Abstract
GC-MS-based metabolomics were used to investigate metabolic changes in prawn shell waste during fermentation. Microbial strains Lactobacillus plantarum and Bacillus subtilis were co-fermented in a shake flask comprising of 5% (w/v) prawn shell waste and 20% (w/v) glucose as a carbon source. Analysis of the prawn shell waste fermentation showed a total of 376 metabolites detected in the culture supernatant, including 14 amino acids, 106 organic acids, and 90 antimicrobial molecules. Results show that the liquid fraction of the co-fermentation is promising for harvesting valuable metabolites for probiotics application.
1. Introduction
The industrial seafood processing industry generates more than 1 million metric tons of dry weight of shellfish waste annually [1]. As the heads and exoskeletons of shellfish that comprise about 50–60% of their total weight are not suitable for human consumption, these shellfish residues are discarded as seafood processing waste by ocean dumping, incineration, or disposal in landfills [2]. This has contributed to both land and sea pollution, hence sparking scientific and environmental interest to develop techniques to recover and utilize the biopolymers in shellfish waste [3].
Prawn shell waste is chemically composed of 20–30% chitin, 20–40% protein, 30–60% minerals, and 0–14% lipids [4]. Currently, crustacean waste serves as the largest source of chitin or its deacetylated derivative chitosan [5]. Chitin, a polysaccharide with a similar structure to cellulose, is an N-acetyl-glucosamine biopolymer with α-1,4 bonds between each monomeric unit [6]. The isolation of chitin involves deproteinization, demineralization, and bleaching [7]. Traditional chemical methods involve the use of highly concentrated sodium hydroxide to carry out deproteinization and highly corrosive hydrochloric acid to carry out demineralization [8]. Other than the formation of toxic waste, undesired by-products such as irregularly deacetylated polymers result [9]. In addition, the protein and carotenoid components of the prawn shell waste are rendered useless [10].
Research has focused on using environmentally friendly processes such as biological co-fermentation by lactic acid bacteria and protease producing bacteria [11]. The lactic acid produced during fermentation reacts with the calcium carbonate in the prawn shell waste, leading to the formation of calcium lactate, which can be separated from the chitin fraction [12]. Proteolytic enzymatic action also simultaneously hydrolyzes the protein fraction of prawn shells to recover chitin [13]. Much attention has been directed at optimizing the extracellular production of the chitinase enzyme by the selection of appropriate micro-organisms [14]. Various factors such as glucose concentrations, inoculum sizes, pH, temperature, and length of fermentation influence the fermentation process as well as deproteinization and demineralization efficiencies [15].
The remains of shellfish heads and exoskeletons are also rich in lipid soluble carotenoid pigments and the recovery of an astaxanthin-rich carotenoprotein concentrate for its antioxidant properties have been a focal point of scientific study [16]. The extraction of protein hydrolysates from prawn shell waste for use as food flavoring agents or for aquaculture diets has received considerable scientific attention [17]. However, the study of these bioactive compounds in the liquor fraction has posed great challenges due to their inherent instability upon analysis [18].
In this study, GC-MS-based metabolomics profiling was performed on the co-culture supernatant of both microbial strains, lactic acid bacteria Lactobacillus plantarum subsp. plantarum ATCC 14,917 and protease producing bacteria Bacillus subtilis subsp. subtilis ATCC 6051, using prawn shell waste as the nitrogen source and 20% glucose in deionized water as the carbon source [19]. Lactobacillus plantarum was selected as previous studies found it to be starch-hydrolyzing, heterofermentative, and proteolytic when tested in skim milk agar, which are important properties for the deproteinization and demineralization of prawn shells [20]. Bacillus subtilis was chosen as it was affirmed in previous studies to produce a high protease yield, which retained maximum protease activity even in the presence of salt, surfactants, metal ions, and solvents [21].
The composition of totals phenols, polysaccharides, reducing sugars, free amino acids, and organic acids in the culture supernatant were determined by GC-MS analysis after GC derivatization to understand the fermentation characteristics of microbial extraction of chitin from prawn shell waste [22]. The remnants of the prawn shell waste were filtered off from the fermented supernatant, washed with deionized water, and sterilized with 70% (v/v) ethanol [23]. After being dried in a vacuum oven at 60°C overnight, chemical analysis was performed and it was found to be chitin [24].
2. Materials and Methods
2.1. Fermentation Conditions and Harvesting of Samples
Single colonies of Lactobacillus plantarum on De Man, Rogosa, and Sharpe (MRS) agar plates were picked to 5 mL MRS broth and cultured at 37 °C, 200 rpm, overnight for 12 to 16 h. Similarly, single colonies of Bacillus subtilis on Luria-Bertani (LB) agar plates were picked to 5 mL LB broth and cultured at 30 °C, 200 rpm, overnight for 12 to 16 h. The Lactobacillus plantarum and Bacillus subtilis bacterial cells were collected by centrifuging at 14,500× g, 25 °C, for 5 min and their respective supernatants were decanted, leaving the cell pellets behind.
A conical flask containing 5 g of prawn shell waste as well as 20 g of glucose dissolved in 100 mL of deionized water were autoclaved at 121 °C for sterilization [25]. The 100 mL 20% (w/v) glucose solution was poured into the sterile conical flask containing 5 g of prawn shell waste. Lactobacillus plantarum cells and Bacillus subtilis cells were picked up from the centrifuged bacterial cell pellets using inoculating loops and inoculated into the fermentation flask. The fermentation setup procedures were repeated twice and the triplicate flasks were incubated at 30 °C, 200 rpm, for 5 days.
2.2. Samples Preparation for Extracellular Metabolites Analysis
First, 1 mL culture supernatant was collected from each of the three fermentation setups after 5 days. Ten microliters of 2 g/L ribitol dissolved in water was added to 50 μL of each supernatant sample and mixed thoroughly in a fresh Eppendorf tube [26]. The addition of ribitol served as an internal standard to correct for metabolite loss during sample preparation [27]. The samples were lyophilized overnight using a Labconco freeze dryer set at −40 °C and 0.0002 mBar and GC-MS derivatization was performed the next day [28].
2.3. GC-MS Analysis of Extracellular Metabolites
GC derivatization was performed for metabolic profiling on the GC-MS [29]. The lyophilized samples were re-dissolved in 100 μL of 20 mg/mL methoxyamine hydrochloride in pyridine and incubated at 37 °C for 1 h for carbonyls protection [30]. One hundred microliters of N-methyl-N-(trimethylsilyl)-trifluoroacetamide (MSTFA) with 1% trimethyl-chlorosilane (TMCS) was added to each sample and silylation was carried out at 70 °C for 30 min [31]. The samples were centrifuged at 14,500× g for 15 min and the supernatant was used for GC-MS analysis [32]. Samples of 1 μL were injected into the HP-5MS capillary column (Agilent Technologies, Singapore) by splitless mode using an auto-injector [33]. Helium was used as a carrier gas at 1.1 mL/min [34]. The injector temperature and ion source temperature were set at 250 °C and 230 °C, respectively, on the GC-MS (Agilent Technologies, Singapore) [35]. The oven temperature was kept at 75 °C for 5 min, raised at 4 °C per minute to a final temperature of 280 °C, and held for 2 min [36]. Data were recorded from m/z 50 to 500 with a scan time of 0.1 s [37]. Metabolites were identified using the NIST08 mass spectral library and normalized using the internal standard ribitol before comparison [38].
2.4. Statistical Analysis of Metabolites
The peak area for ribitol from the GC-MS run was recorded and equated to 20 μg/200 μL. The peak areas for detected metabolites were tabulated and their concentrations calculated via multiplying by ribitol concentration 20 μg/200 μL and dividing over peak area for ribitol. Metabolite measurement results from the triplicate fermentation flasks were expressed as mean ± standard deviation.
2.5. Determination of Chitin Yield and Purity
The mass of the crude chitin obtained was weighed after being dried in the vacuum oven overnight to determine its yield. The Lowry’s test for residual protein was carried out to ascertain the purity of the recovered chitin. Firstly, 5, 10, 15, 20, 25, and 30 μL of 2 mg/mL bovine serum albumin (BSA) was added to 195, 190, 185, 180, 175, and 170 μL of deionized water respectively to form a range of 200 μL protein standards for the construction of a protein calibration curve. Then, 1 mL of Lowry’s solution was added to the protein standards and left to react for 15 min, after which 100 μL of 1 N Folin’s Phenol reagent was added and the protein standards were left to react for another 30 min. Absorbance was measured at 750 nm and the values were plotted into a graph of absorbance versus μg protein. Fifty milligrams of the extracted crude chitin was then treated with 10 mL of 1 M aqueous sodium hydroxide solution for 24 h at 70 °C. 1 mL of Lowry’s solution and 100 μL of 1 N Folin’s Phenol reagent was similarly added to the boiled NaOH supernatant to determine the residual protein content of the recovered chitin [39].
3. Results
3.1. Metabolomics Analysis by GC-MS
A total of 376 metabolites were detected by GC-MS. Fourteen amino acids were detected in the fermentation, with the highest quantity being alanine (4642.67 mg/L), followed by proline (91.76 mg/L), threonine (91.73 mg/L), leucine (63.91 mg/L), norleucine (53.57 mg/L), alanylthreonine (26.39 mg/L), glycine (25.56 mg/L), sarcosine (16.19 mg/L), isoleucine (13.96 mg/L), alloisoleucine (13.86 mg/L), glutamic acid (5.68 mg/L), valine (3.13 mg/L), 1,4-dihydrophenylalanine (2.92 mg/L), and lysine (0.44 mg/L). Ketoisocaproic acid, which is a metabolic intermediate in the metabolic pathway for the amino acid leucine, was detected at 44.06 mg/L; while ketoisovaleric acid, which is a metabolite of the amino acid valine, was detected at 1.2 mg/L.
One hundred and six organic acids were found in the culture supernatant, with the highest quantities being butanoic acid (4399.87 mg/L), mannonic acid (2567.14 mg/L), 2,3-dimethylbutanoic acid (2129.98 mg/L), carbamic acid (1432.07 mg/L), glucopyranuronic acid (1239.42 mg/L), D-glycero-L-manno-heptonic acid (1192.77 mg/L), 3-oxooctanoic acid (1185.00 mg/L), propanoic acid (1184.32 mg/L), and lactic acid (1055.38 mg/L). There were also significant quantities of mandelic acid (443.5 mg/L), gluconic acid (307.05 mg/L), 2-ketobutyric acid (222.57 mg/L), hexanedioic acid (200.31 mg/L), 2-hydroxyisocaproic acid (173.95 mg/L), xylonic acid (156.31 mg/L), butyric acid (153.36 mg/L), hexadecenoic acid (147.85 mg/L), octadecanoic acid (147.83 mg/L), dipropylacetic acid (120.83 mg/L), and 3-deoxy-D-arabino-hexonic acid (112.61 mg/L) detected.
Ninety metabolites were reported in the literature to possess antimicrobial properties, of which 37 metabolites were fatty or organic acids. The remaining 53 reportedly antimicrobial metabolites, which were non-acids, include acetamide (2999.12 mg/L), uridine (1277.01 mg/L), 2-hydroxybenzaldehyde (940.97 mg/L), acethydrazide (366.71 mg/L), 2-propenamide (338.39 mg/L), glycerol (336.23 mg/L), 2-quinolinone (284.36 mg/L), benzenesulfonamide (167.36 mg/L), thymol (68.98 mg/L), quinazoline (66.92 mg/L), sedoheptulose (64.36 mg/L), kaurene (58.27 mg/L), 1,2-benzisothiazole (56.13 mg/L), phenanthroline (49.88 mg/L), ethyl acetate (44.86 mg/L), pyrazine (33.19 mg/L), ethanol (31.01 mg/L), 1,4-benzoquinone (28.12 mg/L), benzoate (24.82 mg/L), benzisothiazolinone (23.64 mg/L), indole (22.32 mg/L), and 2-aminothiadiazole (20.98 mg/L).
Full detailed results for the detected metabolites are shown in Table 1, Table 2, Table 3 and Table 4 below.

Table 1.
Amino acids detected in culture supernatant of dual Lactobacillus plantarum and Bacillus subtilis fermentation in prawn shell waste and 20% glucose in deionized water.

Table 2.
Antimicrobial Compounds detected in culture supernatant of dual Lactobacillus plantarum and Bacillus subtilis fermentation in prawn shell waste and 20% glucose in deionized water.

Table 3.
Other organic compounds detected in culture supernatant of dual Lactobacillus plantarum and Bacillus subtilis fermentation in prawn shell waste and 20% glucose in deionized water.

Table 4.
Sugar derivatives detected in culture supernatant of dual Lactobacillus plantarum and Bacillus subtilis fermentation in prawn shell waste and 20% glucose in deionized water.
3.2. Chitin Yield and Purity Calculations
From 5.0 g of prawn shell waste, 20 g of glucose, and 100 g of deionized water, the dry weight of crude extracted chitin was found to be 0.50 ± 0.01 g, translating to an overall fermentation yield of 0.50/125.0 × 100% = 0.4%.
Lowry’s test was performed on 1 mL of supernatant extracted from 50 mg chitin heated in 10 mL NaOH and its absorbance was found to be 0.213, corresponding to 20 μg of protein when compared against the protein calibration curve (Figure 1). This translates to a residual protein of 200 μg per 50 mg chitin, which is a residual protein content of 200/50,000 × 100% = 0.4%.
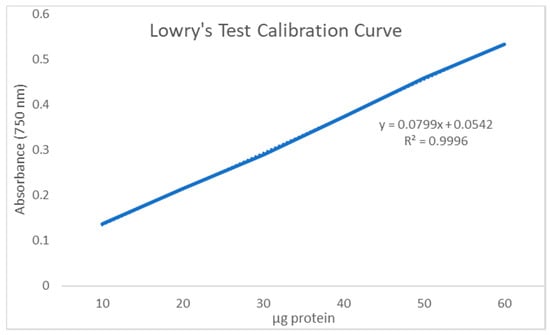
Figure 1.
Lowry’s test calibration curve (absorbance vs. μg protein).
4. Discussion
Bacteria species coexist with neighboring microorganisms in a dynamic community by producing small metabolites in response to environmental changes such as biotic and abiotic stresses. These volatile organic and inorganic compounds are released during interspecies bacteria interactions due to competition and cooperation, forming soluble metabolites in the supernatant [134]. Detection and quantification of these bacteria volatile compounds have always been of great interest in the food, cosmetic, flavor, and fragrance bioprocessing industry as well as in the clinical and medical field. However, analysis of bacteria volatile compounds has remained challenging due to the wide abundance of metabolites and the complexity of the culture medium from where they are extracted.
The co-fermentation of prawn shell waste and 20% glucose by Lactobacillus plantarum and Bacillus subtilis for chitin extraction produced bacteria volatile metabolites of various chemical classes. Fatty acid derivatives such as hydrocarbons, ketones and alcohols, organic acids, as well as sulphur and nitrogen-containing compounds were detected in the culture supernatant. These metabolites were generally produced by different catabolic pathways such as glycolysis, proteolysis, and lipolysis to break down the proteins, fats, and minerals residual in the prawn shell waste [135]. Linear-chained hydrocarbons detected were most probably derived from products of the fatty acid biosynthetic pathway. Both short-chain alkanes and longer-chain hydrocarbons were found in the culture supernatant, testifying to the ability of the microbial strains to synthesize branched hydrocarbons.
Methyl ketones detected were probably produced from the decarboxylation of fatty acids [136]. For example, 3-hydroxy-2-butanone (72.50 mg/L) or acetoin detected might have been derived from pyruvate fermentation. Long-chain aliphatic alcohols such as 1-decanol (1.75 mg/L) were probably produced through the oxidation of fatty acid derivatives. Significant production of butanediol (139.50 mg/L) was detected due to the presence of glucose as the main nutrient in the growth medium. Short-chain branched alcohols such as 3,3-dimethyl-1-butanol (18.60 mg/L) detected might have been produced from the enzymatic conversion of branched chain amino acids such as leucine.
Several short-chain fatty acids were detected in the culture supernatant such as acetic acid (71.94 mg/L), propanoic acid (1184.32 mg/L), and butanoic acid (4399.87 mg/L). These saturated aliphatic organics acids most probably resulted from bacteria fermentation of carbohydrates. Glyoxylic acid (4.68 mg/L) detected could either have been produced in the tricarboxylic acid cycle or generated during amino acid metabolism, for example during the degradation of glycine (25.56 mg/L), threonine (91.73 mg/L), and proline (91.76 mg/L). Indole (22.32 mg/L) biosynthesis, another by-product of amino acid catabolism, was also detected in the fermentation supernatant [137].
An oxidative deamination of many amino acids might have also led to the production of aldehydes, ketones, or alcohols detected. For example, the degradation of 1,4-dihydrophenyalanine (2.92 mg/L) might have served as the first step of aromatic volatile compounds synthesis, producing benzene, its carbohydrate derivatives, as well as other benzenoid volatiles. Many volatile organic compounds produced by Lactobacillus plantarum and Bacillus subtilis have been reported to display antimicrobial activity. Among these known antimicrobial metabolites, benzenoids are the most represented in quantity compared to alkanes, aldehydes, ketones, acids, and alcohols. While a huge majority of antimicrobial benzenoid volatiles have a benzene core linked to a fatty acids derivative, benzenoids are very diverse and can be linked with carbohydrate chains containing nitrogen and sulphur [138].
The antimicrobial mode of action of these bacteria volatile organic compounds might arise from their lipophilic nature, which enables them to destabilize the cell membrane integrity of antagonistic pathogens, inhibiting their growth [139]. Besides benzenoids, nitrogen-containing volatile organic compounds are another important group of antimicrobial metabolites, consisting of non-cyclic amides and amines as well as cyclic azoles, pyrazines, pyridazines, and pyrimidines. Pyrazine (33.19 mg/L), pyridazine (3.29 mg/L), and pyrrolopyrimidine (3.33 mg/L) were detected in the Lactobacillus plantarum and Bacillus subtilis co-fermentation supernatant. Pyrazine, which is the most strongly represented in antimicrobial activity among them, is either formed from the non-enzymatic animation of acyloins or derived from aminoketone intermediates produced from amino acid catabolism. This testifies to the successful breakdown of amino acids from the prawn shell waste.
Antimicrobial active metabolites may have potential use as natural preservatives to control the growth and inactivate undesired microorganisms in food [140]. For example, lactic acid (1055.39 mg/L) and acetic acid (71.94 mg/L) are produced by Lactobacillus plantarum in probiotics to compete for nutrients with other foodborne pathogens. Other organic acids such as propanoic acid (1184.32 mg/L) and butanoic acid (4399.87 mg/L) are also produced, which further reduce the pH of the culture medium. The production of other substances such as ethanol (31.01 mg/L), fatty acids such as 3-hydroxybutyric acid (18.19 mg/L), 3-hydroxysebacic acid (4.09 mg/L), and 3-hydroxpyruvic acid (1.32 mg/L), as well as 3-hydroxy-2-butanone (72.5 mg/L) further intensify its antimicrobial activity. The metabolomics results show that Lactobacillus plantarum is more heterofermentative than homofermentative as a variety of metabolites are generated from the degradation of hexoses.
5. Conclusions
Many useful metabolites are produced when Lactobacillus plantarum and Bacillus subtilis are fermented with prawn shell waste together with 20% glucose as a carbon source. Besides lactic acid, a variety of organic acids such as fatty acids and amino acids as well as several antimicrobial molecules were detected in the culture supernatant. This shows that protease-mediated protein hydrolysis of the prawn shells is successful in removing proteins, minerals, and fats from the prawn shells. While harnessing the solid fraction of the fermentation as chitin, the nutrient-rich liquid fraction may be used for probiotics applications.
Author Contributions
Conceptualization, W.N.C.; methodology, Y.N.T. and J.H.Z.; software, Y.N.T. and J.H.Z.; validation, Y.N.T. and J.H.Z.; formal analysis, Y.N.T. and J.H.Z.; investigation, Y.N.T. and J.H.Z.; resources, W.N.C.; data curation, Y.N.T. and J.H.Z.; writing—original draft preparation, Y.N.T.; writing—review and editing, Y.N.T.; visualization, W.N.C.; supervision, W.N.C.; project administration, W.N.C.; funding acquisition, W.N.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Nanyang Technological University, grant number M4062121.120.703012.
Acknowledgments
We thank Nanyang Technological University for support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kandra, P.; Challa, M.M.; Jyothi, H.K.P. Efficient use of shrimp waste: Present and future trends. Appl. Microbiol. Biotechnol. 2012, 93, 17–29. [Google Scholar] [CrossRef]
- Ferrer, J.; Paez, G.; Marmol, Z.; Ramones, E.; Garcia, H.; Forster, C.F. Acid hydrolysis of shrimp-shell wastes and the production of single cell protein from the hydrolysate. Bioresour. Technol. 1996, 57, 55–60. [Google Scholar] [CrossRef]
- Gomez-Rios, D.; Barrera-Zapata, R.; Rios-Estepa, R. Comparison of process technologies for chitosan production from shrimp shell waste: A techno-economic approach using Aspen Plus. Food Bioprod. Process. 2017, 103, 49–57. [Google Scholar] [CrossRef]
- Manni, L.; Ghorbel-Bellaaj, O.; Jellouli, K.; Younes, I.; Nasri, M. Extraction and Characterization of Chitin, Chitosan, and Protein Hydrolysates Prepared from Shrimp Waste by Treatment with Crude Protease from Bacillus cereus SV1. Appl. Biochem. Biotechnol. 2010, 162, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, M.Y.; Aleman, A.; Calvo, M.M.; Lopez-Caballero, M.E.; Montero, P.; Gomez-Guillen, M.C. Antimicrobial and antioxidant chitosan solutions enriched with active shrimp (Litopenaeus vannamei) waste materials. Food Hydrocoll. 2014, 35, 710–717. [Google Scholar] [CrossRef]
- Mao, X.; Liu, P.; He, S.; Xie, J.; Kan, F.; Yu, C.; Li, Z.; Xue, C.; Lin, H. Antioxidant Properties of Bio-active Substances from Shrimp Head Fermented by Bacillus licheniformis OPL-007. Appl. Biochem. Biotechnol. 2013, 171, 1240–1252. [Google Scholar] [CrossRef] [PubMed]
- Benhabiles, M.S.; Abdi, N.; Drouiche, N.; Lounici, H.; Pauss, A.; Goosen, M.F.A.; Mameri, N. Protein recovery by ultrafiltration during isolation of chitin from shrimp shells Parapenaeus longirostris. Food Hydrocoll. 2013, 32, 28–34. [Google Scholar] [CrossRef]
- El-Beltagy, A.E.; El-Sayed, S.M. Functional and nutritional characteristics of protein recovered during isolation of chitin from shrimp waste. Food Bioprod. Process. 2012, 90, 633–638. [Google Scholar] [CrossRef]
- Prameela, K.; Venkatesh, K.; Immandi, S.B.; Katsuri, A.P.K.; Krishna, C.R.; Mohan, C.M. Next generation nutraceutical from shrimp waste: The convergence of applications with extraction methods. Food Chem. 2017, 237, 121–132. [Google Scholar] [CrossRef]
- Robert, M.; Zatylyn-Gaudin, C.; Fournier, V.; Corre, E.; Corguille, G.L.; Bernay, B.; Henry, J. Transcriptomic and peptidomic analysis of protein hydrolysates from the white shrimp (L. vannamei). J. Biotechnol. 2014, 186, 30–37. [Google Scholar] [CrossRef]
- Bueno-Solano, C.; Lopez-Cervantes, J.; Campas-Baypoli, O.N.; Lauterio-Garcia, R.; Adan-Bante, N.P.; Sanchez-Machado, D.I. Chemical and biological characteristics of protein hydrolysates from fermented shrimp by-products. Food Chem. 2009, 112, 671–675. [Google Scholar] [CrossRef]
- Sila, A.; Nasri, M.; Bougatef, A. Isolation and characterization of carotenoproteins from deep-water pink shrimp processing waste. Int. J. Biol. Macromol. 2012, 51, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Narayan, B.; Velappan, S.P.; Zituji, S.P.; Manjabhatta, S.N.; Gowda, L.R. Yield and chemical composition of fractions from fermented shrimp biowaste. Waste Manag. Res. 2010, 28, 64–70. [Google Scholar] [CrossRef]
- Perez-Santin, E.; Calvo, M.M.; Lopez-Caballero, M.E.; Montero, P.; Gomez-Guillen, M.C. Compositional properties and bioactive potential of waste material from shrimp cooking juice. LWT Food Sci. Technol. 2013, 54, 87–94. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N. In vitro antioxidant activity of liquor from fermented shrimp biowaste. Bioresour. Technol. 2008, 99, 9013–9016. [Google Scholar] [CrossRef]
- Armenta-Lopez, R.; Guerrero, L.I.; Huerta, S. Astaxanthin Extraction from Shrimp Waste by Lactic Acid Fermentation and Enzymatic Hydrolysis of the Carotenoprotein Complex. Food Chem. Toxicol. 2002, 67, 1002–1006. [Google Scholar]
- Gomez-Estaca, J.; Calvo, M.M.; Alvarez-Acero, I.; Montero, P.; Gomez-Guillen, M.C. Characterization and storage stability of astaxanthin esters, fatty acid profile and α-tocopherol of lipid extract from shrimp (L. vannamei) waste with potential applications as food ingredient. Food Chem. 2017, 216, 37–44. [Google Scholar] [CrossRef]
- Sila, A.; Sayari, N.; Balti, R.; Martinez-Alvarez, O.; Nedjar-Arroume, N.; Moncef, N.; Bougatef, A. Biochemical and antioxidant properties of peptidic fraction of carotenoproteins generated from shrimp by-products by enzymatic hydrolysis. Food Chem. 2014, 148, 445–452. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, J.; Kan, F.; Gao, Y.; Lan, J.; Zhang, X.; Hu, Z.; Li, Y.; Lin, H. Antioxidant Production and Chitin Recovery from Shrimp Head Fermentation with Streptococcus thermophilus. Food Sci. Biotechnol. 2013, 22, 1023–1032. [Google Scholar] [CrossRef]
- Francisco, F.C.; Simora, R.M.C.; Nunal, S.N. Deproteination and demineralization of shrimp waste using lactic acid bacteria for the production of crude chitin and chitosan. Aquac. Bioflux 2015, 8, 107–115. [Google Scholar]
- Maruthiah, T.; Somanath, B.; Immanuel, G.; Palavesam, A. Deproteinization potential and antioxidant property of haloalkalophilic organic solvent protease from marine Bacillus sp. APCMST-RS3 using marine shell wastes. Biotechnol. Rep. 2015, 8, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Armenta, R.E.; Guerrero-Legarreta, I. Amino acid profile and enhancement of the enzymatic hydrolysis of fermented shrimp carotenoproteins. Food Chem. 2009, 112, 310–315. [Google Scholar] [CrossRef]
- Jung, W.J.; Jo, G.H.; Kuk, J.H.; Kim, K.Y.; Park, R.D. Extraction of chitin from red crab shell waste by cofermentation with Lactobacillus paracasei subsp. tolerans KCTC-3074 and Serratia marcescens FS-3. Appl. Microbiol. Biotechnol. 2006, 71, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Aytekin, O.; Elibol, M. Cocultivation of Lactococcus lactis and Teredinobacter turnirae for biological chitin extraction from prawn waste. Bioprocess. Biosyst. Eng. 2010, 33, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Phuong, P.T.D.; Minh, N.C.; Cuong, H.N.; Minh, N.V.; Han, N.T.; Hoa, N.V.; Yen, H.T.H.; Trung, T.S. Recovery of protein hydrolysate and chitosan from black tiger shrimp (Penaeus monodon) heads: Approaching a zero waste process. J. Food Sci. Technol. 2017, 54, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Fernando, R.; Munasinghe, D.M.S.; Gunasena, A.R.C.; Abeynayake, P. Determination of nitrofuran metabolites in shrimp muscle by liquid chromatography-photo diode array detection. Food Control 2017, 72, 300–305. [Google Scholar] [CrossRef]
- Chen, D.; Ye, Y.; Chen, J.; Yan, X. Evolution of metabolomics profile of crab paste during fermentation. Food Chem. 2016, 192, 886–892. [Google Scholar] [CrossRef]
- Xiao, M.; Qian, K.; Wang, Y.; Bao, F. GC-MS metabolomics reveals metabolic differences of the farmed Mandarin fish Siniperca chuatsi in recirculating ponds aquaculture system and pond. Sci. Rep. 2020, 10, 6090. [Google Scholar] [CrossRef]
- Ma, Q.Q.; Wang, X.D.; Cui, Y.Y.; Zhang, N.N.; Qin, J.G.; Du, Z.Y.; Chen, L.Q. Untargeted GC-MS metabolomics reveals metabolic differences in the Chinese mitten-hand crab (Eriocheir sinensis) fed with dietary palm oil or olive oil. Aquacult. Nutr. 2018, 24, 1623–1637. [Google Scholar] [CrossRef]
- Zang, J.; Xu, Y.; Xia, W.; Jiang, Q.; Yang, F.; Wang, B. Phospholipid molecular species composition of Chinese traditional low-salt fermented fish inoculated with different starter cultures. Food Res. Int. 2018, 111, 87–96. [Google Scholar] [CrossRef]
- Ming, T.; Han, J.; Li, Y.; Lu, C.; Qiu, D.; Li, Y.; Zhou, J.; Su, X. A metabolomics and proteomics study of the Lactobacillus plantarum in the grass carp fermentation. BMC Microbiol. 2018, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cervantes, J.; Sanchez-Machado, D.I.; Rosas-Rodriguez, J.A. Analysis of free amino acids in fermented shrimp waste by high-performance liquid chromatography. J. Chromatogr. A 2005, 1105, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cervantes, J.; Sanchez-Machado, D.I.; Rosas-Rodriguez, J.A. High-performance liquid chromatography method for the simultaneous quantification of retinol, α-tocopherol, and cholesterol in shrimp waste hydrolysate. J. Chromatogr. A 2006, 1105, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Yoo, S.A.; Seo, S.H.; Lee, K.I.; Na, C.S.; Son, H.S. GC-MS based metabolomics approach of Kimchi for the understanding of Lactobacillus plantarum fermentation characteristics. LWT Food Sci. Technol. 2016, 68, 313–321. [Google Scholar] [CrossRef]
- Yang, X.; Hu, W.; Xiu, Z.; Jiang, A.; Yang, X.; Sarengaowa; Ji, Y.; Guan, Y.; Feng, K. Microbial dynamics and volatilome profiles during the fermentation of Chinese northeast sauerkraut by Leuconostoc mesenteroides ORC 2 and Lactobacillus plantarum HBUAS 51041 under different salt concentrations. Food Res. Int. 2020, 130, 108926. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, J.; Ma, Z.; Li, X.; Liu, L.; Hu, X. Microbial succession and metabolite changes during traditional serofluid dish fermentation. LWT Food Sci. Technol. 2017, 84, 771–779. [Google Scholar] [CrossRef]
- Li, P.; Tang, H.; Shi, C.; Xie, Y.; Zhou, H.; Xia, B.; Zhang, C.; Chen, L.; Jiang, L. Untargeted metabolomics analysis of Mucor racemosus Douchi fermentation by gas chromatography with time-of-flight mass spectrometry. Food Sci. Nutr. 2019, 7, 1865–1874. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, P.R.; Mishra, S.K. Identification of Metabolites through GC/LC-MS Processed Data using Different Reference Libraries and Their Comparison. J. Pharm. Biomed. Sci. 2016, 6, 363–368. [Google Scholar]
- Devi, R.; Dhamodharan, R. Pretreatment in Hot Glycerol for Facile and Green Separation of Chitin from Prawn Shell Waste. ACS Sustain. Chem. Eng. 2018, 6, 846–853. [Google Scholar] [CrossRef]
- Toche, R.B.; Janrao, R.A. Synthesis and characterization and antimicrobial evaluation of novel urea, sulfonamide and acetamide 3,4-dihydropyrazinol[1,2-a]indol-1(2H)-one derivates. Arab. J. Chem. 2019, 12, 3406–3416. [Google Scholar] [CrossRef]
- Popiolek, L.; Biernasiuk, A. Design, synthesis, and in vitro antimicrobial activity of hydrazide-hydrazones of 2-substituted acetic acid. Chem. Bio. Drug Des. 2016, 88, 873–883. [Google Scholar] [CrossRef] [PubMed]
- Fraise, A.P.; Wilkinson, M.A.C.; Bradley, C.R.; Oppenheim, B.; Moiemen, N. The antibacterial activity and stability of acetic acid. J. Hosp. Infect. 2013, 84, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Josephrajan, T.; Ramakrishnan, V.T.; Kathiravan, G.; Muthumary, J. Synthesis and antimicrobial studies of some acridinediones and their thiourea derivatives. Arkivoc 2005, 11, 124–136. [Google Scholar]
- Gratzl, G.; Paulik, C.; Hild, S.; Guggenbichler, J.P.; Lackner, M. Antimicrobial activity of poly(acrylic acid) block copolymers. Mater. Sci. Eng. C 2014, 38, 94–100. [Google Scholar] [CrossRef]
- Meeta, M.; Kumar, P.; Narasimhan, B. Synthesis, antimicrobial evaluation and QSAR studies of p-amino benzoic acid derivatives. J. Pharm. Technol. Res. Manag. 2014, 2, 339–356. [Google Scholar]
- Sariguney, A.B.; Kocabas, E.; Erci, F.; Torlak, E.; Coskun, A. Synthesis and Antimicrobial Activity of Some 2-aminothiazole and 2-aminothiadiazine Derivatives. J. Heterocycl. Chem. 2018, 55, 2107–2110. [Google Scholar] [CrossRef]
- Das, U.N. Arachidonic acid and other unsaturated fatty acids and some of their metabolites function as endogenous antimicrobial molecules: A review. J. Adv. Res. 2018, 11, 57–66. [Google Scholar] [CrossRef]
- Leong, H.J.; Oh, S.G. Preparation of antibacterial TiO2 particles by hybridization with azelaic acid for applications in cosmetics. J. Ind. Eng. Chem. 2018, 66, 242–247. [Google Scholar] [CrossRef]
- Nuta, D.C.; Chifiriuc, M.C.; Draghici, C.; Limban, C.; Missir, A.V.; Morusciag, L. Synthesis, characterization and antimicrobial activity evaluation of new agents from benzamides class. Farmacia 2013, 61, 966–974. [Google Scholar]
- Kim, M.G.; Lee, H.S. 1,2-benzenediol isolated from persimmon roots and its structural analogues show antimicrobial activities against food-borne bacteria. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 429–433. [Google Scholar] [CrossRef]
- Abdelkader, M.S.A.; Rateb, M.E.; Mohamed, G.A.; Jaspars, M. Harpulliasides A and B: Two new benzeneacetic acid derivatives from Harpullia pendula. Phytochem. Lett. 2016, 15, 131–135. [Google Scholar] [CrossRef]
- Li, J.; Duan, M.; Yao, X.; Tian, D.; Tang, J. Prenylated benzenepropanoic acid analogues from the Citrus grandis (L.) Osbeck and their anti-neuroinflammatory activity. Fitoterapia 2019, 139, 104410. [Google Scholar] [CrossRef] [PubMed]
- Igwe, C.N.; Okoro, U.C. Synthesis, Characterization and Evaluation for Antibacterial and Antifungal Activites of N-Heteroaryl Substituted Benzene Sulphonamides. Org. Chem. Int. 2014. [Google Scholar] [CrossRef][Green Version]
- Ambala, A.; Lincoln, C.A. Synthesis, characterization, antimicrobial activity and DNA cleavage study of (E)-2-(((2-(P-Tolyloxy)Quinolin-3-Yl)Methylene)Amino)Benzenethiol Schiff base metal complexes. Chem. Data Collect. 2020, 27. [Google Scholar] [CrossRef]
- Vicini, P.; Zani, F.; Cozzini, P.; Doytchinova, I. Hydrazones of 1,2-benzisothiazole hydrazides: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2002, 37, 553–564. [Google Scholar] [CrossRef]
- Alex, D.; Gay-Andrieu, F.; May, J.; Thampi, L.; Dou, D.; Mooney, A.; Groutas, W.; Calderone, R. Amino Acid-Derived 1,2-Benzisothiazolinone Derivatives as Novel Small-Molecule Antifungal Inhibitors: Identification of Potential Genetic Targets. Antimicrob. Agents Chemother. 2012, 56, 4630–4639. [Google Scholar] [CrossRef]
- Rakesh, K.P.; Shantharam, C.S.; Sridhara, M.B.; Manukumar, H.M.; Qin, H.L. Benzisoxazole: A privileged scaffold for medicinal chemistry. Med. Chem. Commun. 2017, 8, 2023–2039. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, Q. Antibacterial activity of acidified sodium benzoate against Escherichia coli O157:H7, Salmonella enterica, and Listeria monocytogenes in tryptic soy broth and on cherry tomatoes. Int. J. Food Microbiol. 2018, 274, 38–44. [Google Scholar] [CrossRef]
- Leite, A.C.L.; da Silva, K.P.; de Souza, I.A.; de Araujo, J.M.; Brondani, D.J. Synthesis, antitumour and antimicrobial activities of new peptidyl derivatives containing the 1,3-benzodioxole system. Eur. J. Med. Chem. 2004, 39, 1059–1065. [Google Scholar] [CrossRef]
- Park, E.S.; Moon, W.S.; Song, M.J.; Kim, M.N.; Chung, K.H.; Yoon, J.S. Antimicrobial activity of phenol and benzoic acid derivatives. Int. Biodeterior. Biodegrad. 2001, 47, 209–214. [Google Scholar] [CrossRef]
- Carcamo-Noriega, E.N.; Sathyamoorthi, S.; Banerjee, S.; Gnanamani, E.; Mendoza-Trujillo, M.; Mata-Espinosa, D.; Hernandez-Pando, R.; Veytia-Bucheli, J.I.; Possani, L.D.; Zare, R.N. 1,4-Benzoquinone antimicrobial agents against Staphylococcus aureus and Mycobacterium tuberculosis derived from scorpion venom. Proc. Natl. Acad. Sci. USA 2019, 116, 12642–12647. [Google Scholar] [CrossRef] [PubMed]
- Rida, S.M.; Ashour, F.A.; El-Hawash, S.A.M.; El-Semary, M.M.; Badr, M.H.; Shalaby, M.A. Synthesis of some novel benzoxazole derivatives as anticancer, anti-HIV-1 and antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 949–959. [Google Scholar] [CrossRef] [PubMed]
- Kashid, A.M.; Dube, P.N.; Alkutkar, P.G.; Bothara, K.G.; Mokale, S.N.; Dhawale, S.C. Synthesis, biological screening and ADME prediction of benzylindole derivatives as novel anti-HIV-1, anti-fungal and anti-bacterial agents. Med. Chem. Res. 2013, 22, 4633–4640. [Google Scholar] [CrossRef]
- Gouda, K.G.M.; Kavitha, M.D.; Sarada, R. Antihyperglycemic, antioxidant and antimicrobial activities of the butanol extract from Spirulina Platensis. J. Food Biochem. 2015, 39, 594–602. [Google Scholar] [CrossRef]
- Namkung, H.; Yu, H.; Gong, J.; Leeson, S. Antimicrobial activity of butyrate glycerides towards Salmonella Typhimurium and Clostridium perfringens. Poultr. Sci. 2011, 90, 2217–2222. [Google Scholar] [CrossRef]
- Kratky, M.; Vinsova, J. Salicylanilide N-monosubstituted carbamates: Synthesis and in vitro antimicrobial activity. Bioorg. Med. Chem. 2016, 24, 1322–1330. [Google Scholar] [CrossRef]
- Zanatta, N.; Borchhardt, D.M.; Alves, S.H.; Coelho, H.S.; Squizani, A.M.C.; Marchi, T.M.; Bonacorso, H.G.; Martins, M.A.P. Synthesis and antimicrobial activity of new (4,4,4-trihalo-3-oxo-but-1-enyl)-carbamic acid ethyl esters, (4,4,4-trihalo-3-hydroxy-butyl)-carbamic acid ethyl esters, and 2-oxo-6-trihalomethyl-[1,3]oxazinane-3-carboxylic acid ethyl esters. Bioorg. Med. Chem. 2006, 14, 3174–3184. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Jain, S.; Kumar, G. Synthesis, antimicrobial evaluation, QSAR and in silico ADMET studies of decanoic acid derivatives. Pol. Pharm. Soc. Drug Res. 2011, 68, 191–204. [Google Scholar]
- Al-Dhabi, N.A.; Arasu, M.V.; Rejiniemon, T.S. In Vitro Antibacterial, Antifungal, Antibiofilm, Antioxidant, and Anticancer Properties of Isosteviol Isolated from Endangered Medicinal Plant Pittosporum tetraspermum. Evid.-Based Complem. Altern. Med. 2015, 2015, 164261. [Google Scholar] [CrossRef]
- Shin, S.Y.; Bajpai, V.K.; Kim, H.R.; Kang, S.C. Antibacterial activity of bioconverted eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) against foodborne pathogenic bacteria. Int. J. Food Microbiol. 2007, 113, 233–236. [Google Scholar] [CrossRef]
- Lakshmi, S.A.; Bhaskar, J.P.; Krishnan, V.; Sethupathy, S.; Pandipriya, S.; Aruni, W.; Pandian, S.K. Inhibition of biofilm and biofilm-associated virulence factor production in methicillin-resistant Staphylococcus aureus by docosanol. J. Biotechnol. 2020, 317, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Abdalha, A.A.; Mekawey, A.A.I. Antimicrobial Susceptibility of Certain Fungal and Bacterial Strains to Dodecanamide and Quinazolinone Derivatives. World Appl. Sci. J. 2013, 24, 312–319. [Google Scholar]
- Oh, D.H.; Marshall, D.L. Antimicrobial activity of ethanol, glycerol monolaurate or lactic acid against List. Monocytogenes. Int. J. Food Microbiol. 1993, 20, 239–246. [Google Scholar] [CrossRef]
- Tamokou, J.D.D.; Mpetga, D.J.S.; Lunga, P.K.; Tene, M.; Tane, P.; Kuiate, J.R. Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia (Mimosoideae). Bmc Complem. Altern. Med. 2012, 12. [Google Scholar] [CrossRef]
- Huisjes, E.H.; de Hulster, E.; van Dam, J.C.; Pronk, J.T.; van Maris, A.J.A. Galacturonic Acid Inhibits the Growth of Saccharomyces cerevisiae on Galactose, Xylose, and Arabinose. Appl. Environ. Microbiol. 2012, 78, 5052–5059. [Google Scholar] [CrossRef]
- Nieto-Penalver, C.G.; Savino, M.J.; Bertini, E.V.; Sanchez, L.A.; de Figueroa, L.I.C. Gluconic acid produced by Gluconacetobacter diazotrophicus Pal5 possesses antimicrobial properties. Res. Microbiol. 2014, 165, 549–558. [Google Scholar] [CrossRef]
- Berger, F.M.; Hubbard, C.V.; Ludwig, B.J. The Antimicrobial Action of Certain Glycerol Ethers and Related Compounds. Appl. Microbiol. 1953, 1, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Nurullaeva, M.K.; Azizov, U.M.; Mikhlina, E.E.; Turchin, K.F.; Silin, V.A.; Yakhontov, L.N. Synthesis of (3-pyridyl)glyoxylic acid derivatives and their antimicrobial properties. Pharmaceut. Chem. J. 1987, 20, 563–567. [Google Scholar] [CrossRef]
- Liu, H.; Lepoittevin, B.; Roddier, C.; Guerineau, V.; Bech, L.; Herry, J.M.; Bellon-Fontaine, M.N.; Roger, P. Facile synthesis and promising antibacterial properties of a new guaiacol-based polymer. Polymer 2011, 52, 1908–1916. [Google Scholar] [CrossRef]
- Vasudevan, A.; Vijayan, D.; Mandal, P.; Haridas, M. Anti-inflammatory Property of n-Hexadecanoic Acid: Structural Evidence and Kinetic Assessment. Chem. Biol. Drug Des. 2010, 9, 1236–1240. [Google Scholar]
- Narasimhan, B.; Judge, V.; Narang, R.; Ohlan, R.; Ohlan, S. Quantitative structure-activity relationship studies for prediction of antimicrobial activity of synthesized 2,4-hexadienoic acid derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 5835–5845. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.H.; Jiang, M.H. Evaluation of antibacterial activity of hexanedioic acid isolated from Hermetia illucens larvae. J. Appl. Biomed. 2014, 12, 179–189. [Google Scholar] [CrossRef]
- Alva-Murillo, N.; Ochoa-Zarzosa, A.; Lopez-Meza, J.E. Short chain fatty acids (propionic and hexanoic) decrease Staphylococcus aureus internalization into bovine mammary epithelial cells and modulate antimicrobial peptide expression. Vet. Microbiol. 2012, 155, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Kratky, M.; Vinsova, J.; Volkova, M.; Buchta, V.; Trejtnar, F.; Stolarikova, J. Antimicrobial activity of sulfonamides containing 5-chloro-2-hydroxybenzaldehyde and 5-chloro-2-hydroxybenzoic acid scaffold. Eur. J. Med. Chem. 2012, 50, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, Z.; Li, J.; Yang, X.; Fei, B.; Leung, P.H.M.; Tao, X. A New Antimicrobial Agent: Poly (3-hydroxybutyric acid) Oligomer. Macromol. Biosci. 2019, 19. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, A.K. Synthesis and antimicrobial activity of some new diphenylamine derivatives. J. Pharm. Bioallied Sci. 2015, 7, 81–85. [Google Scholar] [CrossRef]
- Sakko, M.; Moore, C.; Novak-Fraser, L.; Rautemaa, V.; Sorsa, T.; Hietala, P.; Jarvinen, A.; Bowyer, P.; Tjaderhane, L.; Rautemaa, R. 2-hydroxyisocaproic acid is fungicidal for Candida and Aspergillus species. Mycoses 2014, 57, 214–221. [Google Scholar] [CrossRef]
- Sundar, L.; Chang, F.N. Antimicrobial activity and biosynthesis of indole antibiotics produced by Xenorhabdus nematophilus. J. Gen. Microbiol. 1993, 139, 3139–3148. [Google Scholar] [CrossRef]
- Himaja, M.; Jose, T.; Ramana, M.V.; Anand, R.; Munirajasekhar, D. Synthesis and biological evaluation of indole-3-carboxylic acid derivatives of amino acids and peptides. Int. Res. J. Pharm. 2010, 1, 436–440. [Google Scholar]
- In, Y.W.; Kim, J.J.; Kim, H.J.; Oh, S.W. Antimicrobial activities of acetic acid, citric acid and lactic acid against Shigella species. J. Food Saf. 2013, 33, 79–85. [Google Scholar] [CrossRef]
- Ferrazzano, L.; Viola, A.; Lonati, E.; Bulbarelli, A.; Musumeci, R.; Cocuzza, C.; Lombardo, M.; Tolomelli, A. New isoxazolidinone and 3,4-dehydro-β-proline derivatives as antibacterial agents as MAO-inhibitors: A complex balance between two activities. Eur. J. Med. Chem. 2010, 124, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Velikova, M.; Bankova, V.; Tsvetkova, I.; Kujumgiev, A.; Marcucci, M.C. Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 2000, 71, 693–696. [Google Scholar] [CrossRef]
- Wang, C.; Chang, T.; Yang, H.; Cui, M. Antibacterial mechanism of lactic acid on physiological and morphological properties of Salmonella Enteritidis, Escherichia coli and Listeria monocytogenes. Food Control 2015, 47, 231–236. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, Y.S.; Shin, D.H. Antimicrobial Synergistic Effect of Linolenic Acid and Monoglyceride against Bacillus cereus and Staphylococcus aureus. J. Agric. Food Chem. 2002, 50, 2193–2199. [Google Scholar] [CrossRef]
- Orhan, I.; Ozeelik, B.; Aslan, S.; Kartal, M.; Karaoglu, T.; Sener, B.; Terzioglu, S.; Choudhary, M.I. Antioxidant and antimicrobial actions of the clubmoss Lycopodium clavatum L. Phytochem. Rev. 2007, 6, 189–196. [Google Scholar] [CrossRef]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martin-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella Enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control 2009, 20, 105–112. [Google Scholar] [CrossRef]
- Motamedifar, M.; Bazargani, A.; Namazi, M.R.; Sarai, H.S.E. Antimicrobial Activity of Mandelic Acid Against Methicillin-Resistant Staphylococcus aureus: A Novel Finding with Important Practical Implications. World Appl. Sci. J. 2014, 31, 925–929. [Google Scholar]
- Ristovski, J.T.; Jankovic, N.; Borcic, V.; Jain, S.; Bugarcic, Z.; Mikov, M. Evaluation of antimicrobial activity and retention behavior of newly synthesized vanilidene derivatives of Meldrum’s acids using QSRR approach. J. Pharm. Biomed. Anal. 2018, 155, 42–49. [Google Scholar] [CrossRef]
- Karaman, I.; Sahin, F.; Gulluce, M.; Ogutcu, H.; Sengul, M.; Adiguzel, A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J. Ethnopharmacol. 2003, 85, 231–235. [Google Scholar] [CrossRef]
- Kern, E.R.; Kushner, N.L.; Hartline, C.B.; Williams-Aziz, S.L.; Harden, E.A.; Zhou, S.; Zemlicka, J.; Prichard, M.N. In Vitro Activity and Mechanism of Action of Methylenecyclopropane Analogs of Nucleosides against Herpesvirus Replication. Antimicrob. Agents Chemother. 2005, 49, 1039–1045. [Google Scholar] [CrossRef]
- Yoo, J.C.; Han, J.M.; Nam, S.K.; Ko, O.H.; Choi, C.H.; Kee, K.H.; Sohng, J.K.; Jo, J.S.; Seong, C.N. Characterization and Cytotoxic Activities of Nonadecanoic Acid Produced by Streptomyces scabiei subsp. chosunensi M0137 (KCTC 9927). J. Microbiol. 2002, 40, 331–334. [Google Scholar]
- Sahin, N.; Kula, I.; Erdogan, Y. Investigation of Antimicrobial Activities of Nonanoic Acid Derivatives. Fresenius Environ. Bull. 2006, 15, 141–143. [Google Scholar]
- Pu, Z.H.; Zhang, Y.Q.; Yin, Z.Q.; Xu, J.; Jia, R.Y.; Lu, Y.; Yang, F. Antibacterial Activity of 9-Octadecanoic Acid-Hexadecanoic Acid-Tetrahydrofuran-3,4-Diyl Ester from Neem Oil. Agric. Sci. China 2010, 9, 1236–1240. [Google Scholar] [CrossRef]
- Hilgren, J.D.; Salverda, J.A. Antimicrobial Efficacy of a Peroxyacetic/Octanoic Acid Mixture in Fresh-Cut-Vegetable Process Waters. J. Food Sci. 2000, 65, 1376–1379. [Google Scholar] [CrossRef]
- De Lucena, J.M.V.M.; Decker, E.M.; Walter, C.; Boeira, L.S.; Lost, C.; Weiger, R. Antimicrobial effectiveness of intracanal medicaments on Enterococcus faecalis: Chlorhexidine versus octenidine. Int. Endod. J. 2013, 46, 53–61. [Google Scholar] [CrossRef]
- Kuhrt, M.F.; Fancher, M.J.; McKinlay, M.A.; Lennert, S.D. Virucidal Activity of Glutaric Acid and Evidence for Dual Mechanism of Action. Antimicrob. Agents Chemother. 1984, 26, 924–927. [Google Scholar] [CrossRef]
- Roy, S.; Hagen, K.D.; Maheswari, P.U.; Lutz, M.; Spek, A.L.; Reedijk, J.; van Wezel, G.P. Phenanthroline Derivatives with Improved Selectivity as DNA-Targeting Anticancer or Antimicrobial Drugs. ChemMedChem 2008, 3, 1427–1434. [Google Scholar] [CrossRef]
- Mazimba, O.; Wale, K.; Loeto, D.; Kwape, T. Antioxidant and antimicrobial studies on fused-ring pyrazolones and isoxazolones. Bioorg. Med. Chem. 2014, 22, 6564–6569. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Ferrer-Luque, C.M.; Espigares-Rodriguez, E.; Liebana-Urena, J.; Espigares-Garcia, M. Bactericidal activity of phosphoric acid, citric acid, and EDTA solutions against Enterococcus faecalis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 84–89. [Google Scholar] [CrossRef]
- Evren, A.E.; Yurttas, L.; Yilmaz-Cankilic, M. Synthesis of novel N-(naphthalen-1-yl)propanamide derivatives and evaluation of their antimicrobial activity. Phosphorussulfur Silicon Relat. Elem. 2020, 195, 158–164. [Google Scholar] [CrossRef]
- Desai, N.C.; Pandya, D.; Vaja, D. Synthesis, characterization and antimicrobial studies on 3-((4-(4-nitrophenyl)-6-aryl-1,6-dihydropyrimindin-2-yl)thio)propanenitriles and their derivatives. Med. Chem. Res. 2017, 26, 1089–1097. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Jiang, X.; Jiang, F.; Zhuang, H.; Fu, L. Design, synthesis and antimicrobial activity of chiral 2-(substituted-hydroxyl)-3-(benzo[d]oxazol-5-yl)propanoic acid derivates. Eur. J. Med. Chem. 2011, 46, 3639–3650. [Google Scholar] [CrossRef] [PubMed]
- Helal, M.H.; Abbas, S.Y.; Salem, M.H.; Farag, A.A.; Ammar, Y.A. Synthesis and characterization of new types of 2-(6-methoxy-2-naphthyl)propionamide derivatives as potential antibacterial and antifungal agents. Med. Chem. Res. 2013, 22, 5598–5609. [Google Scholar] [CrossRef]
- El Azab, I.H.; Khalifa, M.E.; Gobouri, A.A.; Altalhi, T.A. Synthesis, Characterization and Pharmacological Evaluation of Some New Pteridine-Based Heterocycles as Antimicrobial Agents. J. Heterocycl. Chem. 2019, 56, 1352–1361. [Google Scholar] [CrossRef]
- Elaasser, M.M.; Abdel-Aziz, M.M.; El-Kassas, R.A. Antioxidant, antimicrobial, antiviral and antitumour activities of pyranone derivative obtained from Aspergillus candidus. J. Microbiol. Biotech. Res. 2011, 1, 5–17. [Google Scholar]
- Premkumar, T.; Govindarajan, S. Antimicrobial study of pyrazine, pyrazole and imidazole carboxylic acids and their hydrazinium salts. World J. Microbiol. Biotechnol. 2005, 21, 479–480. [Google Scholar] [CrossRef]
- Kandile, N.G.; Mohamed, M.I.; Zaky, H.; Mohamed, H.M. Novel pyridazine derivatives: Synthesis and antimicrobial activity evaluation. Eur. J. Med. Chem. 2009, 44, 1989–1996. [Google Scholar] [CrossRef]
- Yurttas, L.; Ozkay, Y.; Kaplancikli, Z.A.; Tunali, Y.; Karaca, H. Synthesis and antimicrobial activity of some new hydrazone-bridged thiazole-pyrrole derivatives. J. Enzym. Inhib. Med. Chem. 2013, 28, 830–835. [Google Scholar] [CrossRef]
- Jacobson, J.G.; Renau, T.E.; Nassiri, M.R.; Sweier, D.G.; Breitenbach, J.M.; Townsend, L.B.; Drach, J.C. Nonnucleoside Pyrrolopyrimidines with a Unique Mechanism of Action against Human Cytomegalovirus. Antimicrob. Agents Chemother. 1999, 43, 1888–1894. [Google Scholar] [CrossRef]
- Purohit, A.; Mohan, A. Antimicrobial effects of pyruvic and succinic acids on Salmonella survival in ground chicken. LWT Food Sci. Technol. 2019, 116, 108596. [Google Scholar] [CrossRef]
- Jafari, E.; Khajouei, M.R.; Hassanzadeh, F.; Hakimelahi, G.H.; Khodarahmi, G.A. Quinazolinone and quinazoline derivatives: Recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci. 2016, 11, 1–14. [Google Scholar] [PubMed]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009, 44, 4637–4647. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.R. Quinolone Molecular Structure-Activity Relationships: What We Have Learned about improving Antimicrobial Activity. Clin. Infect. Dis. 2001, 33, S180–S186. [Google Scholar] [CrossRef]
- Brilisauer, K.; Rapp, J.; Rath, P.; Schollhorn, A.; Bleul, L.; Weiβ, E.; Stahl, M.; Grond, S.; Forchhammer, K. Cyanobacterial antimetabolite 7-deoxy-sedoheptulose blocks the shikimate pathway to inhibit the growth of prototrophic organisms. Nat. Commun. 2019, 10, 545. [Google Scholar] [CrossRef] [PubMed]
- Hans, S.; Sharma, S.; Hameed, S.; Fatima, Z. Sesamol exhibits potent antimycobacterial activity: Underlying mechanisms and impact on virulence traits. J. Glob. Antimicrob. Resist. 2017, 10, 228–237. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Abdel-Hay, F.I.; Shahada, L.; El-Shanshoury, A.E.R.; El-Newehy, M.H. Biologically Active Polymers. IV. Synthesis and Antimicrobial Activity of Tartaric Acid Polyamides. J. Appl. Polym. Sci. 2006, 102, 4780–4790. [Google Scholar] [CrossRef]
- Bondock, S.; Fadaly, W.; Metwally, M.A. Synthesis and antimicrobial activity of some new thiazole, thiophene and pyrazole derivatives containing benzothiazole moiety. Eur. J. Med. Chem. 2010, 45, 3692–3701. [Google Scholar] [CrossRef]
- Saeed, S.; Rashid, N.; Jones, P.G.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- Bayrak, H.; Demirbas, A.; Karaoglu, S.A.; Demirbas, N. Synthesis of some new 1,2,4-triazoles, their Mannich and Schiff bases and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 1057–1066. [Google Scholar] [CrossRef]
- Dolezalova, M.; Janis, R.; Svobodova, H.; Kasparkova, V.; Humpolicek, P.; Krejci, J. Antimicrobial properties of 1-monoacylglycerols prepared from undecanoic (C11:0) and undecanoic (C11:1) acid. Eur. J. Lipid Sci. Technol. 2010, 112, 1106–1114. [Google Scholar] [CrossRef]
- Grether-Beck, S.; Felsner, I.; Brenden, H.; Kohne, Z.; Majora, M.; Marini, A.; Jaenicke, T.; Rodriguez-Martin, M.; Trullas, C.; Hupe, M.; et al. Urea Uptake Enhances Barrier Function and Antimicrobial Defense in Humans by Regulating Epidermal Gene Expression. J. Investig. Dermatol. 2012, 132, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Kawsar, S.M.A.; Islam, M.; Jesmin, S.; Manchur, M.A.; Hasan, I.; Rajia, S. Evaluation of the antimicrobial activity and cytotoxic effect of some uridine derivatives. Int. J. Biosci. 2018, 12, 211–219. [Google Scholar]
- Wheatley, R.E. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 2002, 81, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Audrain, B.; Farag, M.A.; Ryu, C.M.; Ghigo, J.M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Caulier, S.; Nannan, C.; Gillis, A.; Licciardi, F.; Bragard, C.; Mahilon, J. Overview of the Antimicrobial Compounds Produced by Members of the Bacillus subtilis Group. Front. Microbiol. 2019, 10, 302. [Google Scholar] [CrossRef]
- Stein, T. Bacillus subtilis antibiotics: Structures, syntheses and specific functions. Mol. Microbiol. 2005, 56, 845–857. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria Antimicrobial Compounds: Characteristics and Applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]
- Niku-Paavola, M.L.; Laitila, A.; Mattila-Sandholm, T.; Haikara, A. New types of antimicrobial compounds produced by Lactobacillus plantarum. J. Appl. Microbiol. 1999, 86, 29–35. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2019, 56, 138–146. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).