Going High to Keep Body Mass Low: How Post-Exercise Exposure to a Simulated High Altitude Influences Energy Balance—A Proof-of-Concept Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
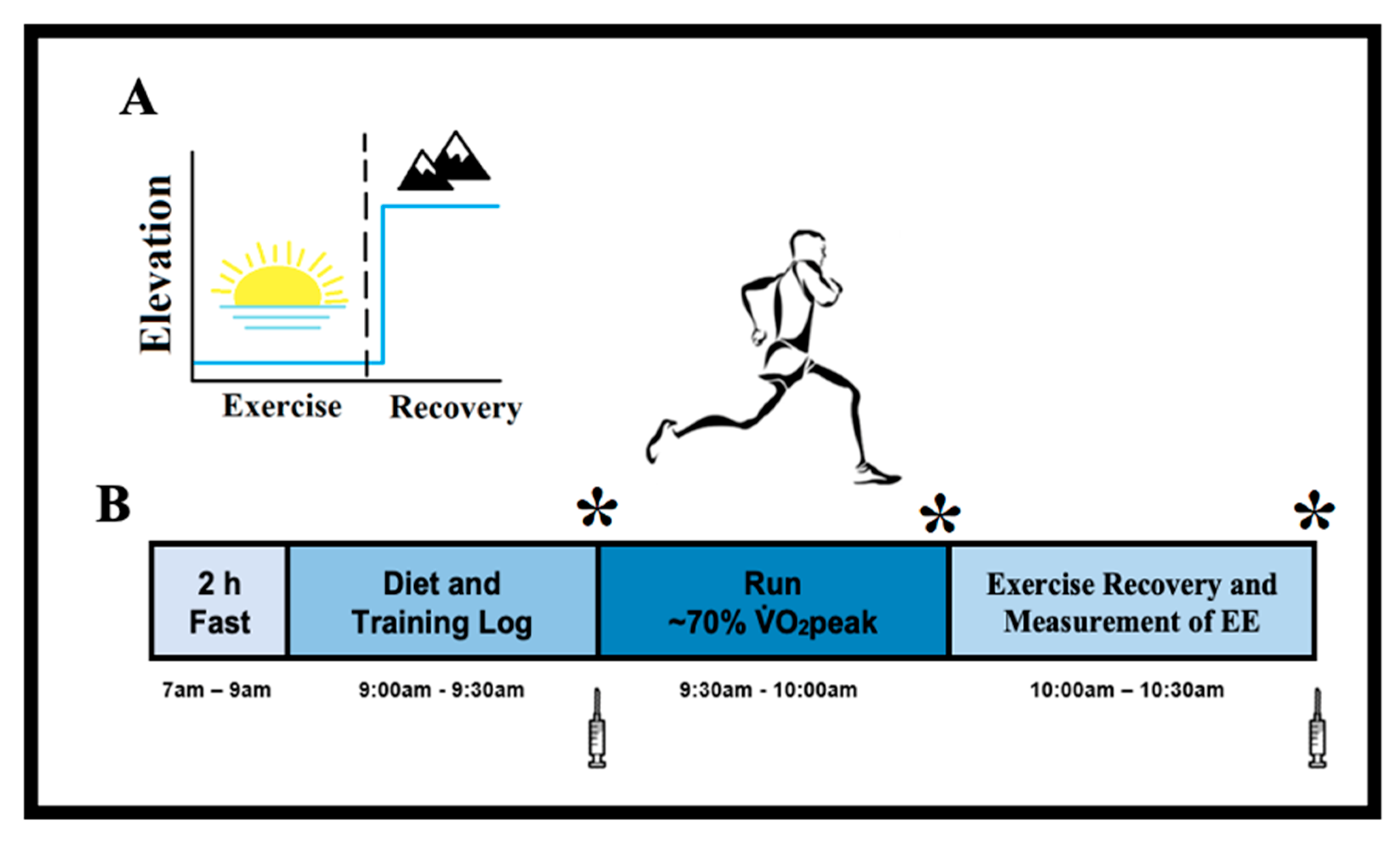
2.2. Study Design
2.3. Metabolic Responses to Exercise
2.4. Hypoxic Delivery System
2.5. Blood Measurements
2.6. Statistical Analysis
3. Results
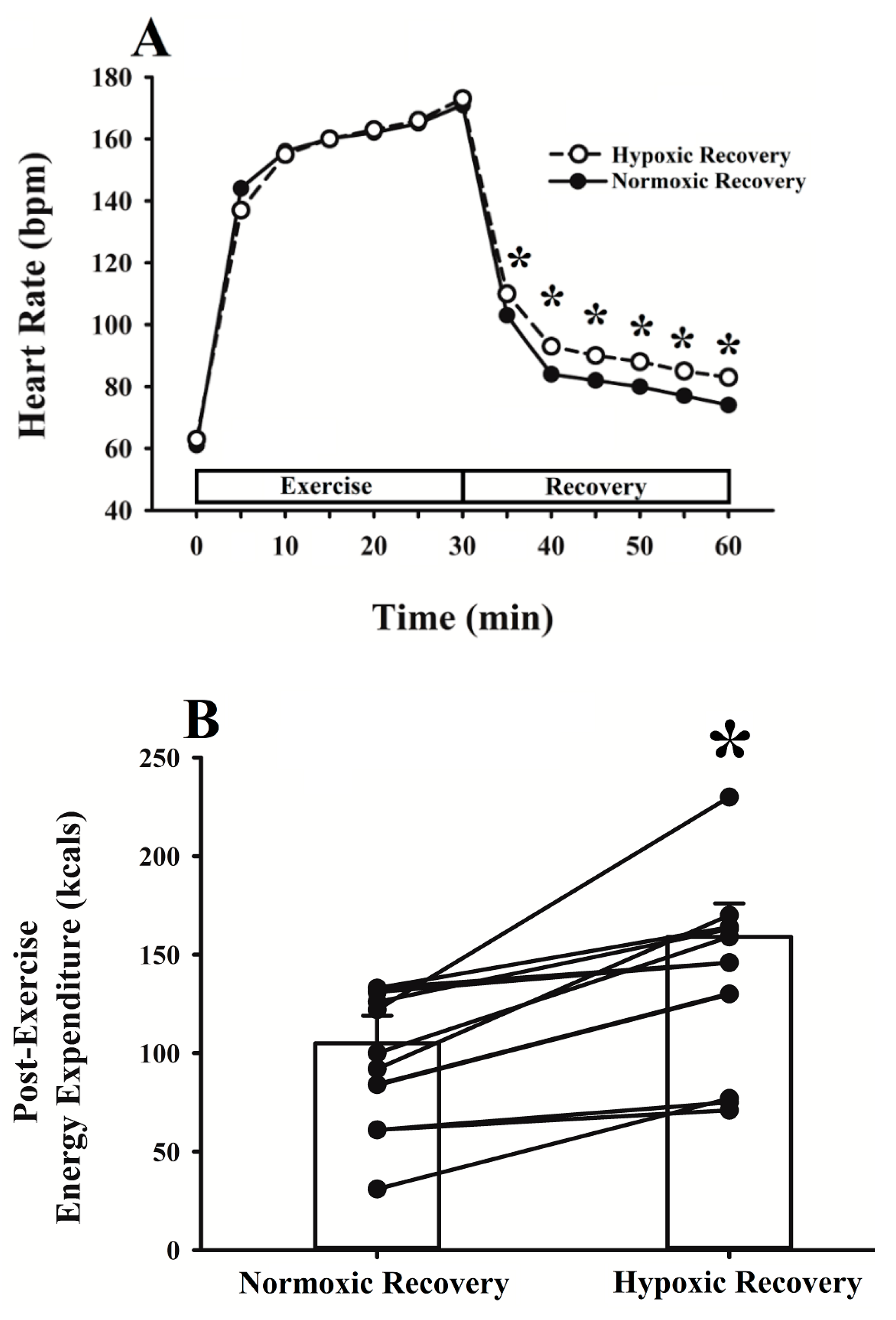
3.1. Energy Expenditure
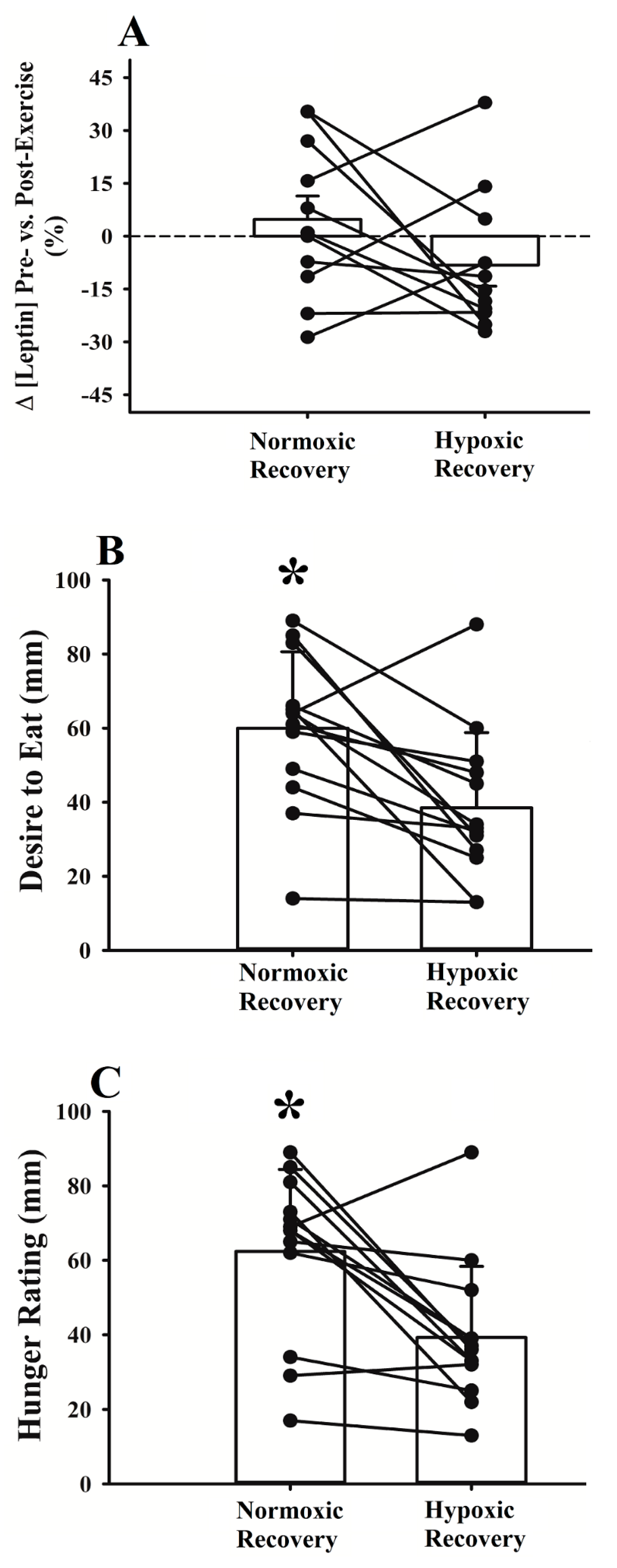
3.2. Energy Intake
3.3. Metabolic and Ventilatory Responses
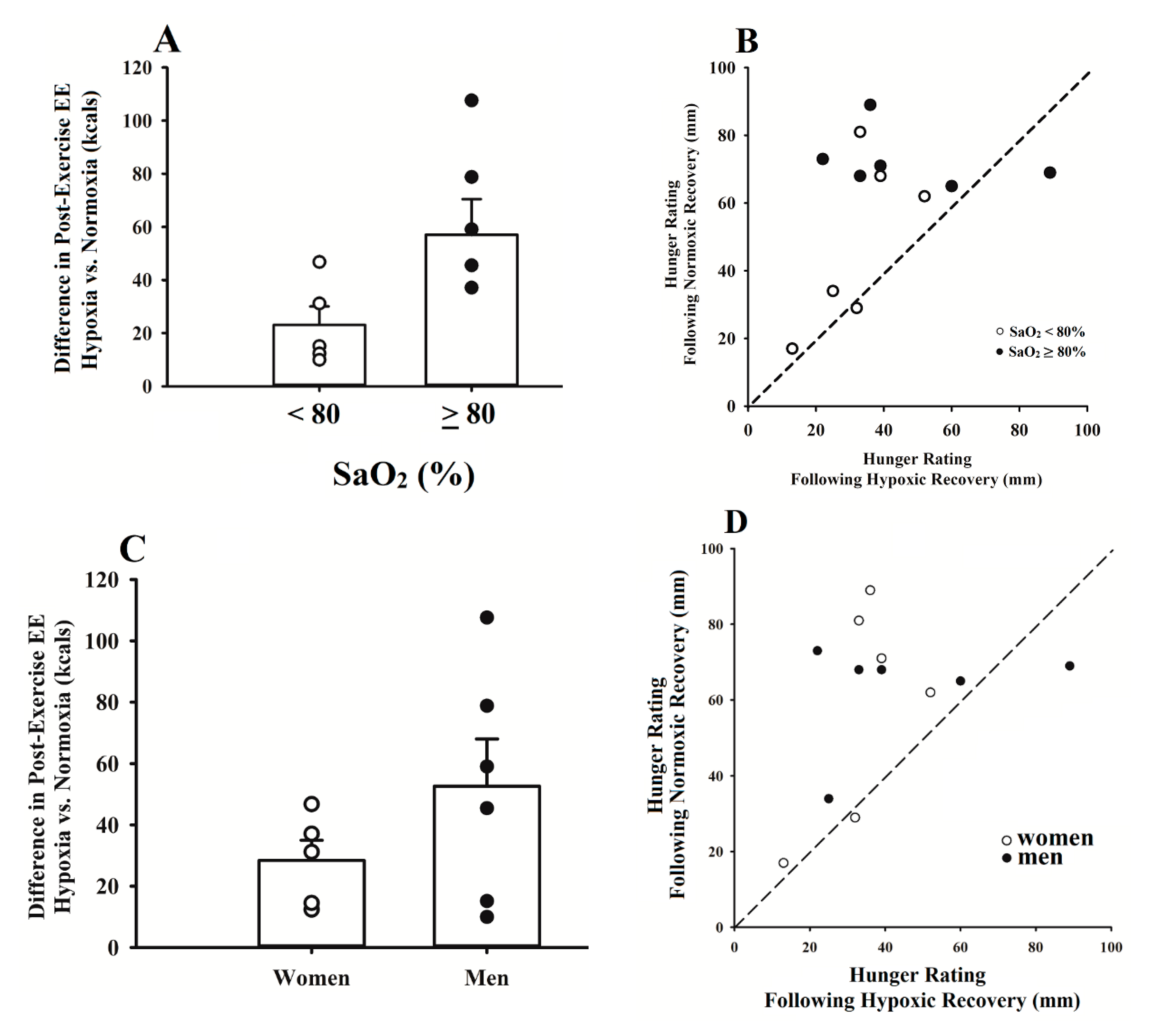
3.4. Group-Specific Responses to Post-Exercise Hypoxia
4. Discussion
4.1. Energy Expenditure
4.2. Energy Intake
4.3. Group-Specific Responses to Post-Exercise Hypoxia
4.4. Methodological Considerations
4.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Beydoun, M.A.; Min, J.; Xue, H.; Kaminsky, L.A.; Cheskin, L.J. Has the prevalence of overweight, obesity and central obesity levelled off in the United States? Trends, patterns, disparities, and future projections for the obesity epidemic. Int. J. Epidemiol. 2020, 49, 810–823. [Google Scholar] [CrossRef] [PubMed]
- World Obesity Federation. World Obesity Atlas. 2022. Available online: https://s3-eu-west-1.amazonaws.com/wof-files/World_Obesity_Atlas_2022.pdf (accessed on 1 July 2023).
- Hill, J.O.; Wyatt, H.R.; Reed, G.W.; Peters, J.C. Obesity and the environment: Where do we go from here? Science 2003, 299, 853–855. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.; Roy, P.; Mitra, K.P. Relation between caloric intake, body weight, and physical work: Studies in an industrial male population in West Bengal. Am. J. Clin. Nutr. 1956, 4, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Gibbons, C.; Beaulieu, K.; Casanova, N.; Duarte, C.; Finlayson, G.; Stubbs, R.J.; Hopkins, M. The drive to eat in homo sapiens: Energy expenditure drives energy intake. Physiol. Behav. 2020, 219, 112846. [Google Scholar] [CrossRef] [PubMed]
- Stutts, W.C. Physical activity determinants in adults: Perceived benefits, barriers, and self efficacy. Aaohn J. 2002, 50, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Crawford, D.; Jeffery, R.W.; French, S.A. Can anyone successfully control their weight? Findings of a three year community-based study of men and women. Int. J. Obes. 2000, 24, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Lonac, M.C.; Richards, J.C.; Schweder, M.M.; Johnson, T.K.; Bell, C. Influence of short-term consumption of the caffeine-free, epigallocatechin-3-gallate supplement, Teavigo, on resting metabolism and the thermic effect of feeding. Obesity 2011, 19, 298–304. [Google Scholar] [CrossRef]
- Astorino, T.; Martin, B.; Wong, K.; Schachtsiek, L. Effect of acute caffeine ingestion on EPOC after intense resistance training. J. Sports Med. Phys. Fit. 2011, 51, 11–17. [Google Scholar]
- Ryan, S.P.P.; Newman, A.A.; Wilburn, J.R.; Rhoades, L.D.; Trikha, S.R.J.; Godwin, E.C.; Schoenberg, H.M.; Battson, M.L.; Ewell, T.R.; Luckasen, G.J.; et al. Sodium Glucose Co-Transporter 2 Inhibition Does Not Favorably Modify the Physiological Responses to Dietary Counselling in Diabetes-Free, Sedentary Overweight and Obese Adult Humans. Nutrients 2020, 12, 510. [Google Scholar] [CrossRef]
- Diepvens, K.; Westerterp, K.R.; Westerterp-Plantenga, M.S. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R77–R85. [Google Scholar] [CrossRef]
- Ravussin, Y.; LeDuc, C.A.; Watanabe, K.; Leibel, R.L. Effects of ambient temperature on adaptive thermogenesis during maintenance of reduced body weight in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R438–R448. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Butterfield, G.E.; Wolfe, R.R.; Groves, B.M.; Mazzeo, R.S.; Sutton, J.R.; Wolfel, E.E.; Reeves, J.T. Increased dependence on blood glucose after acclimatization to 4300 m. J. Appl. Physiol. 1991, 70, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Kayser, B.; Verges, S. Hypoxia, energy balance and obesity: From pathophysiological mechanisms to new treatment strategies. Obes. Rev. 2013, 14, 579–592. [Google Scholar] [CrossRef] [PubMed]
- Hamad, N.; Travis, S.P. Weight loss at high altitude: Pathophysiology and practical implications. Eur. J. Gastroenterol. Hepatol. 2006, 18, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ambrosini, G.; Nath, A.K.; Sierra-Honigmann, M.R.; Flores-Riveros, J. Transcriptional activation of the human leptin gene in response to hypoxia: Involvement of hypoxia-inducible factor 1. J. Biol. Chem. 2002, 277, 34601–34609. [Google Scholar] [CrossRef]
- Tee, C.C.L.; Cooke, M.B.; Chong, M.C.; Yeo, W.K.; Camera, D.M. Mechanisms for combined hypoxic conditioning and divergent exercise modes to regulate inflammation, body composition, appetite, and blood glucose homeostasis in overweight and obese adults: A narrative review. Sports Med. 2023, 53, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, J.; Park, M.Y.; Chung, N.; Hwang, H.; Nam, S.S.; Lim, K. Exposure and exercise training in hypoxic conditions as a new obesity therapeutic modality: A mini review. J. Obes. Metab. Syndr. 2018, 27, 93. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, A.; González-Muniesa, P.; Portillo, M.P.; Martínez, J.A. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J. Physiol. Biochem. 2012, 68, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Ding, S.Q.; Liu, J.L.; Yu, M.T.; Jia, J.H.; Chai, Z.C.; Dai, R.C.; Zhang, S.L.; Li, B.Y.; Pan, L.; et al. Who should not go high: Chronic disease and work at altitude during construction of the Qinghai-Tibet railroad. High Alt. Med. Biol. 2007, 8, 88–107. [Google Scholar] [CrossRef]
- Fulco, C.S.; Rock, P.B.; Cymerman, A. Maximal and submaximal exercise performance at altitude. Aviat. Space Environ. Med. 1998, 69, 793–801. [Google Scholar]
- Butterfield, G.E.; Gates, J.; Fleming, S.; Brooks, G.A.; Sutton, J.R.; Reeves, J.T. Increased energy intake minimizes weight loss in men at high altitude. J. Appl. Physiol. 1992, 72, 1741–1748. [Google Scholar] [CrossRef]
- Slivka, D.; Dumke, C.; Hailes, W.; Ruby, B. Impact of hypoxic exercise recovery on skeletal muscle glycogen and gene expression. High Alt. Med. Biol. 2021, 22, 300–307. [Google Scholar] [CrossRef]
- Shaw, D.M.; Bloomfield, P.M.; Benfell, A.; Hughes, I.; Gant, N. Recovery from acute hypoxia: A systematic review of cognitive and physiological responses during the ‘hypoxia hangover’. PLoS ONE 2023, 18, e0289716. [Google Scholar] [CrossRef]
- Badenhorst, C.E.; Dawson, B.; Goodman, C.; Sim, M.; Cox, G.R.; Gore, C.J.; Tjalsma, H.; Swinkels, D.W.; Peeling, P. Influence of post-exercise hypoxic exposure on hepcidin response in athletes. Eur. J. Appl. Physiol. 2014, 114, 951–959. [Google Scholar] [CrossRef]
- Peters, B.; Ballmann, C.; Mcginnis, G.; Epstein, E.; Hyatt, H.; Slivka, D.; Cuddy, J.; Hailes, W.; Dumke, C.; Ruby, B.; et al. Graded hypoxia and blood oxidative stress during exercise recovery. J. Sports Sci. 2016, 34, 56–66. [Google Scholar] [CrossRef]
- Wiesner, S.; Haufe, S.; Engeli, S.; Mutschler, H.; Haas, U.; Luft, F.C.; Jordan, J. Influences of normobaric hypoxia training on physical fitness and metabolic risk markers in overweight to obese subjects. Obesity 2010, 18, 116–120. [Google Scholar] [CrossRef]
- Fernández Menéndez, A.; Saudan, G.; Sperisen, L.; Hans, D.; Saubade, M.; Millet, G.P.; Malatesta, D. Effects of short-term normobaric hypoxic walking training on energetics and mechanics of gait in adults with obesity. Obesity 2018, 26, 819–827. [Google Scholar] [CrossRef]
- Camacho-Cardenosa, A.; Camacho-Cardenosa, M.; Tomas-Carus, P.; Timón, R.; Olcina, G.; Burtscher, M. Acute physiological response to a normobaric hypoxic exposure: Sex differences. Int. J. Biometeorol. 2022, 66, 1495–1504. [Google Scholar] [CrossRef]
- Harms, C.A.; McCLARAN, S.R.; Nickele, G.A.; Pegelow, D.F.; Nelson, W.B.; Dempsey, J.A. Effect of exercise-induced arterial O2 desaturation on VO2max in women. Med. Sci. Sports Exerc. 2000, 32, 1101–1108. [Google Scholar] [CrossRef]
- Girard, O.; Sciberras, P.; Habrard, M.; Hot, P.; Chevalier, R.; Millet, G. Specific incremental test in elite squash players. Br. J. Sports Med. 2005, 39, 921–926. [Google Scholar] [CrossRef][Green Version]
- Paris, H.L.; Foright, R.M.; Werth, K.A.; Larson, L.C.; Beals, J.W.; Cox-York, K.; Bell, C.; Melby, C.L. Increasing energy flux to decrease the biological drive toward weight regain after weight loss–A proof-of-concept pilot study. Clin. Nutr. ESPEN 2016, 11, e12–e20. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Keytel, L.R.; Goedecke, J.H.; Noakes, T.D.; Hiiloskorpi, H.; Laukkanen, R.; van der Merwe, L.; Lambert, E.V. Prediction of energy expenditure from heart rate monitoring during submaximal exercise. J. Sports Sci. 2005, 23, 289–297. [Google Scholar] [CrossRef]
- Gass, G.; Rogers, S.; Mitchell, R. Blood lactate concentration following maximum exercise in trained subjects. Br. J. Sports Med. 1981, 15, 172–176. [Google Scholar] [CrossRef]
- Workman, C.; Basset, F.A. Post-metabolic response to passive normobaric hypoxic exposure in sedendary overweight males: A pilot study. Nutr. Metab. 2012, 9, 103. [Google Scholar] [CrossRef]
- Snyder, E.M.; Carr, R.D.; Deacon, C.F.; Johnson, B.D. Overnight hypoxic exposure and glucagon-like peptide-1 and leptin levels in humans. Appl. Physiol. Nutr. Metab. 2008, 33, 929–935. [Google Scholar] [CrossRef]
- Stroebele, N.; Hill, J.O.; Willich, S.N. Identifying the energy gap in the German population using results from representative national health surveys (1985–2002). Public Health Nutr. 2011, 14, 44–48. [Google Scholar] [CrossRef][Green Version]
- Mawson, J.T.; Braun, B.; Rock, P.B.; Moore, L.G.; Mazzeo, R.; Butterfield, G.E. Women at altitude: Energy requirement at 4300 m. J. Appl. Physiol. 2000, 88, 272–281. [Google Scholar] [CrossRef]
- Moore, L.G.; Cymerman, A.; Huang, S.Y.; McCullough, R.E.; McCullough, R.G.; Rock, P.B.; Young, A.; Young, P.; Weil, J.V.; Reeves, J.T. Propranolol blocks metabolic rate increase but not ventilatory acclimatization to 4300 m. Respir. Physiol. 1987, 70, 195–204. [Google Scholar] [CrossRef]
- Cunha, F.A.; Midgley, A.W.; McNaughton, L.R.; Farinatti, P.T. Effect of continuous and intermittent bouts of isocaloric cycling and running exercise on excess postexercise oxygen consumption. J. Sci. Med. Sport 2016, 19, 187–192. [Google Scholar] [CrossRef]
- Gibbons, C.; Blundell, J. Appetite regulation and physical activity–an energy balance perspective. Hamdan Med. J. 2015, 8, 33–52. [Google Scholar]
- Westerterp-Plantenga, M.S.; Westerterp, K.R.; Rubbens, M.; Verwegen, C.R.; Richelet, J.-P.; Gardette, B. Appetite at “high altitude”[Operation Everest III (Comex-’97)]: A simulated ascent of Mount Everest. J. Appl. Physiol. 1999, 87, 391–399. [Google Scholar] [CrossRef]
- Tschöp, M.; Morrison, K.M. Weight loss at high altitude. Hypoxia: From genes to the bedside. Adv. Exp. Med. Biol. 2001, 502, 237–247. [Google Scholar]
- Tschöp, M.; Strasburger, C.J.; Hartmann, G.; Biollaz, J.; Bärtsch, P. Raised leptin concentrations at high altitude associated with loss of appetite. Lancet 1998, 352, 1119–1120. [Google Scholar] [CrossRef]
- Kelly, K.R.; Williamson, D.L.; Fealy, C.E.; Kriz, D.A.; Krishnan, R.K.; Huang, H.; Ahn, J.; Loomis, J.L.; Kirwan, J.P. Acute altitude-induced hypoxia suppresses plasma glucose and leptin in healthy humans. Metabolism 2010, 59, 200–205. [Google Scholar] [CrossRef]
- Horton, T.J.; Pagliassotti, M.J.; Hobbs, K.; Hill, J.O. Fuel metabolism in men and women during and after long-duration exercise. J. Appl. Physiol. 1998, 85, 1823–1832. [Google Scholar] [CrossRef]
- Amiel, S.; Maran, A.; Powrie, J.; Umpleby, A.; Macdonald, I. Gender differences in counterregulation to hypoglycaemia. Diabetologia 1993, 36, 460–464. [Google Scholar] [CrossRef]
- Braun, B.; Mawson, J.T.; Muza, S.R.; Dominick, S.B.; Brooks, G.A.; Horning, M.A.; Rock, P.B.; Moore, L.G.; Mazzeo, R.S.; Ezeji-Okoye, S.C.; et al. Women at altitude: Carbohydrate utilization during exercise at 4300 m. J. Appl. Physiol. 2000, 88, 246–256. [Google Scholar] [CrossRef]
- Thackray, A.E.; Deighton, K.; King, J.A.; Stensel, D.J. Exercise, appetite and weight control: Are there differences between men and women? Nutrients 2016, 8, 583. [Google Scholar] [CrossRef]
- Catley, D.M.; Thornton, C.; Jordan, C.; Lehane, J.; Royston, D.; Jones, J. Pronounced, episodic oxygen desaturation in the postoperative period: Its association with ventilatory pattern and analgesic regimen. Anesthesiology 1985, 63, 20–28. [Google Scholar] [CrossRef]
- Louie, A.; Feiner, J.R.; Bickler, P.E.; Rhodes, L.; Bernstein, M.; Lucero, J. Four types of pulse oximeters accurately detect hypoxia during low perfusion and motion. Anesthesiology 2018, 128, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Stanhewicz, A.E.; Wong, B.J. Counterpoint: Investigators should not control for menstrual cycle phase when performing studies of vascular control that include women. J. Appl. Physiol. 2020, 129, 1117–1119. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Castelo-Branco, C.; Chedraui, P.; Lumsden, M.A.; Nappi, R.E.; Shah, D.; Villaseca, P.; Writing Group of the International Menopause Society for World Menopause Day 2012. Understanding weight gain at menopause. Climacteric 2012, 15, 419–429. [Google Scholar] [CrossRef] [PubMed]
| Variable | Mean | ± | SE |
|---|---|---|---|
| Age (y) | 23 | ± | 2 |
| Body mass women (kg) | 61.6 | ± | 1.4 |
| Body mass men (kg) | 71.0 | ± | 4.8 |
| Height women (cm) | 169.5 | ± | 2.2 |
| Height men (cm) | 180.2 | ± | 3.3 |
| O2peak (L∙min−1) | 3.3 | ± | 0.2 |
| O2peak women (mL∙kg−1∙min−1) | 45.4 | ± | 1.5 |
| O2peak men (mL∙kg−1∙min−1) | 52.7 | ± | 2.7 |
| Emax women (L∙min−1) | 102.4 | ± | 5.2 |
| Emax men (L∙min−1) | 144.1 | ± | 7.0 |
| HR (bpm) | E (L∙min−1) | O2 (mL∙kg−1∙min−1) | RPE | |
|---|---|---|---|---|
| Normoxic Recovery | 167 ± 5 | 82.2 ± 3.8 | 42.7 ± 1.5 | 14 ± 1 |
| Hypoxic Recovery | 164 ± 4 | 82.1 ± 4.6 | 42.5 ± 1.8 | 13 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allen, P.E.; Akinwumi, A.D.; Kroeze, E.G.; Leigh, P.Y.; Ramirez, S.N.; Smart, G.L.; Thomas, T.M.; Paris, H.L. Going High to Keep Body Mass Low: How Post-Exercise Exposure to a Simulated High Altitude Influences Energy Balance—A Proof-of-Concept Pilot Study. Obesities 2024, 4, 169-182. https://doi.org/10.3390/obesities4020016
Allen PE, Akinwumi AD, Kroeze EG, Leigh PY, Ramirez SN, Smart GL, Thomas TM, Paris HL. Going High to Keep Body Mass Low: How Post-Exercise Exposure to a Simulated High Altitude Influences Energy Balance—A Proof-of-Concept Pilot Study. Obesities. 2024; 4(2):169-182. https://doi.org/10.3390/obesities4020016
Chicago/Turabian StyleAllen, Peyton E., Akinola D. Akinwumi, Evan G. Kroeze, Paula Y. Leigh, Sahnet N. Ramirez, Gregory L. Smart, Tay M. Thomas, and Hunter L. Paris. 2024. "Going High to Keep Body Mass Low: How Post-Exercise Exposure to a Simulated High Altitude Influences Energy Balance—A Proof-of-Concept Pilot Study" Obesities 4, no. 2: 169-182. https://doi.org/10.3390/obesities4020016
APA StyleAllen, P. E., Akinwumi, A. D., Kroeze, E. G., Leigh, P. Y., Ramirez, S. N., Smart, G. L., Thomas, T. M., & Paris, H. L. (2024). Going High to Keep Body Mass Low: How Post-Exercise Exposure to a Simulated High Altitude Influences Energy Balance—A Proof-of-Concept Pilot Study. Obesities, 4(2), 169-182. https://doi.org/10.3390/obesities4020016






