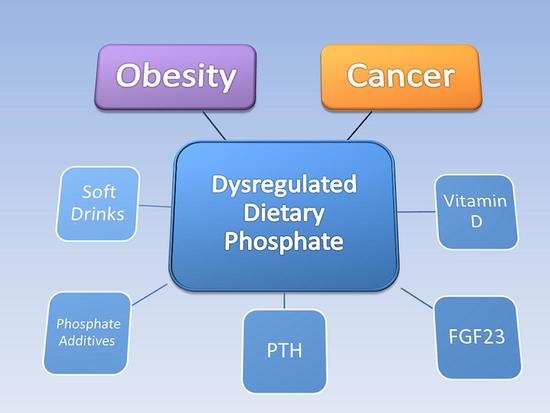
Obesity and Cancer: Potential Mediation by Dysregulated Dietary Phosphate
Abstract
:1. Introduction
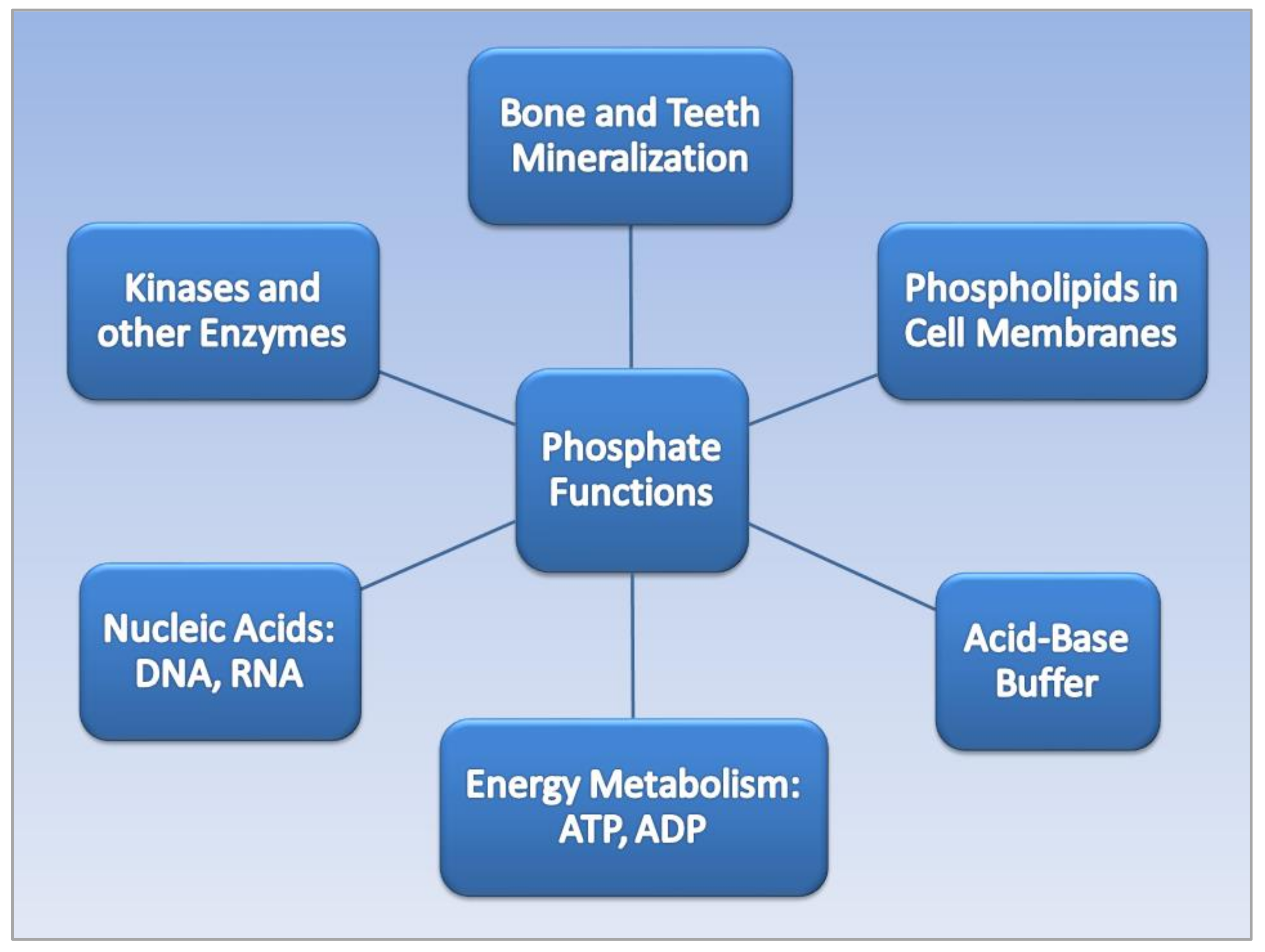
2. Phosphate Metabolism
2.1. Phosphate Functions
2.2. Phosphate Regulation and Dysregulation
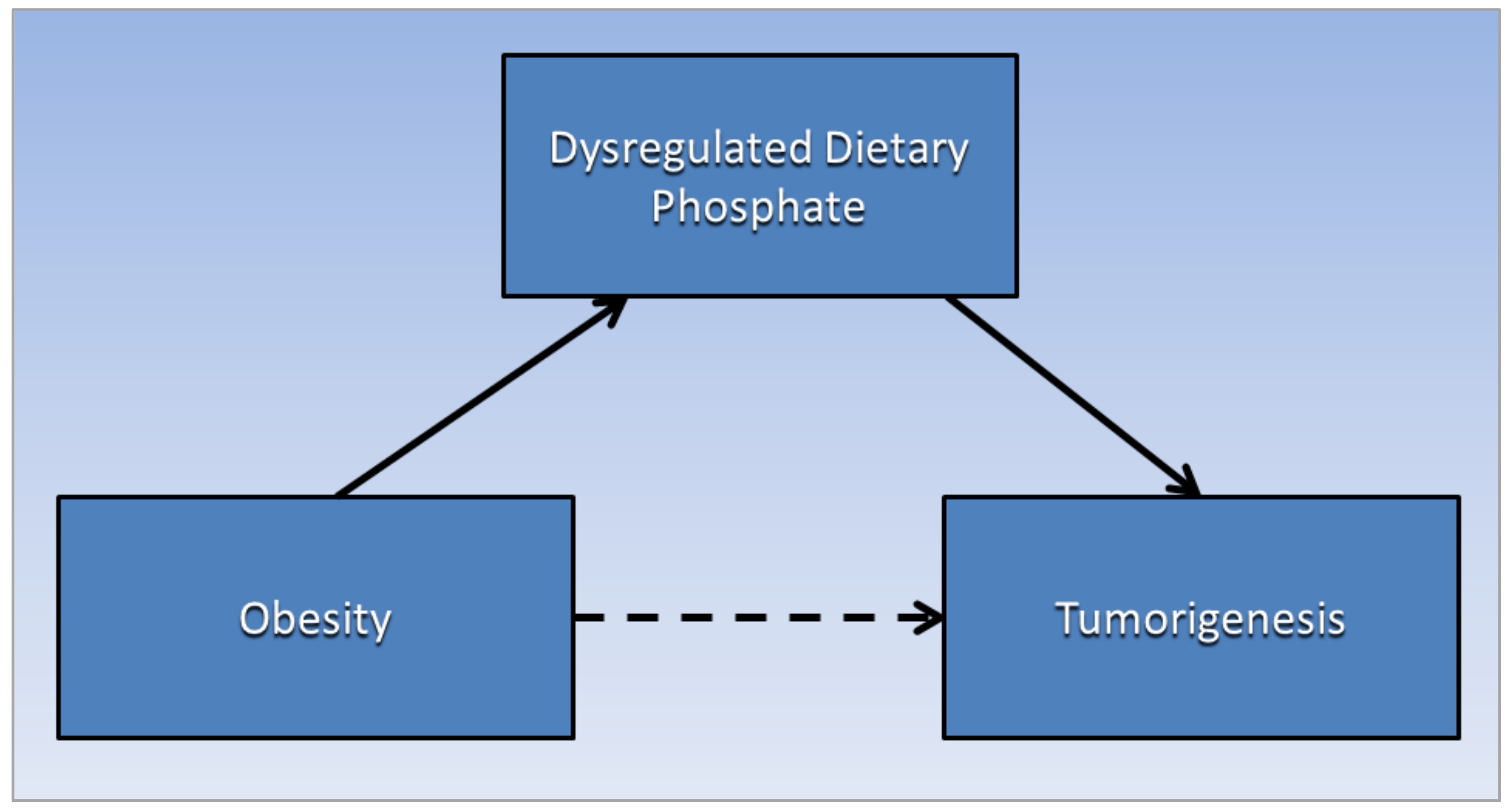
3. Phosphate Toxicity and Tumorigenesis
4. Phosphate Toxicity and Obesity
5. Pi, Ultra-Processed Food, and Obesity
6. Soft Drinks, Phosphoric Acid, and Obesity
Phosphoric Acid
7. Soft Drinks and Cancer
8. Phosphate Additives and Obesity
“In the 1990s, phosphorus-containing food additives contributed an estimated 470 mg per day to the average daily adult diet,” Chou said. “However, phosphates are currently being added much more frequently to a large number of processed foods, including meats, cheeses, beverages, and bakery products. As a result, depending on individual food choices, phosphorus intake could be increased by as much as 1000 mg per day” [84].
“A shift towards healthy dietary patterns has the potential to curtail the current unsustainable high level of obesity, cardiovascular disease, diabetes mellitus and cancer in the United States and around the globe” [85].
9. Summary and Future Directions
10. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- who.int. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 28 November 2021).
- Cancerresearchuk.Org. When could overweight and obesity overtake smoking as the biggest casue of cancer in the UK? Available online: https://www.cancerresearchuk.org/sites/default/files/obesity_tobacco_cross_over_report_final.pdf (accessed on 19 December 2021).
- Renehan, A.G.; Lloyd, K.; Renehan, I. Awareness of the link between obesity and cancer in UK school curricula. Lancet 2019, 393, 1591–1592. [Google Scholar] [CrossRef] [Green Version]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body Fatness and Cancer-Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Giovannucci, E.L. The Obesity Paradox in Cancer: Epidemiologic Insights and Perspectives. Curr. Nutr. Rep. 2019, 8, 175–181. [Google Scholar] [CrossRef]
- Stone, T.W.; McPherson, M.; Gail Darlington, L. Obesity and Cancer: Existing and New Hypotheses for a Causal Connection. EBioMedicine 2018, 30, 14–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, Y.; Hassan, F.; Ostrowski, M.C.; Mehta, K.D. Role of hepatic PKCβ in nutritional regulation of hepatic glycogen synthesis. JCI Insight 2021, 6, e149023. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.J.; Contreras, A.; Nguyen, L.T.; Eldon, E.D.; Klig, L.S. Regulated inositol synthesis is critical for balanced metabolism and development in Drosophila melanogaster. Biol. Open 2021, 10, bio058833. [Google Scholar] [CrossRef]
- Wolfswinkel, J.F.; Furtmueller, E.; Wilderom, C.P.M. Using grounded theory as a method for rigorously reviewing literature. Eur. J. Inf. Syst. 2013, 22, 45–55. [Google Scholar] [CrossRef]
- IOM. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes; National Academy of Sciences: Washington, DC, USA, 1997. [Google Scholar]
- Qadeer, H.A.; Bashir, K. Physiology, Phosphate; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Brown, R.B. Stress, inflammation, depression, and dementia associated with phosphate toxicity. Mol. Biol. Rep. 2020, 47, 9921–9929. [Google Scholar] [CrossRef]
- Saki, F.; Kassaee, S.R.; Salehifar, A.; Omrani, G.H.R. Interaction between serum FGF-23 and PTH in renal phosphate excretion, a case-control study in hypoparathyroid patients. BMC Nephrol. 2020, 21, 176. [Google Scholar] [CrossRef]
- Brown, R.B.; Razzaque, M.S. Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. BoneKEy Rep. 2015, 4, 705. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2018, 1869, 303–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, D.N.; Griffin, A.C. Phosphorus incorporation into nucleic acids and proteins of liver nuclei of normal and azo dye-fed rats. Cancer Res. 1955, 15, 456–461. [Google Scholar] [PubMed]
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R., Jr. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chudek, J.; Nagy, A.; Kokot, F.; Podwinski, A.; Wiecek, A.; Ritz, E.; Kovacs, G. Phosphatemia is related to chromosomal aberrations of parathyroid glands in patients with hyperparathyroidism. J. Nephrol. 2007, 20, 164–172. [Google Scholar]
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial inorganic phosphate as a tumor microenvironment marker for tumor progression. Sci. Rep. 2017, 7, 41233. [Google Scholar] [CrossRef]
- Levan, K.; Mehryar, M.; Mateoiu, C.; Albertsson, P.; Bäck, T.; Sundfeldt, K. Immunohistochemical evaluation of epithelial ovarian carcinomas identifies three different expression patterns of the MX35 antigen, NaPi2b. BMC Cancer 2017, 17, 303. [Google Scholar] [CrossRef]
- D’Arcangelo, M.; Brustugun, O.; Xiao, Y.; Choi, Y.; Behrens, C.; Solis, L.; Wang, Y.; Firestein, R.; Boyle, T.; Lund-Iversen, M. 194P prevalence and prognostic significance of sodium-dependent phosphate transporter 2B (NAPI2B) protein expression in non-small cell lung cancer (NSCLC). Ann. Oncol. 2014, 25, iv66–iv67. [Google Scholar] [CrossRef]
- Elser, J.J.; Kyle, M.M.; Smith, M.S.; Nagy, J.D. Biological stoichiometry in human cancer. PLoS ONE 2007, 2, e1028. [Google Scholar] [CrossRef] [Green Version]
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Papaloucas, C.; Papaloucas, M.; Kouloulias, V.; Neanidis, K.; Pistevou-Gompaki, K.; Kouvaris, J.; Zygogianni, A.; Mystakidou, K.; Papaloucas, A. Measurement of blood phosphorus: A quick and inexpensive method for detection of the existence of cancer in the body. Too good to be true, or forgotten knowledge of the past? Med. Hypotheses 2014, 82, 24–25. [Google Scholar] [CrossRef] [PubMed]
- Wulaningsih, W.; Michaelsson, K.; Garmo, H.; Hammar, N.; Jungner, I.; Walldius, G.; Holmberg, L.; Van Hemelrijck, M. Inorganic phosphate and the risk of cancer in the Swedish AMORIS study. BMC Cancer 2013, 13, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacerda-Abreu, M.A.; Russo-Abrahão, T.; Cosentino-Gomes, D.; Nascimento, M.T.C.; Carvalho-Kelly, L.F.; Gomes, T.; Rodrigues, M.F.; König, S.; Rumjanek, F.D.; Monteiro, R.Q.; et al. H(+)-dependent inorganic phosphate transporter in breast cancer cells: Possible functions in the tumor microenvironment. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Mansinho, A.; Ferreira, A.R.; Casimiro, S.; Alho, I.; Vendrell, I.; Costa, A.L.; Sousa, R.; Abreu, C.; Pulido, C.; Macedo, D.; et al. Levels of Circulating Fibroblast Growth Factor 23 (FGF23) and Prognosis in Cancer Patients with Bone Metastases. Int. J. Mol. Sci. 2019, 20, 695. [Google Scholar] [CrossRef] [Green Version]
- Li, J.R.; Chiu, K.Y.; Ou, Y.C.; Wang, S.S.; Chen, C.S.; Yang, C.K.; Ho, H.C.; Cheng, C.L.; Yang, C.R.; Chen, C.C.; et al. Alteration in serum concentrations of FGF19, FGF21, and FGF23 in patients with urothelial carcinoma. Biofactors 2019, 45, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.T.; Bang, W.J.; Seo, S.P.; Kang, H.W.; Byun, Y.J.; Piao, X.M.; Jeong, P.; Shin, K.S.; Choi, S.Y.; Lee, O.J.; et al. Parathyroid hormone is associated with prostate cancer. Prostate Int. 2020, 8, 116–120. [Google Scholar] [CrossRef]
- Kato, H.; Ito, N.; Makita, N.; Nangaku, M.; Leung, A.M.; Inoue, K. Association of Serum Parathyroid Hormone Levels With All-Cause and Cause-Specific Mortality Among U.S. Adults. Endocr. Pract. 2022, 28, 70–76. [Google Scholar] [CrossRef]
- Palmieri, S.; Roggero, L.; Cairoli, E.; Morelli, V.; Scillitani, A.; Chiodini, I.; Eller-Vainicher, C. Occurrence of malignant neoplasia in patients with primary hyperparathyroidism. Eur. J. Intern. Med. 2017, 43, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.A.; Alsiö, J.; Hammarstedt, A.; Erben, R.G.; Michaëlsson, K.; Tivesten, A.; Marsell, R.; Orwoll, E.; Karlsson, M.K.; Ljunggren, O.; et al. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Ministrini, S.; Ricci, M.A.; Daviddi, G.; Scavizzi, M.; De Vuono, S.; D’Abbondanza, M.; Paganelli, M.T.; Boni, M.; Roscini, A.R.; Scarponi, A.M.; et al. Determinants of High Parathyroid Hormone Levels in Patients With Severe Obesity and Their Relationship With the Cardiometabolic Risk Factors, Before and After a Laparoscopic Sleeve Gastrectomy Intervention. Obes. Surg. 2020, 30, 2225–2232. [Google Scholar] [CrossRef]
- Coluzzi, I.; Raparelli, L.; Guarnacci, L.; Paone, E.; Del Genio, G.; le Roux, C.W.; Silecchia, G. Food Intake and Changes in Eating Behavior After Laparoscopic Sleeve Gastrectomy. Obes. Surg. 2016, 26, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Jo, K.; Lim, D.J.; Lee, J.M.; Chang, S.A.; Kang, M.I.; Cha, B.Y.; Kim, M.H. Parathyroid hormone and vitamin D are associated with the risk of metabolic obesity in a middle-aged and older Korean population with preserved renal function: A cross-sectional study. PLoS ONE 2017, 12, e0175132. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ma, X.; Li, M.; Yan, S.; Zhao, H.; Pan, Y.; Wang, C.; Yao, Y.; Jin, L.; Li, B. Association between dietary mineral nutrient intake, body mass index, and waist circumference in U.S. adults using quantile regression analysis NHANES 2007-2014. PeerJ 2020, 8, e9127. [Google Scholar] [CrossRef] [PubMed]
- Hartman, M.-L.; Groppo, F.; Ohnishi, M.; Goodson, J.M.; Hasturk, H.; Tavares, M.; Yaskell, T.; Floros, C.; Behbehani, K.; Razzaque, M.S. Can salivary phosphate levels be an early biomarker to monitor the evolvement of obesity. In Phosphate and Vitamin D in Chronic Kidney Disease; Karger Publishers: Basel, Switzerland, 2013; Volume 180, pp. 138–148. [Google Scholar]
- Håglin, L.; Törnkvist, B.; Bäckman, L. Obesity, smoking habits, and serum phosphate levels predicts mortality after life-style intervention. PLoS ONE 2020, 15, e0227692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoian, M.; Stoica, V. The role of disturbances of phosphate metabolism in metabolic syndrome. Maedica 2014, 9, 255–260. [Google Scholar] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- García-Estévez, L.; Cortés, J.; Pérez, S.; Calvo, I.; Gallegos, I.; Moreno-Bueno, G. Obesity and Breast Cancer: A Paradoxical and Controversial Relationship Influenced by Menopausal Status. Front. Oncol. 2021, 11, 705911. [Google Scholar] [CrossRef]
- Piiroinen, O.; Kaihola, H.L. Uterine size measured by ultrasound during the menstrual cycle. Acta Obstet. Gynecol. Scand. 1975, 54, 247–250. [Google Scholar] [CrossRef]
- Gorczyca, A.M.; Sjaarda, L.A.; Mitchell, E.M.; Perkins, N.J.; Schliep, K.C.; Wactawski-Wende, J.; Mumford, S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016, 55, 1181–1188. [Google Scholar] [CrossRef]
- Shanmugalingam, T.; Crawley, D.; Bosco, C.; Melvin, J.; Rohrmann, S.; Chowdhury, S.; Holmberg, L.; Van Hemelrijck, M. Obesity and cancer: The role of vitamin D. BMC Cancer 2014, 14, 712. [Google Scholar] [CrossRef] [Green Version]
- Olanbiwonnu, T.; Holden, R.M. Inorganic phosphate as a potential risk factor for chronic disease. Cmaj 2018, 190, E784–E785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritz, E.; Hahn, K.; Ketteler, M.; Kuhlmann, M.K.; Mann, J. Phosphate additives in food—A health risk. Dtsch. Arztebl. Int. 2012, 109, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Adams, J. Addressing socioeconomic inequalities in obesity: Democratising access to resources for achieving and maintaining a healthy weight. PLoS Med. 2020, 17, e1003243. [Google Scholar] [CrossRef] [PubMed]
- Rauber, F.; Chang, K.; Vamos, E.P.; da Costa Louzada, M.L.; Monteiro, C.A.; Millett, C.; Levy, R.B. Ultra-processed food consumption and risk of obesity: A prospective cohort study of UK Biobank. Eur. J. Nutr. 2021, 60, 2169–2180. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Srour, B.; Sellem, L.; Kesse-Guyot, E.; Allès, B.; Méjean, C.; Deschasaux, M.; Fassier, P.; Latino-Martel, P.; Beslay, M.; et al. Consumption of ultra-processed foods and cancer risk: Results from NutriNet-Santé prospective cohort. BMJ 2018, 360, k322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, J.; Smith, N.; Chung, J.; Kalantar-Zadeh, K. Evaluation Of Serum Phosphorus Levels And Obesity. Kidney Res. Clin. Pract. 2012, 31, A74. [Google Scholar] [CrossRef] [Green Version]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; et al. Ultra-Processed Diets Cause Excess Calorie Intake and Weight Gain: An Inpatient Randomized Controlled Trial of Ad Libitum Food Intake. Cell Metab. 2019, 30, 67–77.e63. [Google Scholar] [CrossRef] [Green Version]
- Hu, F.B. Resolved: There is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes. Rev. 2013, 14, 606–619. [Google Scholar] [CrossRef]
- Sánchez-Pimienta, T.G.; Batis, C.; Lutter, C.K.; Rivera, J.A. Sugar-Sweetened Beverages Are the Main Sources of Added Sugar Intake in the Mexican Population. J. Nutr. 2016, 146, 1888S–1896S. [Google Scholar] [CrossRef] [Green Version]
- Malik, V.S.; Schulze, M.B.; Hu, F.B. Intake of sugar-sweetened beverages and weight gain: A systematic review. Am. J. Clin. Nutr. 2006, 84, 274–288. [Google Scholar] [CrossRef]
- Keller, A.; Bucher Della Torre, S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Child. Obes. 2015, 11, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Luger, M.; Lafontan, M.; Bes-Rastrollo, M.; Winzer, E.; Yumuk, V.; Farpour-Lambert, N. Sugar-Sweetened Beverages and Weight Gain in Children and Adults: A Systematic Review from 2013 to 2015 and a Comparison with Previous Studies. Obes. Facts. 2017, 10, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Ruanpeng, D.; Thongprayoon, C.; Cheungpasitporn, W.; Harindhanavudhi, T. Sugar and artificially sweetened beverages linked to obesity: A systematic review and meta-analysis. QJM: An. Int. J. Med. 2017, 110, 513–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, P.; Li, Q.; Zhao, Y.; Chen, Q.; Sun, X.; Liu, Y.; Li, H.; Wang, T.; Chen, X.; Zhou, Q.; et al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: A dose-response meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 655–671. [Google Scholar] [CrossRef]
- Mullee, A.; Romaguera, D.; Pearson-Stuttard, J.; Viallon, V.; Stepien, M.; Freisling, H.; Fagherazzi, G.; Mancini, F.R.; Boutron-Ruault, M.C.; Kühn, T.; et al. Association Between Soft Drink Consumption and Mortality in 10 European Countries. JAMA Intern. Med. 2019, 179, 1479–1490. [Google Scholar] [CrossRef] [Green Version]
- Amato, D.; Maravilla, A.; García-Contreras, F.; Paniagua, R. Soft-drinks and health. Rev. Investig. Clin. 1997, 49, 387–395. [Google Scholar]
- Alkhedaide, A.; Soliman, M.M.; Salah-Eldin, A.E.; Ismail, T.A.; Alshehiri, Z.S.; Attia, H.F. Chronic effects of soft drink consumption on the health state of Wistar rats: A biochemical, genetic and histopathological study. Mol. Med. Rep. 2016, 13, 5109–5117. [Google Scholar] [CrossRef] [Green Version]
- Elsherif, M.T.; Tawfik, M.S.; Gomaa, R.S.; elmalkey, n.f. Evaluation of Chronic Cola Consumption Effect on the Liver and Kidney Functions in Adult Male Rats: Role of Serum 25-hydroxyvitamin D. Zagazig Univ. Med. J. 2021, 27, 305–315. [Google Scholar] [CrossRef]
- Mazariegos-Ramos, E.; Guerrero-Romero, F.; Rodríguez-Morán, M.; Lazcano-Burciaga, G.; Paniagua, R.; Amato, D. Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in children: A case-control study. J. Pediatr. 1995, 126, 940–942. [Google Scholar] [CrossRef]
- Mazariegos-Ramos, E.; Rodriguez-Moran, M.; Guerrero-Romero, J.; Paniagua, R. Calcium and phosphate metabolism disorders secondary to consumption of soft drinks with phosphoric acid. Bol. Med.-Hosp. Infant. De Mex. 1995, 52, 6. [Google Scholar]
- Fernando, G.R.; Martha, R.M.; Evangelina, R. Consumption of soft drinks with phosphoric acid as a risk factor for the development of hypocalcemia in postmenopausal women. J. Clin. Epidemiol. 1999, 52, 1007–1010. [Google Scholar] [CrossRef]
- Guarnotta, V.; Riela, S.; Massaro, M.; Bonventre, S.; Inviati, A.; Ciresi, A.; Pizzolanti, G.; Benvenga, S.; Giordano, C. The Daily Consumption of Cola Can Determine Hypocalcemia: A Case Report of Postsurgical Hypoparathyroidism-Related Hypocalcemia Refractory to Supplemental Therapy with High Doses of Oral Calcium. Front. Endocrinol. 2017, 8, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tucker, K.L.; Morita, K.; Qiao, N.; Hannan, M.T.; Cupples, L.A.; Kiel, D.P. Colas, but not other carbonated beverages, are associated with low bone mineral density in older women: The Framingham Osteoporosis Study. Am. J. Clin. Nutr. 2006, 84, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Park, Y.K. Sugar-sweetened beverage consumption and bone health: A systematic review and meta-analysis. Nutr. J. 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Yoo, J.H. Associations between cola consumption and bone mineral density in Korean adolescents and young adults: A cross-sectional study using data from the Korea National Health and Nutrition Examination Survey, 2008–2011. J. Nutr. Sci. 2020, 9, e56. [Google Scholar] [CrossRef]
- Belpoggi, F.; Soffritti, M.; Tibaldi, E.; Falcioni, L.; Bua, L.; Trabucco, F. Results of long-term carcinogenicity bioassays on Coca-Cola administered to Sprague-Dawley rats. Ann. N. Y. Acad. Sci. 2006, 1076, 736–752. [Google Scholar] [CrossRef] [Green Version]
- Chazelas, E.; Srour, B.; Desmetz, E.; Kesse-Guyot, E.; Julia, C.; Deschamps, V.; Druesne-Pecollo, N.; Galan, P.; Hercberg, S.; Latino-Martel, P.; et al. Sugary drink consumption and risk of cancer: Results from NutriNet-Santé prospective cohort. BMJ 2019, 366, l2408. [Google Scholar] [CrossRef] [Green Version]
- Inoue-Choi, M.; Robien, K.; Mariani, A.; Cerhan, J.R.; Anderson, K.E. Sugar-sweetened beverage intake and the risk of type I and type II endometrial cancer among postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2384–2394. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.; Otegbeye, E.; Joh, H.K.; Nimptsch, K.; Ng, K.; Ogino, S.; Meyerhardt, J.A.; Chan, A.T.; Willett, W.C.; Wu, K.; et al. Sugar-sweetened beverage intake in adulthood and adolescence and risk of early-onset colorectal cancer among women. Gut 2021, 70, 2330–2336. [Google Scholar] [CrossRef]
- Li, Y.; Guo, L.; He, K.; Huang, C.; Tang, S. Consumption of sugar-sweetened beverages and fruit juice and human cancer: A systematic review and dose-response meta-analysis of observational studies. J. Cancer 2021, 12, 3077–3088. [Google Scholar] [CrossRef]
- Jatho, A.; Cambia, J.M.; Myung, S.K. Consumption of artificially sweetened soft drinks and risk of gastrointestinal cancer: A meta-analysis of observational studies. Public Health Nutr. 2021, 24, 6122–6136. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Duarte-Salles, T.; Fedirko, V.; Trichopoulou, A.; Lagiou, P.; Bamia, C.; Overvad, K.; Tjønneland, A.; Hansen, L.; Boutron-Ruault, M.C.; et al. Consumption of soft drinks and juices and risk of liver and biliary tract cancers in a European cohort. Eur. J. Nutr. 2016, 55, 7–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, M.C.; Louzada, M.L.; de Sá, T.H.; Laverty, A.A.; Parra, D.C.; Garzillo, J.M.; Monteiro, C.A.; Millett, C. Artificially Sweetened Beverages and the Response to the Global Obesity Crisis. PLoS Med. 2017, 14, e1002195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulgoni, K.; Fulgoni, V.L., 3rd. Trends in Total, Added, and Natural Phosphorus Intake in Adult Americans, NHANES 1988-1994 to NHANES 2015-2016. Nutrients 2021, 13, 2249. [Google Scholar] [CrossRef]
- Beydoun, M.A.; Fanelli-Kuczmarski, M.T.; Beydoun, H.A.; Dore, G.A.; Canas, J.A.; Evans, M.K.; Zonderman, A.B. Dairy product consumption and its association with metabolic disturbance in a prospective study of urban adults. Br. J. Nutr. 2018, 119, 706–719. [Google Scholar] [CrossRef] [Green Version]
- Beydoun, M.A.; Gary, T.L.; Caballero, B.H.; Lawrence, R.S.; Cheskin, L.J.; Wang, Y. Ethnic differences in dairy and related nutrient consumption among US adults and their association with obesity, central obesity, and the metabolic syndrome. Am. J. Clin. Nutr. 2008, 87, 1914–1925. [Google Scholar] [CrossRef] [Green Version]
- Pereira Dde, C.; Lima, R.P.; de Lima, R.T.; Gonçalves Mda, C.; de Morais, L.C.; Franceschini Sdo, C.; Filizola, R.G.; de Moraes, R.M.; Asciutti, L.S.; Costa, M.J. Association between obesity and calcium:phosphorus ratio in the habitual diets of adults in a city of Northeastern Brazil: An epidemiological study. Nutr. J. 2013, 12, 90. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.; Kumagai, T.; Kimura, M.; Koike, S.; Shimizu, T. Dietary Intake in Body Mass Index Differences in Community-Based Japanese Patients with Schizophrenia. Iran. J. Public Health 2015, 44, 639–645. [Google Scholar]
- Sciencedaily.com. Common Food Additive Found To Increase Risk And Speed Spread Of Lung Cancer. Amercian Thoracic Society. Available online: https://www.sciencedaily.com/releases/2008/12/081229080851.htm#:~:text=to%20the%20disease.-,New%20research%20in%20an%20animal%20model%20suggests%20that%20a%20diet,tumors%20in%20individuals%20predisposed%20to (accessed on 22 December 2021).
- Neuhouser, M.L. The importance of healthy dietary patterns in chronic disease prevention. Nutr. Res. 2019, 70, 3–6. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Kotsis, V.; Martinez, F.; Trakatelli, C.; Redon, J. Impact of Obesity in Kidney Diseases. Nutrients 2021, 13, 4482. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.; Turley, E.A.; Baum, M. A new biological framework for cancer research. Lancet 1996, 348, 1149–1151. [Google Scholar] [CrossRef]
- Molina, P.; Gavela, E.; Vizcaíno, B.; Huarte, E.; Carrero, J.J. Optimizing Diet to Slow CKD Progression. Front. Med. 2021, 8, 654250. [Google Scholar] [CrossRef] [PubMed]
| Bone | Kidney | Parathyroid | Intestine |
|---|---|---|---|
| FGF23 inhibits serum Pi reabsorption in kidneys. | Regulates serum Pi reabsorption and Pi urinary excretion. | PTH increases serum calcium through bone resorption. | Pi absorption from dietary sources. |
| Increases Pi urinary excretion. | Biosynthesis of calcitriol from vitamin D3 increases Pi intestinal absorption. | Increases Pi urinary excretion with FGF23. | Type II sodium-dependent phosphate cotransporters. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, R.B. Obesity and Cancer: Potential Mediation by Dysregulated Dietary Phosphate. Obesities 2022, 2, 64-75. https://doi.org/10.3390/obesities2010007
Brown RB. Obesity and Cancer: Potential Mediation by Dysregulated Dietary Phosphate. Obesities. 2022; 2(1):64-75. https://doi.org/10.3390/obesities2010007
Chicago/Turabian StyleBrown, Ronald B. 2022. "Obesity and Cancer: Potential Mediation by Dysregulated Dietary Phosphate" Obesities 2, no. 1: 64-75. https://doi.org/10.3390/obesities2010007
APA StyleBrown, R. B. (2022). Obesity and Cancer: Potential Mediation by Dysregulated Dietary Phosphate. Obesities, 2(1), 64-75. https://doi.org/10.3390/obesities2010007