and Elevated Temperatures Annual Report. PWA-FR-4566. 30 June 1971. Available online: https://ntrs.nasa.gov/citations/19710024252 (accessed on 4 May 2023).
- Jewett, R.P.; Walter, R.J.; Chandler, W.T.; Frohmberg, R.P. Hydrogen Environment Embrittlement of Metals. NASA-CR-2163. 1 March 1973. Available online: https://ntrs.nasa.gov/citations/19730012717 (accessed on 4 May 2023).
- Report on Hydrogen Tanks, Pipe and Pipeline Stds—ASME. Available online: https://www.asme.org/codes-standards/find-codes-standards/stp-pt-003-hydrogen-standardization-interim-report-for-tanks-piping-and-pipelines (accessed on 9 May 2023).
- CHMC 1-2014; Test Methods for Evaluating Material Compatibility in Compressed Hydrogen Applications-Metals. CSA Group: Toronto, ON, Canada, 2014.
- Kaushal, G.; Bala, N.; Kaur, N.; Singh, H.; Prakash, S. Comparative high-temperature corrosion behavior of Ni-20Cr coatings on T22 boiler steel produced by HVOF, D-Gun, and cold spraying. Metall. Mater. Trans. A 2014, 45, 395–410. [Google Scholar] [CrossRef]
- Singh, G.; Bala, N.; Chawla, V. Oxidation behaviour of HVOF sprayed NiCrAlY and NiCrAlY-20SiC coatings on T-91 boiler tube steel. Prot. Met. Phys. Chem. Surf. 2020, 56, 134–150. [Google Scholar] [CrossRef]
- Eklund, J.; Phother, J.; Sadeghi, E.; Joshi, S.; Liske, J. High-temperature corrosion of HVAF-sprayed Ni-based coatings for boiler applications. Oxid. Met. 2019, 91, 729–747. [Google Scholar] [CrossRef]
- Wetegrove, M.; Duarte, M.J.; Taube, K.; Rohloff, M.; Gopalan, H.; Scheu, C.; Dehm, G.; Kruth, A. Preventing Hydrogen Embrittlement: The Role of Barrier Coatings for the Hydrogen Economy. Hydrogen 2023, 4, 307–322. [Google Scholar] [CrossRef]
- Nemanič, V. Hydrogen permeation barriers: Basic requirements, materials selection, deposition methods, and quality evaluation. Nucl. Mater. Energy 2019, 19, 451–457. [Google Scholar] [CrossRef]
- Levchuk, D.; Koch, F.; Maier, H.; Bolt, H. Deuterium permeation through Eurofer and α-alumina coated Eurofer. J. Nucl. Mater. 2004, 328, 103–106. [Google Scholar] [CrossRef]
- Serra, E.; Bini, A.C.; Cosoli, G.; Pilloni, L. Hydrogen Permeation Measurements on Alumina. J. Am. Ceram. Soc. 2005, 88, 15–18. [Google Scholar] [CrossRef]
- Stöver, D.; Buchkremer, H.P.; Hecker, R. Hydrogen and deuterium permeation through metallic and surface-oxidized chromium. Surf. Coat. Technol. 1986, 28, 281–290. [Google Scholar] [CrossRef]
- Levchuk, D.; Levchuk, S.; Maier, H.; Bolt, H.; Suzuki, A. Erbium oxide as a new promising tritium permeation barrier. J. Nucl. Mater. 2007, 367–370, 1033–1037. [Google Scholar] [CrossRef]
- Checchetto, R.; Gratton, L.M.; Miotello, A.; Tosello, C. Deuterium permeation through SiO2 thin film deposited on stainless steel substrate. J. Non-Cryst. Solids 1997, 216, 65–70. [Google Scholar] [CrossRef]
- Levchuk, D.; Bolt, H.; Döbeli, M.; Eggenberger, S.; Widrig, B.; Ramm, J. Al–Cr–O thin films as an efficient hydrogen barrier. Surf. Coat. Technol. 2008, 202, 5043–5047. [Google Scholar] [CrossRef]
- RLee, W.; Frank, R.C.; Swets, D.E. Diffusion of hydrogen and deuterium in fused quartz. J. Chem. Phys. 1962, 36, 1062–1071. [Google Scholar]
- Tamura, M.; Noma, M.; Yamashita, M. Characteristic change of hydrogen permeation in stainless steel plate by BN coating. Surf. Coat. Technol. 2014, 260, 148–154. [Google Scholar] [CrossRef]
- Tamura, M. Hydrogen permeation characteristics of TiN-Coated stainless steels. J. Mater. Sci. Eng. 2015, 5, 197–201. [Google Scholar]
- Forcey, K.S.; Perujo, A.; Reiter, F.; Lolli-Ceroni, P.L. The formation of tritium permeation barriers by CVD. J. Nucl. Mater. 1993, 200, 417–420. [Google Scholar] [CrossRef]
- Wang, P.; Liu, J.; Wang, Y.; Shi, B. Investigation of SiC films deposited onto stainless steel and their retarding effects on tritium permeation. Surf. Coat. Technol. 2000, 128–129, 99–104. [Google Scholar] [CrossRef]
- Checchetto, R.; Bonelli, M.; Gratton, L.; Miotello, A.; Sabbioni, A.; Guzman, L.; Horino, Y.; Benamati, G. Analysis of the hydrogen permeation properties of TiN-TiC bilayers deposited on martensitic stainless steel. Surf. Coat. Technol. 1996, 83, 40–44. [Google Scholar] [CrossRef]
- Archakov, Y.I.; Grebeshkova, I.D. Ability of clad steels to resist hydrogen attack. Chem. Pet. Eng. 1966, 2, 388–395. [Google Scholar] [CrossRef]
- Nugent, M.; Silfies, T.; Kowalski, P.; Sutton, N. Recent Applications of Evaluations of Equipment in HTHA Service. In NACE CORROSION; NACE, OnePetro: Richardson, TX, USA, 2018; p. NACE-2018. Available online: https://onepetro.org/NACECORR/proceedings-abstract/CORR18/All-CORR18/125786 (accessed on 21 February 2024).
- Saini, N.; Pandey, C.; Mahapatra, M.M. Effect of diffusible hydrogen content on embrittlement of P92 steel. Int. J. Hydrogen Energy 2017, 42, 17328–17338. [Google Scholar] [CrossRef]
- American Welding Society; Committee on Filler Metal. Standard Methods for the Determination of Diffusible Hydrogen Content of Martinsitic, Bainitic, and Ferritic Steel Weld Metal Produced by Arc Welding. The Society. 1986. Available online: https://pubs.aws.org/Download_PDFS/A4.3-93_R2006PV.pdf (accessed on 7 January 2024).
- Fangnon, E.; Malitckii, E.; Latypova, R.; Vilaça, P. Prediction of hydrogen concentration responsible for hydrogen-induced mechanical failure in martensitic high-strength steels. Int. J. Hydrogen Energy 2023, 48, 5718–5730. [Google Scholar] [CrossRef]
- Malitckii, E.; Fangnon, E.; Vilaça, P. Study of correlation between the steels susceptibility to hydrogen embrittlement and hydrogen thermal desorption spectroscopy using artificial neural network. Neural Comput. Appl. 2020, 32, 14995–15006. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, X.; Xiong, Y.; Shi, Y.; Song, J.; Luo, D. Mechanism for adsorption, dissociation and diffusion of hydrogen in hydrogen permeation barrier of α-Al2O3: A density functional theory study. Int. J. Hydrogen Energy 2013, 38, 1157–1165. [Google Scholar] [CrossRef]
- Zhang, G.; Dou, S.; Lu, Y.; Shi, Y.; Lai, X.; Wang, X. Mechanisms for adsorption, dissociation and diffusion of hydrogen in hydrogen permeation barrier of α-Al2O3: The role of crystal orientation. Int. J. Hydrogen Energy 2014, 39, 610–619. [Google Scholar] [CrossRef]
- Depover, T.; Escobar, D.P.; Wallaert, E.; Zermout, Z.; Verbeken, K. Effect of hydrogen charging on the mechanical properties of advanced high strength steels. Int. J. Hydrogen Energy 2014, 39, 4647–4656. [Google Scholar] [CrossRef]
- Danford, M.D. Hydrogen Trapping and the Interaction of Hydrogen with Metals. 1 July 1987. Available online: https://ntrs.nasa.gov/citations/19870016030 (accessed on 8 January 2024).
- Mertens, G.; Duprez, L.; Cooman, B.C.D.; Verhaege, M. Hydrogen Absorption and Desorption in Steel by Electrolytic Charging. Adv. Mater. Res. 2007, 15–17, 816–821. [Google Scholar] [CrossRef]
- Vernerey, F.J.; McVeigh, C.; Liu, W.K.; Moran, B.; Tewari, D.; Parks, D.M.; Olson, G.B. The 3-D computational modeling of shear-dominated ductile failure in steel. JOM 2006, 58, 45–51. [Google Scholar] [CrossRef]
- Needleman, A. Computational Modeling of Material Failure. Appl. Mech. Rev. 1994, 47, S34–S42. [Google Scholar] [CrossRef]
- Dujc, J.; Brank, B.; Ibrahimbegovic, A. Multi-scale computational model for failure analysis of metal frames that includes softening and local buckling. Comput. Methods Appl. Mech. Eng. 2010, 199, 1371–1385. [Google Scholar] [CrossRef]
- Barrera, O.; Bombac, D.; Chen, Y.; Daff, T.D.; Galindo-Nava, E.; Gong, P.; Haley, D.; Horton, R.; Katzarov, I.; Kermode, J.R.; et al. Understanding and mitigating hydrogen embrittlement of steels: A review of experimental, modelling and design progress from atomistic to continuum. J. Mater. Sci. 2018, 53, 6251–6290. [Google Scholar] [CrossRef]
- Cheng, G.; Chen, K.; Zhang, Y.; Venkatesh, T.; Wang, X.; Li, Z.; Yang, J. Probing the effects of hydrogen on the materials used for large-scale transport of hydrogen through multi-scale simulations. Renew. Sustain. Energy Rev. 2023, 182, 113353. [Google Scholar] [CrossRef]
- Roadmap on Multiscale Materials Modeling—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/1361-651X/ab7150/meta (accessed on 8 January 2024).
- Al-Jabr, K.A.; Multiscale Modeling of Hydrogen Embrittlement for Multiphase Material. May 2014. Available online: http://hdl.handle.net/10754/316598 (accessed on 8 January 2024).
- Morrissey, L. A Multiscale Analysis on the Effect of Atomistic Processes on Mechanical Properties. Ph.D. Thesis, Memorial University of Newfoundland, St. John’s, NL, Canada, 2020. Available online: https://research.library.mun.ca/15073/ (accessed on 8 January 2024).
- Chavoshi, S.Z.; Hill, L.T.; Bagnoli, K.E.; Holloman, R.L.; Nikbin, K.M. A combined fugacity and multi-axial ductility damage approach in predicting high temperature hydrogen attack in a reactor inlet nozzle. Eng. Fail. Anal. 2020, 117, 104948. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J. Phase field modeling of defects and deformation. Acta Mater. 2010, 58, 1212–1235. [Google Scholar] [CrossRef]
- Martínez-Pañeda, E.; Golahmar, A.; Niordson, C.F. A phase field formulation for hydrogen assisted cracking. Comput. Methods Appl. Mech. Eng. 2018, 342, 742–761. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Nguyen, V.P.; Nguyen, C.T.; Sutula, D.; Sinaie, S.; Bordas, S.P.A. Chapter One—Phase-field modeling of fracture. Adv. Appl. Mech. 2020, 53, 1–183. [Google Scholar] [CrossRef]
- Moelans, N.; Blanpain, B.; Wollants, P. An introduction to phase-field modeling of microstructure evolution. Calphad 2008, 32, 268–294. [Google Scholar] [CrossRef]
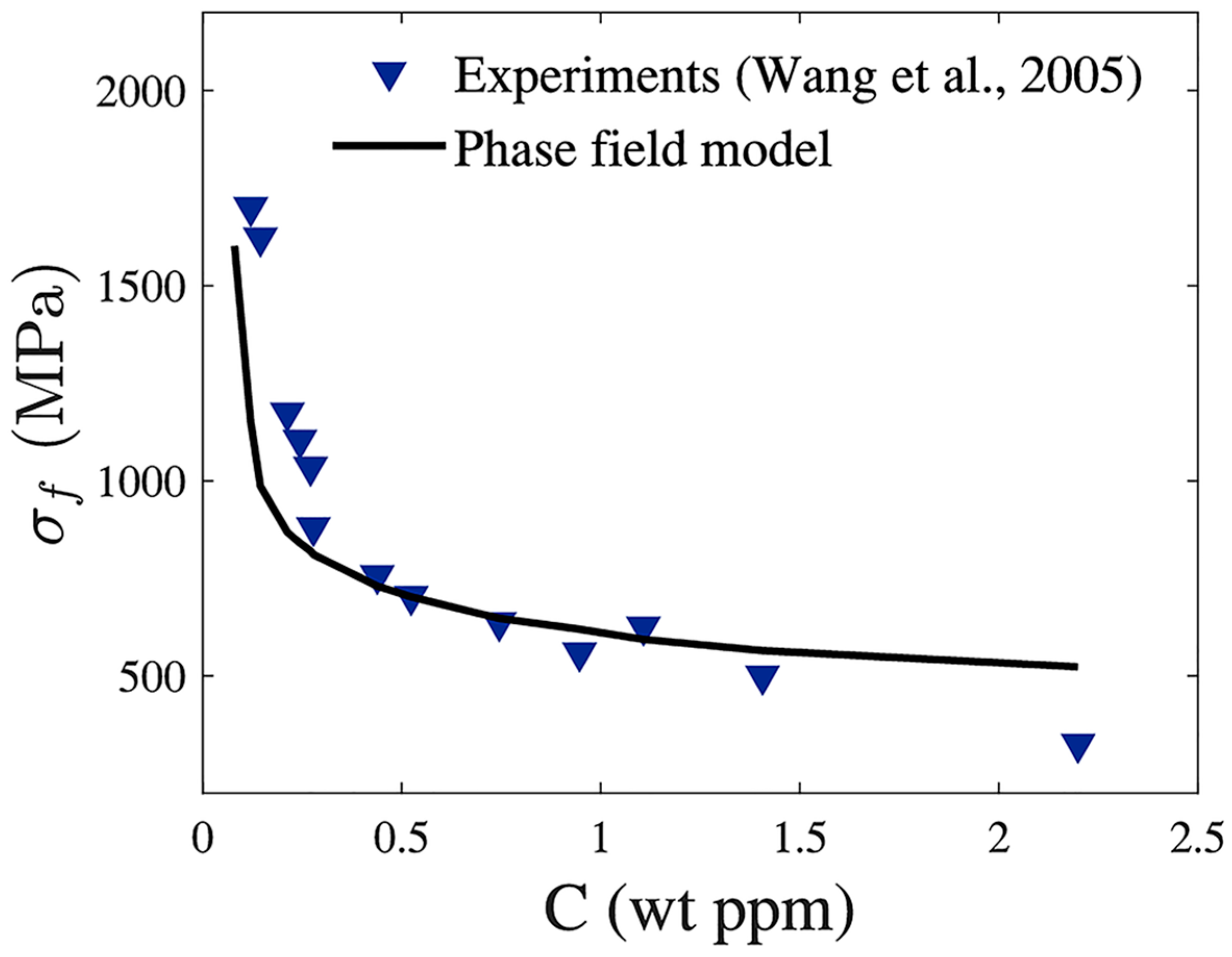
- Wang, M.; Akiyama, E.; Tsuzaki, K. Effect of hydrogen and stress concentration on the notch tensile strength of AISI 4135 steel. Mater. Sci. Eng. A 2005, 398, 37–46. [Google Scholar] [CrossRef]
- Olden, V.; Thaulow, C.; Johnsen, R.; Østby, E.; Berstad, T. Influence of hydrogen from cathodic protection on the fracture susceptibility of 25%Cr duplex stainless steel—Constant load SENT testing and FE-modelling using hydrogen influenced cohesive zone elements. Eng. Fract. Mech. 2009, 76, 827–844. [Google Scholar] [CrossRef]
- Thomas, R.L.S.; Scully, J.R.; Gangloff, R.P. Internal hydrogen embrittlement of ultrahigh-strength AERMET 100 steel. Metall. Mater. Trans. A 2003, 34, 327–344. [Google Scholar] [CrossRef]
- Alvaro, A.; Jensen, I.T.; Kheradmand, N.; Løvvik, O.M.; Olden, V. Hydrogen embrittlement in nickel, visited by first principles modeling, cohesive zone simulation and nanomechanical testing. Int. J. Hydrogen Energy 2015, 40, 16892–16900. [Google Scholar] [CrossRef]
- Valverde-González, A.; Martínez-Pañeda, E.; Quintanas-Corominas, A.; Reinoso, J.; Paggi, M. Computational modelling of hydrogen assisted fracture in polycrystalline materials. Int. J. Hydrogen Energy 2022, 47, 32235–32251. [Google Scholar] [CrossRef]
- Harris, Z.D.; Lawrence, S.K.; Medlin, D.L.; Guetard, G.; Burns, J.T.; Somerday, B.P. Elucidating the contribution of mobile hydrogen-deformation interactions to hydrogen-induced intergranular cracking in polycrystalline nickel. Acta Mater. 2018, 158, 180–192. [Google Scholar] [CrossRef]
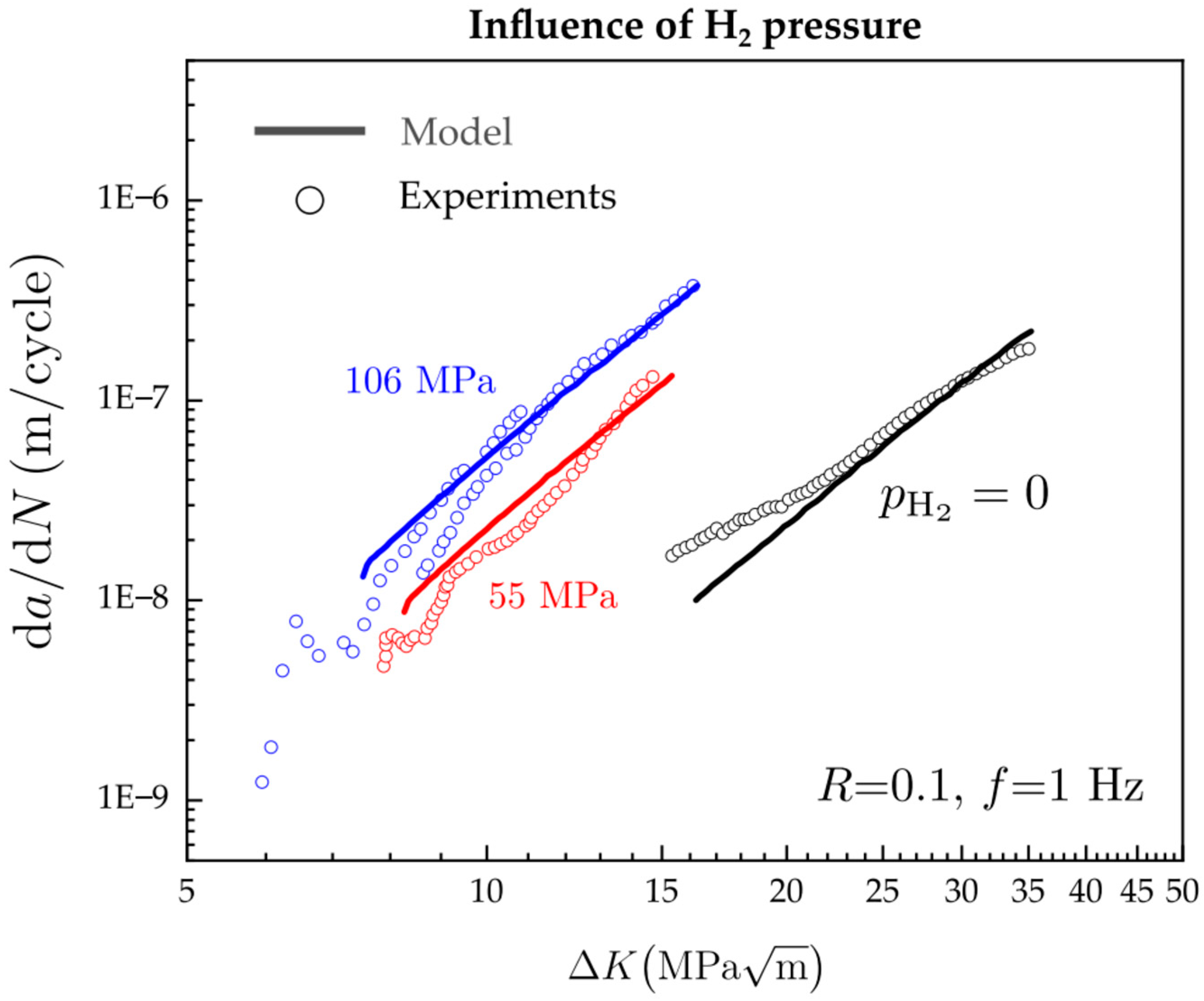
- Cui, C.; Bortot, P.; Ortolani, M.; Martínez-Pañeda, E. Computational predictions of hydrogen-assisted fatigue crack growth. Int. J. Hydrogen Energy 2024, 72, 315–325. [Google Scholar] [CrossRef]
- Kumar, G.R.; Muralidharan, A.; Rajyalakshmi, G.; Swaroop, S. Atom probe tomography analysis of hydrogen distribution in laser peened Ti6Al4V alloy to control hydrogen embrittlement. Int. J. Adv. Manuf. Technol. 2021, 114, 1395–1408. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, Y.; Wang, C.; Xue, D.; Bai, Y.; Antonov, S.; Dai, L.; Lookman, T.; Su, Y. Machine learning assisted design of high entropy alloys with desired property. Acta Mater. 2019, 170, 109–117. [Google Scholar] [CrossRef]
- Feng, S.; Zhou, H.; Dong, H. Using deep neural network with small dataset to predict material defects. Mater. Des. 2019, 162, 300–310. [Google Scholar] [CrossRef]
- Xiong, J.; Shi, S.-Q.; Zhang, T.-Y. A machine-learning approach to predicting and understanding the properties of amorphous metallic alloys. Mater. Des. 2020, 187, 108378. [Google Scholar] [CrossRef]
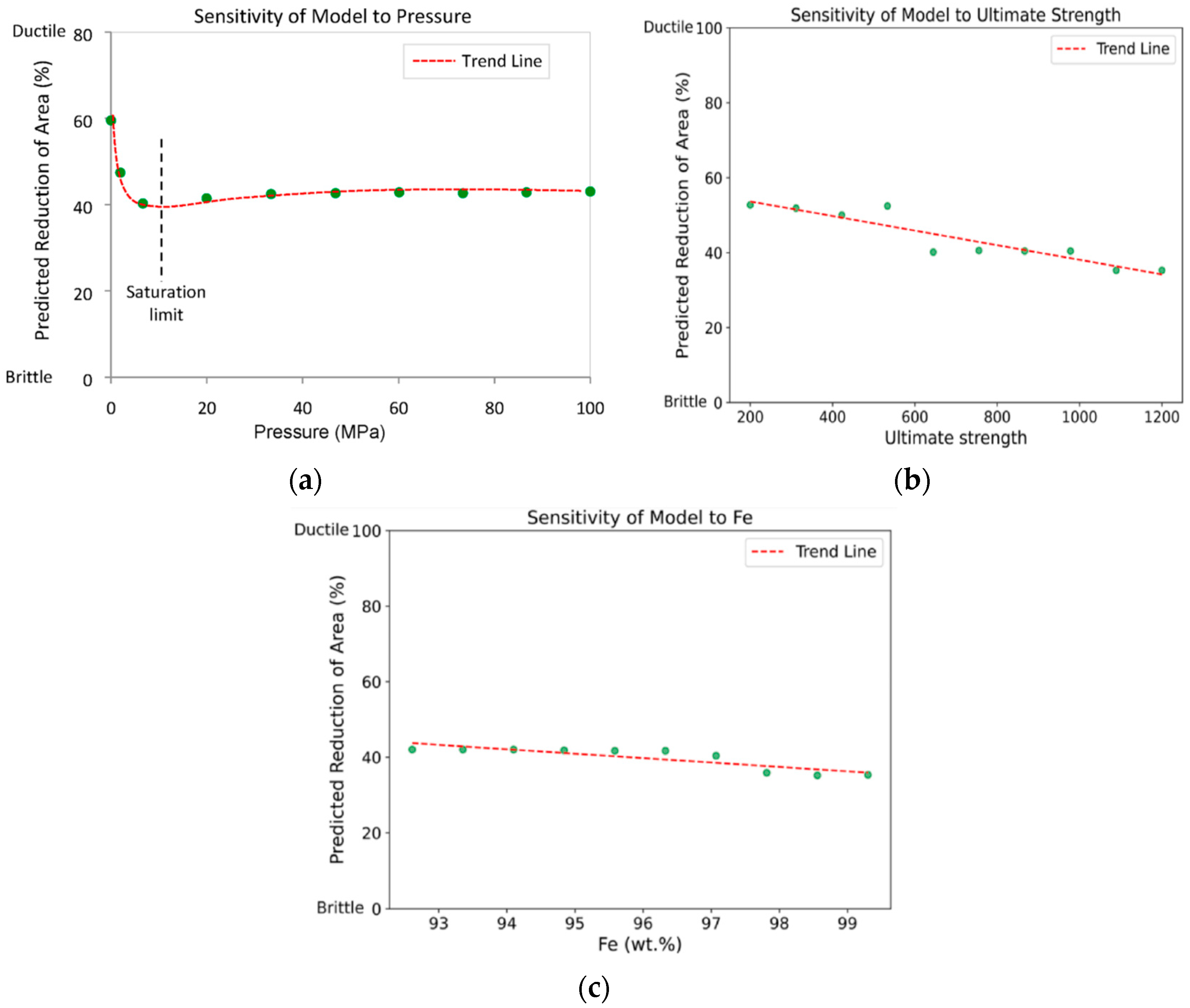
- Ahmed, N.; Aldaw, M.; Ahmed, R.; Teodoriu, C. Modeling of necking area reduction of carbon steel in hydrogen environment using machine learning approach. Eng. Fail. Anal. 2024, 156, 107864. [Google Scholar] [CrossRef]
- Campari, A.; Darabi, M.A.; Alvaro, A.; Ustolin, F.; Paltrinieri, N.; A Machine Learning Approach to Predict. the Materials’ Susceptibility to Hydrogen Embrittlement. 2023. Available online: https://ntnuopen.ntnu.no/ntnu-xmlui/bitstream/handle/11250/3105129/033.pdf?sequence=1 (accessed on 13 April 2024).
- Subedi, A.; Campari, A.; Alvaro, A.; Thapa, B.S.; Paltrinieri, N. Evaluation of the Factors Determining Hydrogen Embrittlement in Pipeline Steels: An Artificial Intelligence Approach. 2023. Available online: https://rpsonline.com.sg/proceedings/esrel2023/pdf/P510.pdf (accessed on 13 April 2024).
- Kim, S.-G.; Shin, S.-H.; Hwang, B. Machine learning approach for prediction of hydrogen environment embrittlement in austenitic steels. J. Mater. Res. Technol. 2022, 19, 2794–2798. [Google Scholar] [CrossRef]
- Xu, K. Hydrogen embrittlement of carbon steels and their welds. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2012; pp. 526–561. Available online: https://www.sciencedirect.com/science/article/pii/B9781845696771500144 (accessed on 14 August 2024).
- Subedi, A.; Campari, A.; Thapa, B.S.; Paltrinieri, N. Safety Evaluation of Hydrogen Pipeline Transport: An Approach Based on Machine Learning. Chem. Eng. Trans. 2023, 105, 121–126. [Google Scholar]
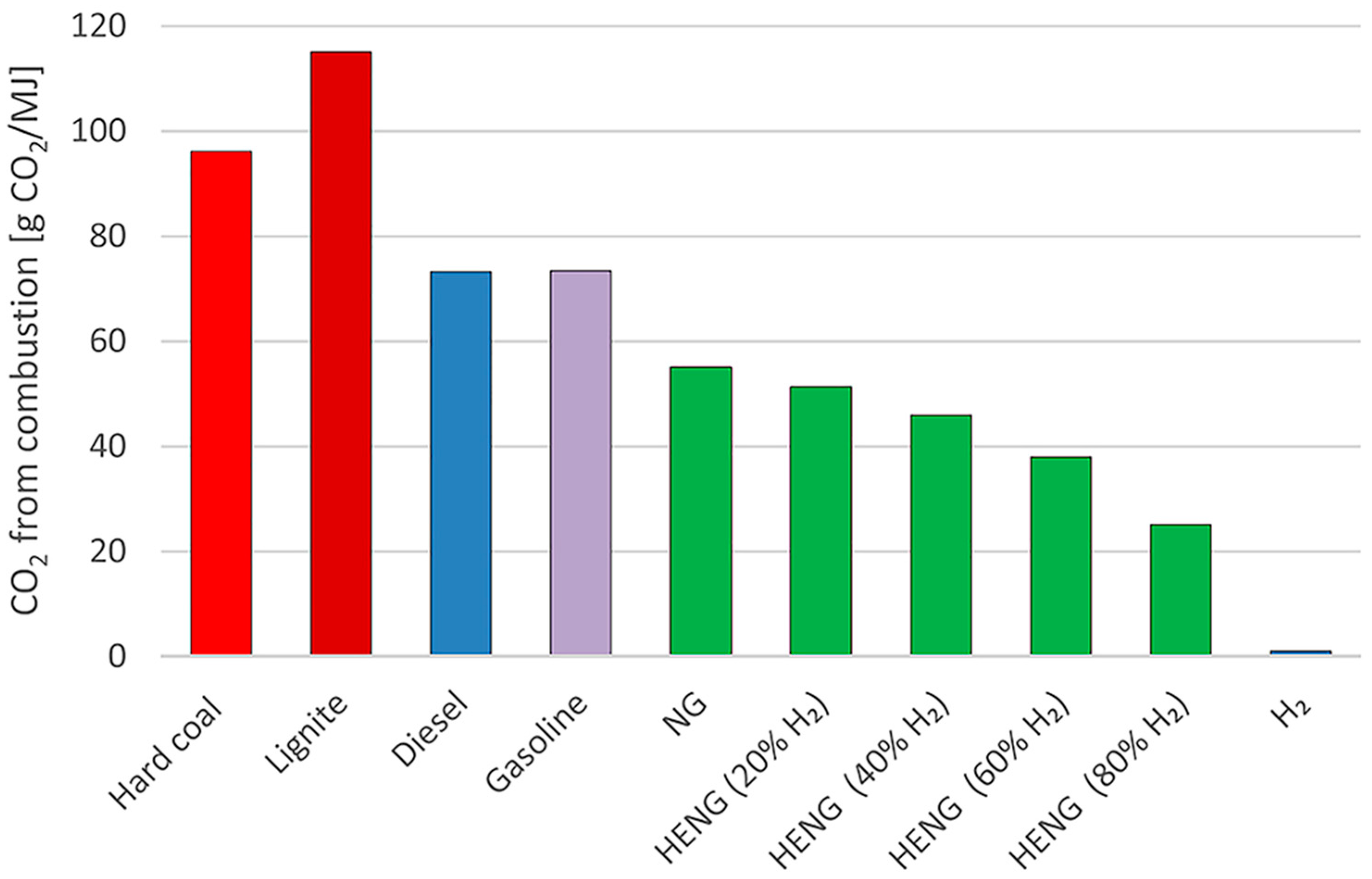
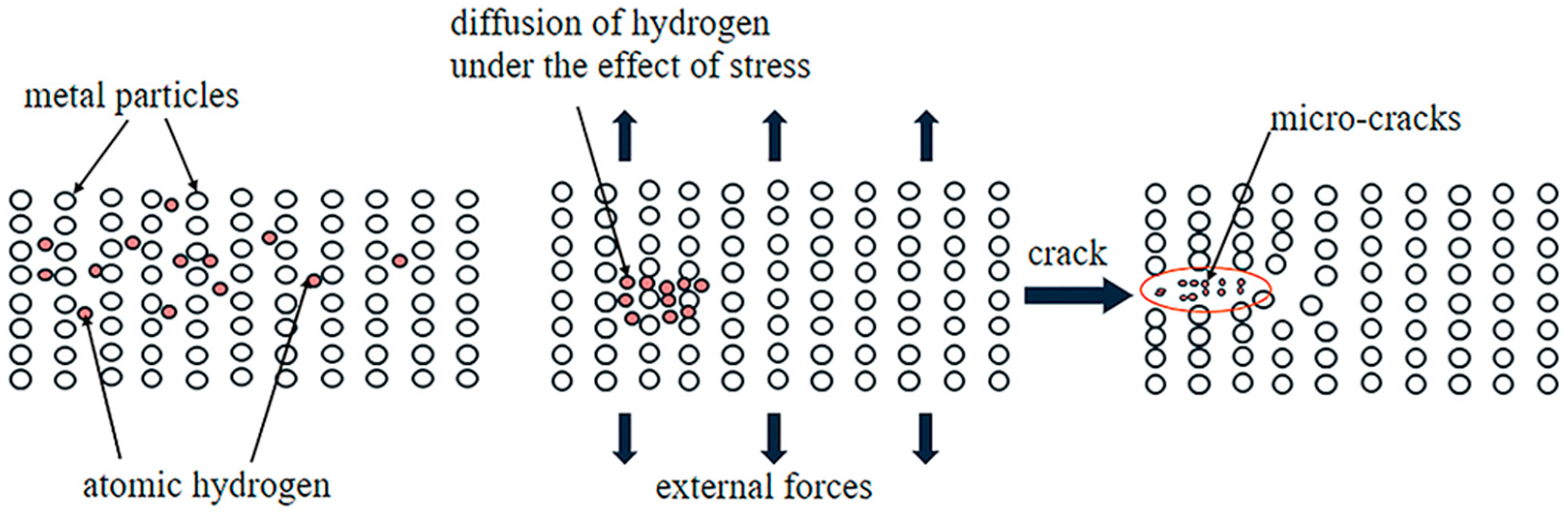
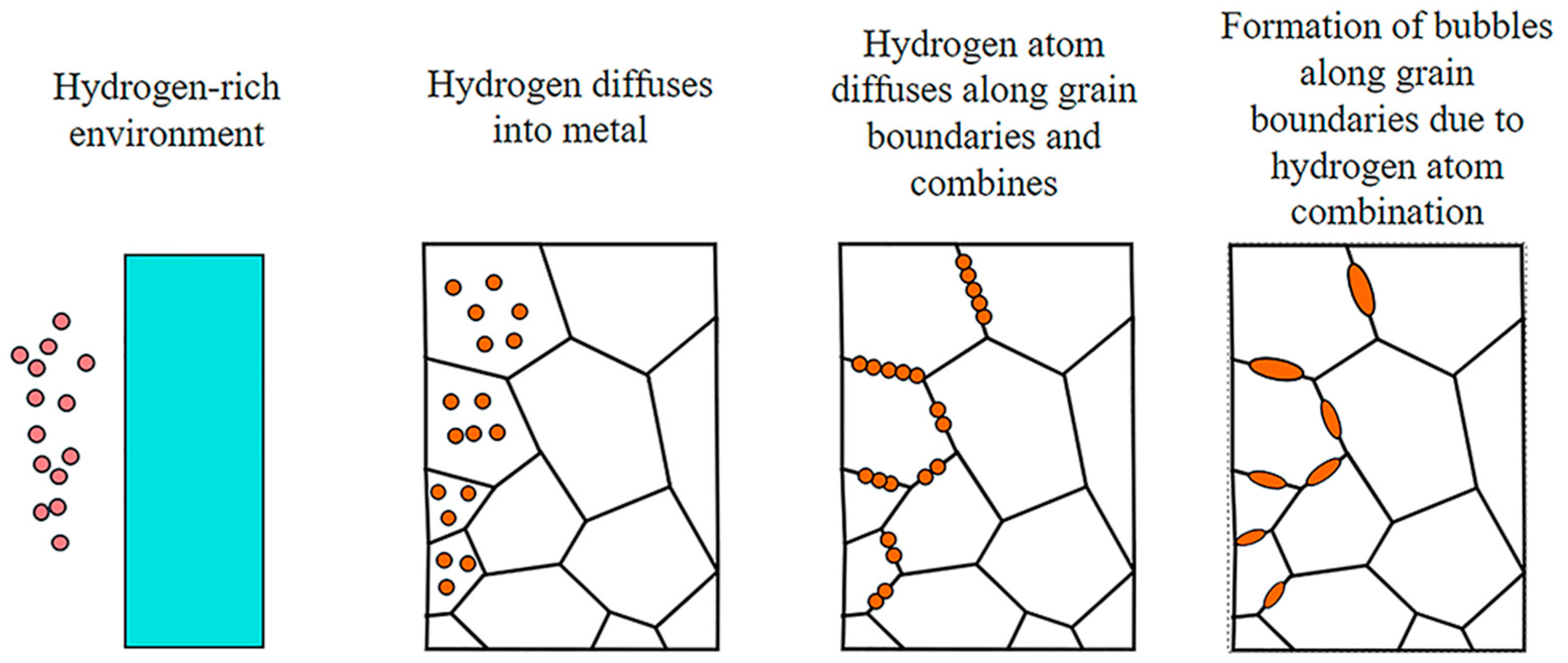
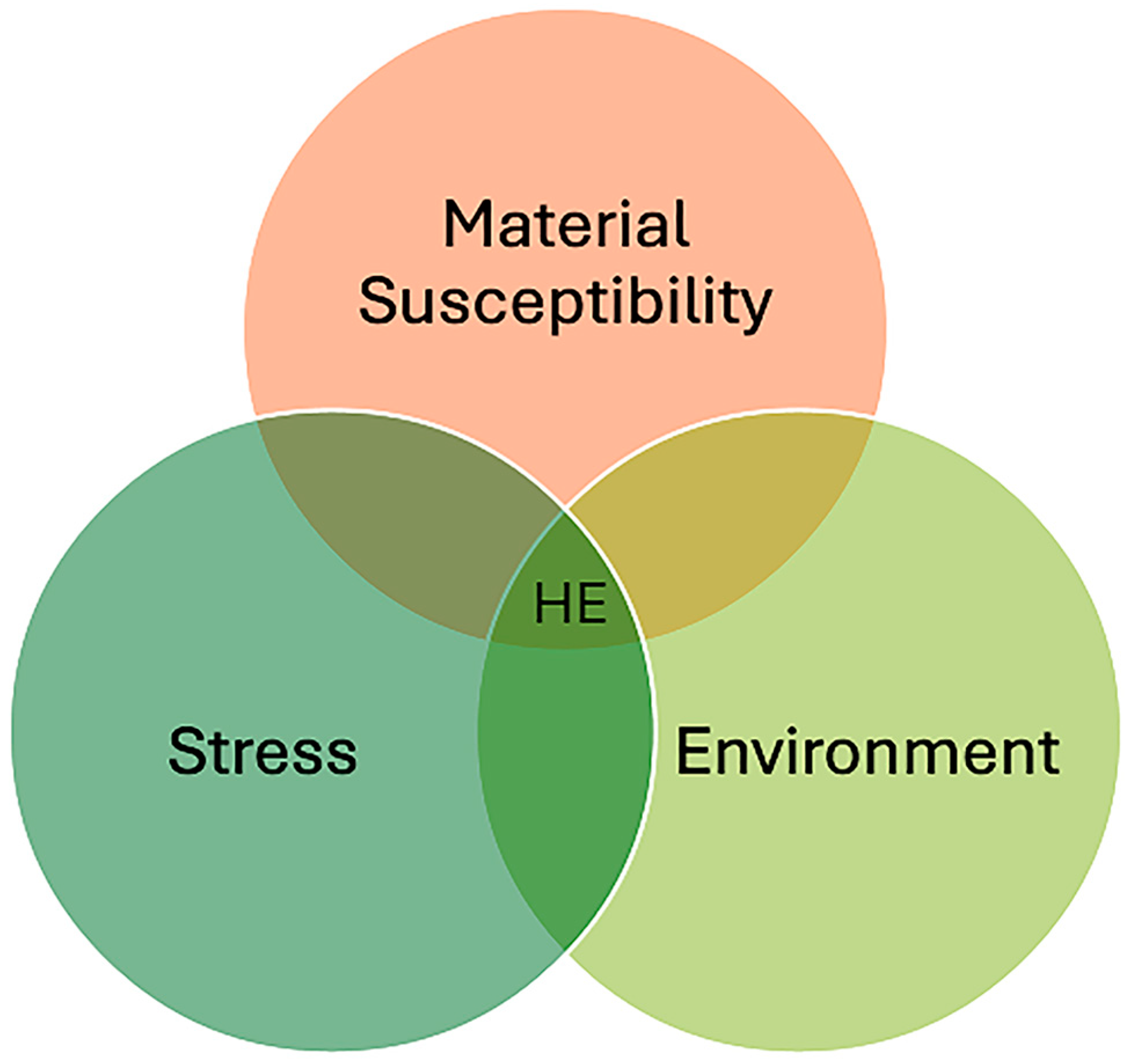




| Property | CH4 | H2 |
|---|---|---|
| Stoichiometric air/fuel ratio (kg) | 17.2 | 34.3 |
| Combustible range (%) | 5–15 | 6.7–36.0 |
| Flame temperature (°C) | 1914 | 2207 |
| Minimum spark ignition energy (mJ) | 0.30 | 0.017 |
| Autoignition temperature (°C) | 600 | 585 |
| Fuel | Energy Content [MJ/kg] | |
|---|---|---|
| Lower Heating Value | Higher Heating Value | |
| Gaseous hydrogen | 119.96 | 141.88 |
| Liquid hydrogen | 120.04 | 141.77 |
| Natural gas | 47.13 | 52.21 |
| Liquified natural gas | 48.62 | 55.19 |
| Still gas | 46.89 | 50.94 |
| Crude oil | 42.68 | 45.93 |
| Liquified petroleum gas (LPG) | 46.60 | 50.14 |
| Conventional gasoline | 43.44 | 46.52 |
| Reformulated or Low-sulfur gasoline (RFG) | 42.35 | 45.42 |
| Conventional diesel | 42.78 | 45.76 |
| Low-sulfur diesel | 42.60 | 45.56 |
| Coal (wet basis) | 22.73 | 23.96 |
| Bituminous coal (wet basis) | 26.12 | 27.26 |
| Coking coal (wet basis) | 28.60 | 29.86 |
| Methanol | 20.09 | 22.88 |
| Ethanol | 26.95 | 29.84 |
| Model | Key Features | Strengths | Limitations |
|---|---|---|---|
| HPT | Hydrogen atoms accumulate at defect sites, forming molecular hydrogen; which leads to increased local pressure and potential cracking. | Provides a clear mechanism for hydrogen-induced cracking (HIC) in non-austenitic steels. | Limited to non-austenitic steels; less applicable to other metal classes; questionable accuracy in general HE characterization. |
| HESIV | Hydrogen increases vacancy density, causing clusters that limit dislocation mobility and lead to premature fracture. | Highlights the role of vacancies in HE; explains premature fractures under strain. | Focuses on vacancy behavior, which might not capture all aspects of HE, particularly in different metal types. |
| HEDE | Hydrogen reduces interatomic strength, leading to intergranular fractures. | Provides insight into hydrogen-induced intergranular fracture and interface decohesion; Simple to apply. | Difficulty in measuring cohesive forces; may oversimplify the HE phenomenon. |
| HELP | Hydrogen facilitates dislocation motion; dislocation buildup at the crack tip leads to brittle fractures. | Widely accepted model; explains hydrogen-induced plastic deformation and brittle fracture. | Highly dependent on microstructure and stress intensity; limited by hydrogen clustering assumptions. |
| AIDE | Hydrogen adsorption weakens interatomic bonds near crack tips; combines elements of HEDE and HELP. | Integrates aspects of both decohesion and dislocation-based models, providing a more comprehensive view. | Complex mechanism; it may require more experimental validation to fully capture its applicability. |
| Dielectrics | PRF | Ds (mm) | Df m) | Ps ×10−11molH2 )−1 | Pf ×10−11molH2 )−1 |
|---|---|---|---|---|---|
| Al2O3 | 1000 | 0.5 | 1 | 1.30 | 25.9 |
| Cr2O3 | 1000 | 1.6 | 10 1 | 0.017 | 0.72 1 |
| Cr2O3/Al2O | 3500 | 0.5 | 1 | 1.30 | 7.41 |
| Er2O3 | 1000 | 0.5 | 1 | 1.30 | 25.9 |
| Er2O3 | 1000 | 0.5 | 1.3 | 1.30 | 33.7 |
| SiO2 | 1 | 0.15 | 0.2 | 0.13 | 1711 |
| BN | 100 | 0.1 | 1.5 | 0.13 | 193 |
| TiN | 100 | 0.1 | 1.5 | 0.13 | 193 |
| TiN | 1100 | 0.35 | 1.7 | 0.13 | 5.7 |
| TiN | 1000 | 0.1 | 1.7 | 0.13 | 21.8 |
| TiAlN | 6800 | 0.35 | 1.7 | 0.13 | 0.92 |
| TiAlN | 20,000 | 0.5 | 5 | 1.30 | 6.5 |
| SiN | 2000 | 0.5 | 0.5 | 1.30 | 6.5 |
| WN | 38 | 0.5 | 2.3 | 1.30 | 1570 |
| CrWN | 100 | 0.5 | 4.4 | 1.30 | 1140 |
| CrN | 117 | 0.5 | 2.6 | 1.30 | 576 |
| Cr2N | 286 | 0.5 | 2.2 | 1.30 | 241 |
| ALCrN | 350 | 0.5 | 4.5 | 1.30 | 333 |
| ZrN | 4600 | 0.5 | 1.4 | 1.30 | 7.9 |
| TiC | 10 | 0.1 | 1 | 0.27 | 2750 |
| TiN+TiC | 100 | 0.5 | 1 + 0.25 | 1.30 | 324 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honu, E.; Guo, S.; Rahman, S.; Zeng, C.; Mensah, P. Advancing Hydrogen Gas Utilization in Industrial Boilers: Impacts on Critical Boiler Components, Mitigation Measures, and Future Perspectives. Hydrogen 2024, 5, 574-623. https://doi.org/10.3390/hydrogen5030032
Honu E, Guo S, Rahman S, Zeng C, Mensah P. Advancing Hydrogen Gas Utilization in Industrial Boilers: Impacts on Critical Boiler Components, Mitigation Measures, and Future Perspectives. Hydrogen. 2024; 5(3):574-623. https://doi.org/10.3390/hydrogen5030032
Chicago/Turabian StyleHonu, Edem, Shengmin Guo, Shafiqur Rahman, Congyuan Zeng, and Patrick Mensah. 2024. "Advancing Hydrogen Gas Utilization in Industrial Boilers: Impacts on Critical Boiler Components, Mitigation Measures, and Future Perspectives" Hydrogen 5, no. 3: 574-623. https://doi.org/10.3390/hydrogen5030032







