Intermittent Energy Restriction Combined with a High-Protein/Low-Protein Diet: Effects on Body Weight, Satiety, and Inflammation: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
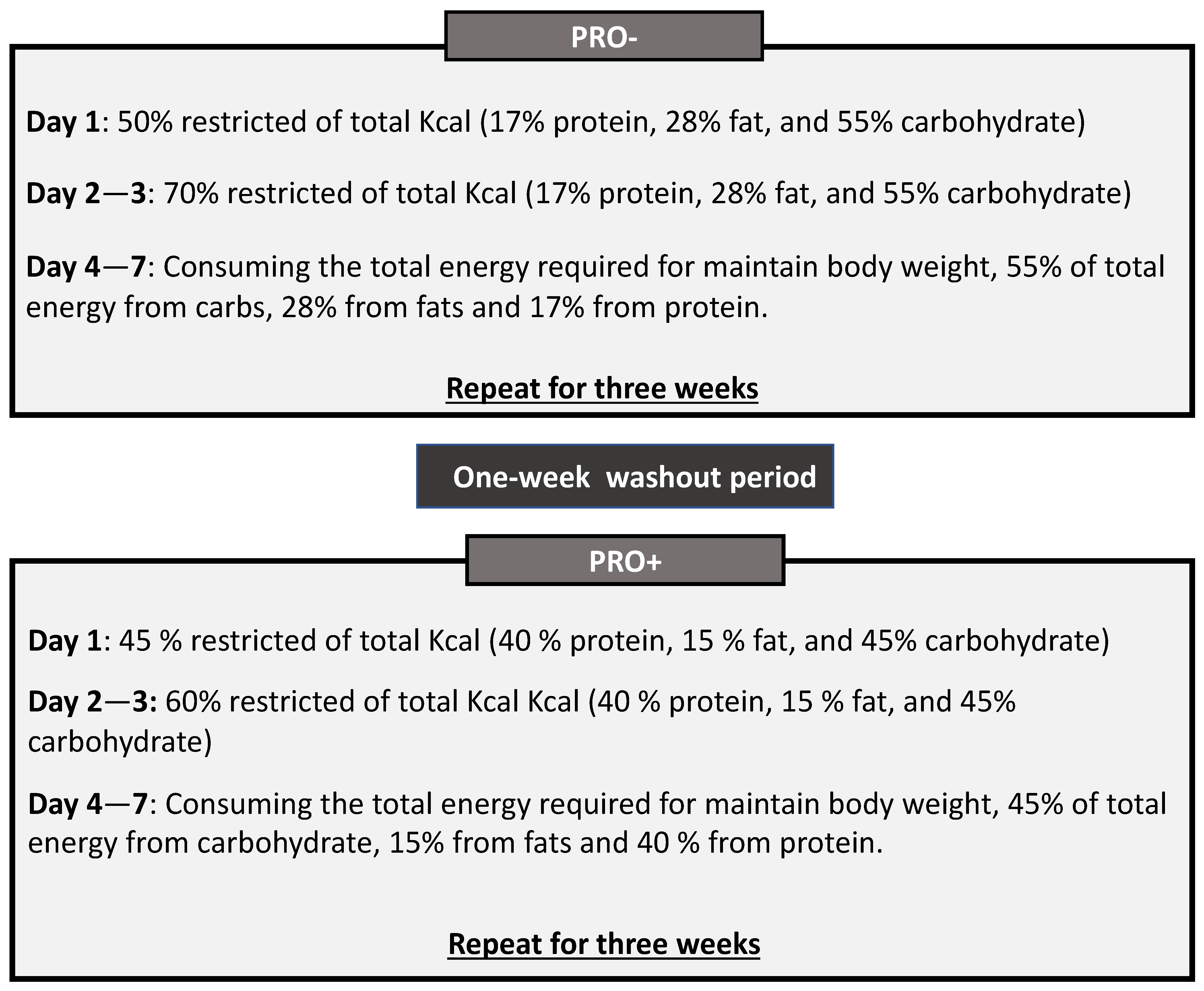
2.3. Dietary Interventions
2.3.1. PRO− Diet
2.3.2. PRO+ Diet
2.4. Anthropometric Measures
2.5. Blood Tests
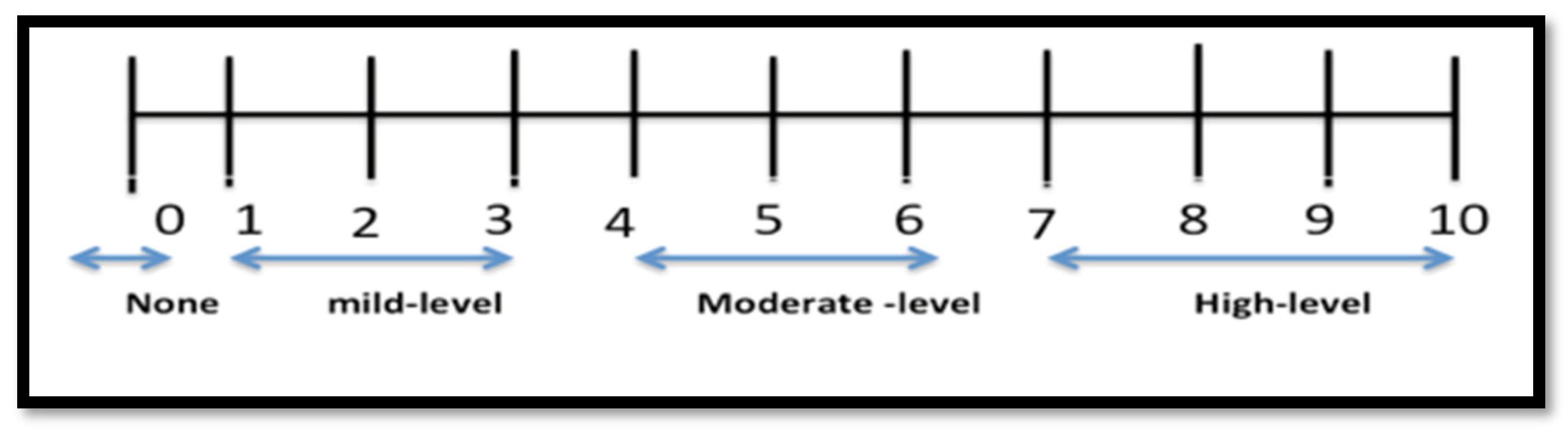
2.6. Hunger, Satisfaction, and Fullness
2.7. Adherence
2.8. Statistical Analysis
3. Results
3.1. Participants
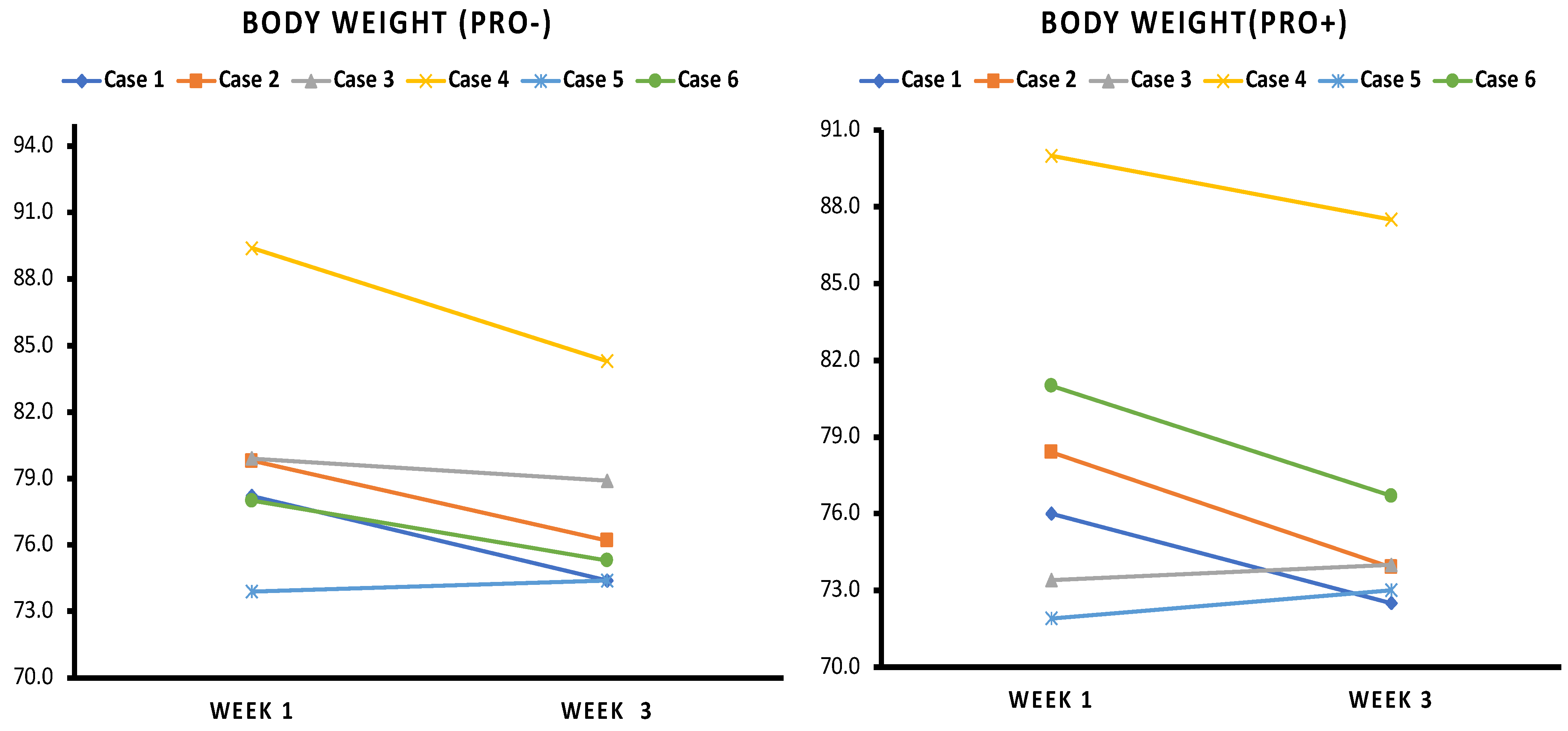
3.2. Body Weight
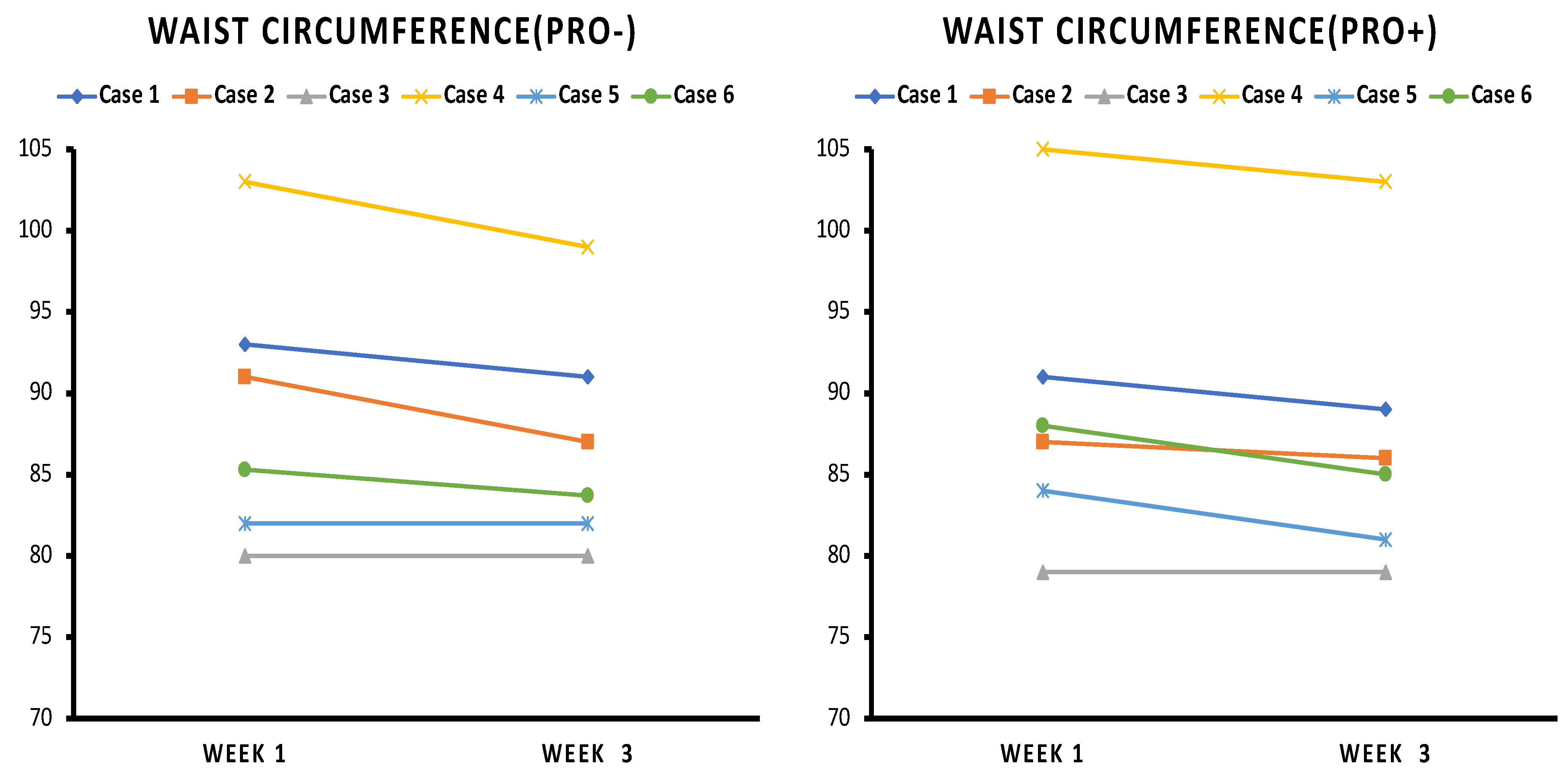
3.3. Waist Circumference
3.4. CRP
3.5. Fasting Glucose
3.6. Satiety
3.7. Effect of Order of Rotation on Results
3.8. Additional Observations
4. Discussion
4.1. Weight Loss and Waist Circumference
4.2. CRP
4.3. Glucose
4.4. Adherence
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Astrup, A. Carbohydrates as Macronutrients in Relation to Protein and Fat for Body Weight Control. Int. J. Obes. 2006, 30, S4–S9. [Google Scholar] [CrossRef]
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, Weight Management, and Satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef] [PubMed]
- Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 22 May 2021).
- NCD Risk Factor Collaboration (NCD-RisC). Trends in Adult Body-Mass Index in 200 Countries from 1975 to 2014: A Pooled Analysis of 1698 Population-Based Measurement Studies with 19·2 Million Participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Oliveira, T.; Fernandes, R. Biochemistry of Adipose Tissue: An Endocrine Organ. Arch. Med. Sci. 2013, 9, 191–200. [Google Scholar] [CrossRef]
- Kroeger, C.M.; Klempel, M.C.; Bhutani, S.; Trepanowski, J.F.; Tangney, C.C.; Varady, K.A. Improvement in Coronary Heart Disease Risk Factors during an Intermittent Fasting/Calorie Restriction Regimen: Relationship to Adipokine Modulations. Nutr. Metab. 2012, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Harvie, M.; Wright, C.; Pegington, M.; McMullan, D.; Mitchell, E.; Martin, B.; Cutler, R.G.; Evans, G.; Whiteside, S.; Maudsley, S.; et al. The Effect of Intermittent Energy and Carbohydrate Restriction v. Daily Energy Restriction on Weight Loss and Metabolic Disease Risk Markers in Overweight Women. Br. J. Nutr. 2013, 110, 1534–1547. [Google Scholar] [CrossRef]
- Stanek, A.; Brożyna-Tkaczyk, K.; Zolghadri, S.; Cholewka, A.; Myśliński, W. The Role of Intermittent Energy Restriction Diet on Metabolic Profile and Weight Loss among Obese Adults. Nutrients 2022, 14, 1509. [Google Scholar] [CrossRef]
- Muñoz-Hernández, L.; Márquez-López, Z.; Mehta, R.; Aguilar-Salinas, C.A. Intermittent Fasting as Part of the Management for T2DM: From Animal Models to Human Clinical Studies. Curr. Diab. Rep. 2020, 20, 13. [Google Scholar] [CrossRef]
- Keenan, S.; Cooke, M.B.; Chen, W.S.; Wu, S.; Belski, R. The Effects of Intermittent Fasting and Continuous Energy Restriction with Exercise on Cardiometabolic Biomarkers, Dietary Compliance, and Perceived Hunger and Mood: Secondary Outcomes of a Randomised, Controlled Trial. Nutrients 2022, 14, 3071. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, F.; Chen, H.; Liu, L.; Zhang, S.; Luo, W.; Wang, G.; Hu, X. Comparison of the Effects of Intermittent Energy Restriction and Continuous Energy Restriction among Adults with Overweight or Obesity: An Overview of Systematic Reviews and Meta-Analyses. Nutrients 2022, 14, 2315. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Cooper, A.; Lee, I.; Cernoch, C.A.; Huntoon, G.; Hodek, B.; Christian, H.; Chao, A.M. Intermittent Energy Restriction for Weight Loss: A Systematic Review of Cardiometabolic, Inflammatory and Appetite Outcomes. Biol. Res. Nurs. 2022, 24, 410–428. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, L.; Chiara, M.; Sesca, E.; Premoselli, F.; Binasco, V.; Dianzani, M.U. Fasting during Promotion, but Not during Initiation, Enhances the Growth of Methylnitrosourea-Induced Mammary Tumours. Carcinogenesis 1997, 18, 1679–1681. [Google Scholar] [CrossRef] [PubMed]
- Rosas Fernández, M.; Concha Vilca, C.; Batista, L.O.; Tavares do Carmo, M.d.G.; de Albuquerque, K.T. Intermittent Food Restriction Upregulates Critical Hypothalamic Genes Involved in Energy Regulation Imbalance. Nutrition 2023, 110, 112006. [Google Scholar] [CrossRef]
- Gibson, A.A.; Sainsbury, A. Strategies to Improve Adherence to Dietary Weight Loss Interventions in Research and Real-World Settings. Behav. Sci. 2017, 7, E44. [Google Scholar] [CrossRef]
- Dombrowski, S.U.; Knittle, K.; Avenell, A.; Araújo-Soares, V.; Sniehotta, F.F. Long Term Maintenance of Weight Loss with Non-Surgical Interventions in Obese Adults: Systematic Review and Meta-Analyses of Randomised Controlled Trials. BMJ 2014, 348, g2646. [Google Scholar] [CrossRef]
- Lemstra, M.; Bird, Y.; Nwankwo, C.; Rogers, M.; Moraros, J. Weight Loss Intervention Adherence and Factors Promoting Adherence: A Meta-Analysis. Patient Prefer. Adherence 2016, 10, 1547–1559. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The Role of Protein in Weight Loss and Maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef]
- Jéquier, E. Carbohydrates as a Source of Energy. Am. J. Clin. Nutr. 1994, 59, 682S–685S. [Google Scholar] [CrossRef]
- Holdcroft, A. Gender Bias in Research: How Does It Affect Evidence Based Medicine? J. R. Soc. Med. 2007, 100, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.S.; Lejeune, M.P.G.M.; Smeets, A.J.P.G.; Luscombe-Marsh, N.D. Sex Differences in Energy Homeostatis Following a Diet Relatively High in Protein Exchanged with Carbohydrate, Assessed in a Respiration Chamber in Humans. Physiol. Behav. 2009, 97, 414–419. [Google Scholar] [CrossRef]
- Bédard, A.; Hudon, A.-M.; Drapeau, V.; Corneau, L.; Dodin, S.; Lemieux, S. Gender Differences in the Appetite Response to a Satiating Diet. J. Obes. 2015, 2015, 140139. [Google Scholar] [CrossRef] [PubMed]
- Rolls, B.J.; McDermott, T.M. Effects of Age on Sensory-Specific Satiety. Am. J. Clin. Nutr. 1991, 54, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Pilgrim, A.L.; Robinson, S.M.; Sayer, A.A.; Roberts, H.C. An Overview of Appetite Decline in Older People. Nurs. Older People 2015, 27, 29–35. [Google Scholar] [CrossRef]
- Kitahara, C.M.; Flint, A.J.; Berrington de Gonzalez, A.; Bernstein, L.; Brotzman, M.; MacInnis, R.J.; Moore, S.C.; Robien, K.; Rosenberg, P.S.; Singh, P.N.; et al. Association between Class III Obesity (BMI of 40–59 Kg/M2) and Mortality: A Pooled Analysis of 20 Prospective Studies. PLoS Med. 2014, 11, e1001673. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean Diet Attenuates Inflammation and Coagulation Process in Healthy Adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Comparison of Waist Circumference Using the World Health Organization and National Institutes of Health Protocols. Available online: https://www150.statcan.gc.ca/n1/pub/82-003-x/2012003/article/11707-eng.htm (accessed on 16 February 2022).
- Nichols, S.D.; Crichlow, H. An Evaluation of the Diagnostic Utility of Anthropometric and Body Composition Cut-off Values in Assessing Elevated Fasting Blood Sugar and Blood Pressure. West Indian Med. J. 2010, 59, 253–258. [Google Scholar]
- Witjaksono, F.; Jutamulia, J.; Annisa, N.G.; Prasetya, S.I.; Nurwidya, F. Comparison of Low Calorie High Protein and Low Calorie Standard Protein Diet on Waist Circumference of Adults with Visceral Obesity and Weight Cycling. BMC Res. Notes 2018, 11, 674. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Petermann, F.; Steell, L.; Anderson, J.; Welsh, P.; Mackay, D.F.; Iliodromiti, S.; Lyall, D.M.; Lean, M.E.; Pell, J.P.; et al. Associations of Dietary Protein Intake with Fat-Free Mass and Grip Strength: A Cross-Sectional Study in 146,816 UK Biobank Participants. Am. J. Epidemiol. 2018, 187, 2405–2414. [Google Scholar] [CrossRef]
- Shivappa, N.; Hébert, J.R.; Rietzschel, E.R.; De Buyzere, M.L.; Langlois, M.; Debruyne, E.; Marcos, A.; Huybrechts, I. Associations between Dietary Inflammatory Index and Inflammatory Markers in the Asklepios Study. Br. J. Nutr. 2015, 113, 665–671. [Google Scholar] [CrossRef]
- Hart, M.J.; Torres, S.J.; McNaughton, S.A.; Milte, C.M. Dietary Patterns and Associations with Biomarkers of Inflammation in Adults: A Systematic Review of Observational Studies. Nutr. J. 2021, 20, 24. [Google Scholar] [CrossRef]
- Rankin, J.W.; Turpyn, A.D. Low Carbohydrate, High Fat Diet Increases c-Reactive Protein during Weight Loss. J. Am. Coll. Nutr. 2007, 26, 163–169. [Google Scholar] [CrossRef]
- Pereira, M.; Swain, J.; Goldfine, A.; Rifai, N.; Ludwig, D. Effects of a Low-Glycemic Load Diet on Resting Energy Expenditure and Heart Disease Risk Factors during Weight Loss-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15562127/ (accessed on 1 April 2023).
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-Term Effects of a Very-Low-Carbohydrate Weight Loss Diet Compared with an Isocaloric Low-Fat Diet after 12 Mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Selvin, E.; Paynter, N.P.; Erlinger, T.P. The Effect of Weight Loss on C-Reactive Protein: A Systematic Review. Arch. Intern. Med. 2007, 167, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Kusminski, C.M.; Bickel, P.E.; Scherer, P.E. Targeting Adipose Tissue in the Treatment of Obesity-Associated Diabetes. Nat. Rev. Drug Discov. 2016, 15, 639–660. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The Effects of Intermittent or Continuous Energy Restriction on Weight Loss and Metabolic Disease Risk Markers: A Randomized Trial in Young Overweight Women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Georg Jensen, M.; Kristensen, M.; Astrup, A. Effect of Alginate Supplementation on Weight Loss in Obese Subjects Completing a 12-Wk Energy-Restricted Diet: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2012, 96, 5–13. [Google Scholar] [CrossRef]
- Steven, S.; Taylor, R. Restoring Normoglycaemia by Use of a Very Low Calorie Diet in Long- and Short-Duration Type 2 Diabetes. Diabet. Med. J. Br. Diabet. Assoc. 2015, 32, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of Type 2 Diabetes: Normalisation of Beta Cell Function in Association with Decreased Pancreas and Liver Triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef] [PubMed]
- Ritzel, R.A.; Butler, A.E.; Rizza, R.A.; Veldhuis, J.D.; Butler, P.C. Relationship Between β-Cell Mass and Fasting Blood Glucose Concentration in Humans. Diabetes Care 2006, 29, 717–718. [Google Scholar] [CrossRef] [PubMed]
- Tuomilehto, J.; Indstrom, J.; Eriksson, J.G.; Valle, T.T.; Hämäläinen, H.; Ilanne-Parikka, P.; Keinänen-Kiukaanniemi, S.; Laakso, M.; Louheranta, A.; Rastas, M.; et al. Prevention of Type 2 Diabetes Mellitus by Changes in Lifestyle among Subjects with Impaired Glucose Tolerance. N. Engl. J. Med. 2001, 344, 1343–1350. [Google Scholar] [CrossRef]
- Magkos, F.; Fraterrigo, G.; Yoshino, J.; Luecking, C.; Kirbach, K.; Kelly, S.C.; de Las Fuentes, L.; He, S.; Okunade, A.L.; Patterson, B.W.; et al. Effects of Moderate and Subsequent Progressive Weight Loss on Metabolic Function and Adipose Tissue Biology in Humans with Obesity. Cell Metab. 2016, 23, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Nakagami, T.; Oya, J.; Takahashi, K.; Isago, C.; Kurita, M.; Tanaka, Y.; Ito, A.; Kasahara, T.; Uchigata, Y. Body Weight Reduction of 5% Improved Blood Pressure and Lipid Profiles in Obese Men and Blood Glucose in Obese Women: A Four-Year Follow-up Observational Study. Metab. Syndr. Relat. Disord. 2019, 17, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Williamson, D.F.; Liu, S. Weight Change and Diabetes Incidence: Findings from a National Cohort of US Adults. Am. J. Epidemiol. 1997, 146, 214–222. [Google Scholar] [CrossRef]
- Halton, T.L.; Hu, F.B. The Effects of High Protein Diets on Thermogenesis, Satiety and Weight Loss: A Critical Review. J. Am. Coll. Nutr. 2004, 23, 373–385. [Google Scholar] [CrossRef]

| Participant ID | Age (y) | Body Weight (kg) | WC (cm) | BMI (kg/m2) |
|---|---|---|---|---|
| Case 1 | 49 | 78.2 | 93 | 28.9 |
| Case 2 | 47 | 79.8 | 91 | 29.5 |
| Case 3 | 37 | 79.9 | 80 | 29.2 |
| Case 4 | 54 | 90.0 | 105 | 33.9 |
| Case 5 | 51 | 71.9 | 84 | 29.4 |
| Case 6 | 44 | 81.0 | 88 | 31.5 |
| CRP | CRP | |||
|---|---|---|---|---|
| Baseline (PRO−) | Week 3 (PRO−) | Baseline (PRO+) | End Week 7 (PRO+) | |
| Case 1 | Negative | Negative | Negative | Negative |
| Case 2 | Moderate | Negative | Negative | Negative |
| Case 3 | Moderate | Negative | Negative | Negative |
| Case 4 | Moderate | Moderate | High | Moderate |
| Case 5 | Negative | Negative | Moderate | Negative |
| Case 6 | Moderate | Moderate | Moderate | Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzhrani, N.E.; Bryant, J.M. Intermittent Energy Restriction Combined with a High-Protein/Low-Protein Diet: Effects on Body Weight, Satiety, and Inflammation: A Pilot Study. Obesities 2023, 3, 180-192. https://doi.org/10.3390/obesities3020015
Alzhrani NE, Bryant JM. Intermittent Energy Restriction Combined with a High-Protein/Low-Protein Diet: Effects on Body Weight, Satiety, and Inflammation: A Pilot Study. Obesities. 2023; 3(2):180-192. https://doi.org/10.3390/obesities3020015
Chicago/Turabian StyleAlzhrani, Nada Eid, and Jo M. Bryant. 2023. "Intermittent Energy Restriction Combined with a High-Protein/Low-Protein Diet: Effects on Body Weight, Satiety, and Inflammation: A Pilot Study" Obesities 3, no. 2: 180-192. https://doi.org/10.3390/obesities3020015
APA StyleAlzhrani, N. E., & Bryant, J. M. (2023). Intermittent Energy Restriction Combined with a High-Protein/Low-Protein Diet: Effects on Body Weight, Satiety, and Inflammation: A Pilot Study. Obesities, 3(2), 180-192. https://doi.org/10.3390/obesities3020015






