Removal of Lead, Cadmium, and Aluminum Sulfate from Simulated and Real Water with Native and Oxidized Starches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
- the heavy metal ion and sulfate ion concentrations in simulated wastewater. Thus, 5 standards were used: Standard 1: S (100 mg/L); Standard 2: Pb, Al, Cd, and S (each 50 mg/L), Standard 3: Pb, Al, Cd, and S (each 10 mg/L), Standard 4: Pb, Al, Cd, and S (each 5 mg/L), Standard 5: Pb, Al, Cd, and S (each 1 mg/L), Standard 6: Pb, Al, Cd, and S (each 0.5 mg/L). Each standard contained 4 wt % HNO3. To each sample of 8 mL, we added 2 mL of 20 wt % nitric acid prior to analysis. From the threefold measurement of all standards, we constructed a calibration curve. All samples were measured three times. The detection limits in the measurement solutions were given with 0.05 mg/L Al, 0.01 mg/L Cd, 0.05 mg/L Pb, and 0.06 mg/L S.
- potential inorganic impurities in the starch samples. First, 100 mg of each sample was digested by 3.5 mL 5 M HNO3 and 0.5 mL 30 wt % H2O2 in a 10 mL microwave vessel using a Discover SP microwave oven (CEM Corporation, Matthews, NC, USA) in a two-step dynamic, pressurized process (first step: 50 W max./85 °C max./17 bar max./5 min/high magnetic stirring; depressurizing; second step: 100 W max./100 °C max./17 bar max./5 min/high magnetic stirring) (modified method from Gonzalez et. al. [51]). Afterwards, the sample was quantitatively transferred into a 50 mL measuring flask with water and filled up to a total volume of 50 mL. The sample was shaken for 24 h. To each ICP-OES sample of 8 mL, we added 2 mL of 12 wt % HNO3. The following standards were used: Standard 1: Ca, Na, K, S, P, and Si (each 500 mg/L); Standard 2: Ca, Na, K, S, P, and Si (each 100 mg/L); Standard 3: Ca, Na, K, S, P, and Si (each 50 mg/L); Standard 4: Ca, Na, K, S, P, and Si (each 10 mg/L); Standard 1: Ca, Na, K, S, P, and Si (each 5 mg/L); Standard 5: Ca, Na, K, S, P, and Si (each 1 mg/L). Each standard contained 4 wt % HNO3. From the threefold measurement of all standards, we constructed a calibration curve. All samples were measured three times. The given detection limits were calculated by the software QtegraTM from Thermo Scientific, Waltham, MA, USA. The detection limits in the measurement solutions were given with 0.05 mg/L Ca, 0.09 mg/L Na, 0.55 mg/L K, 0.14 mg/L S, 0.05 mg/L P, and 0.03 mg/L for Si.
2.3. Adsorption Experiments
2.3.1. Simulated Water Samples
2.3.2. Real Water Samples
2.4. Theoretical Model
3. Results and Discussion
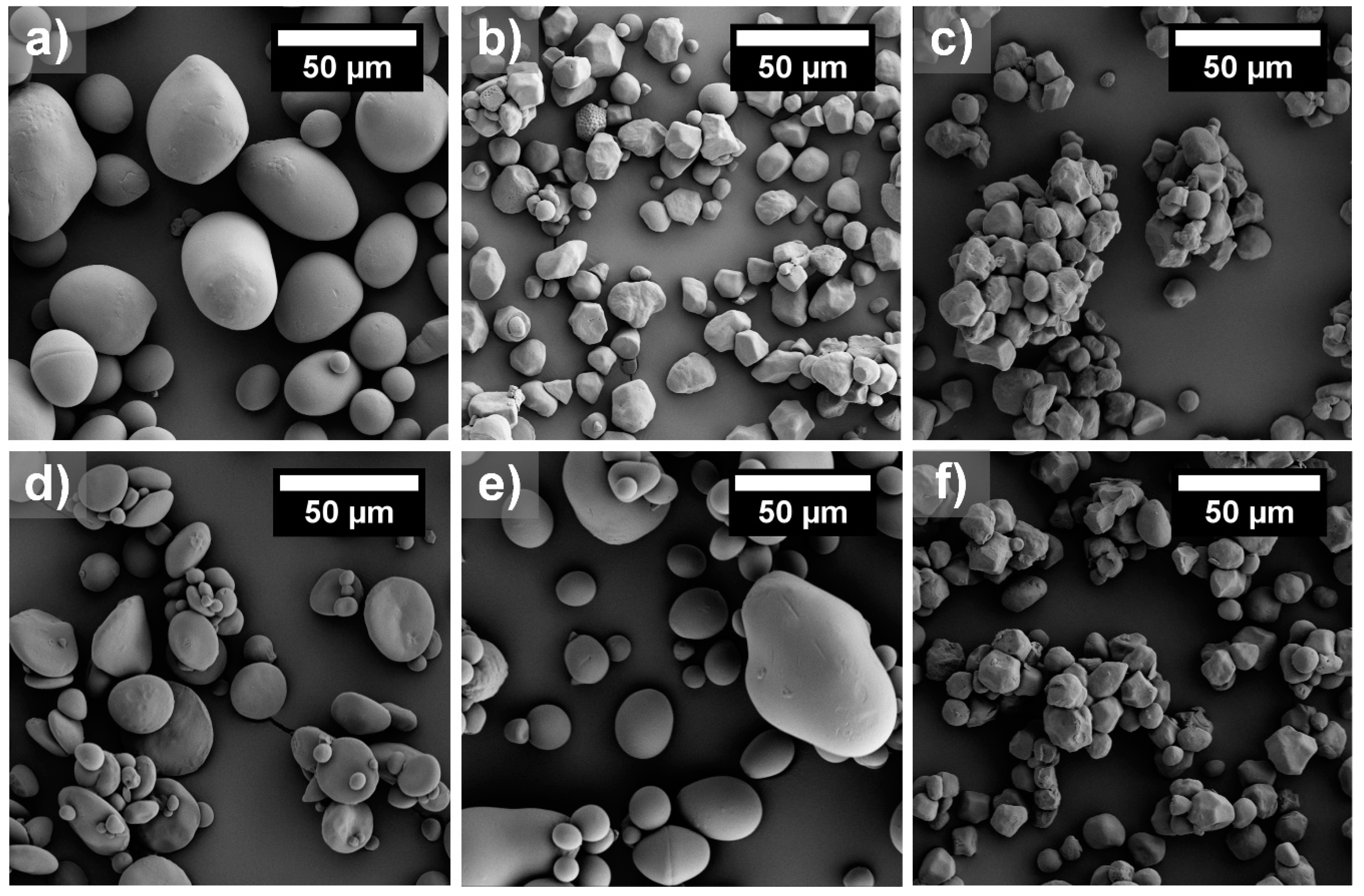
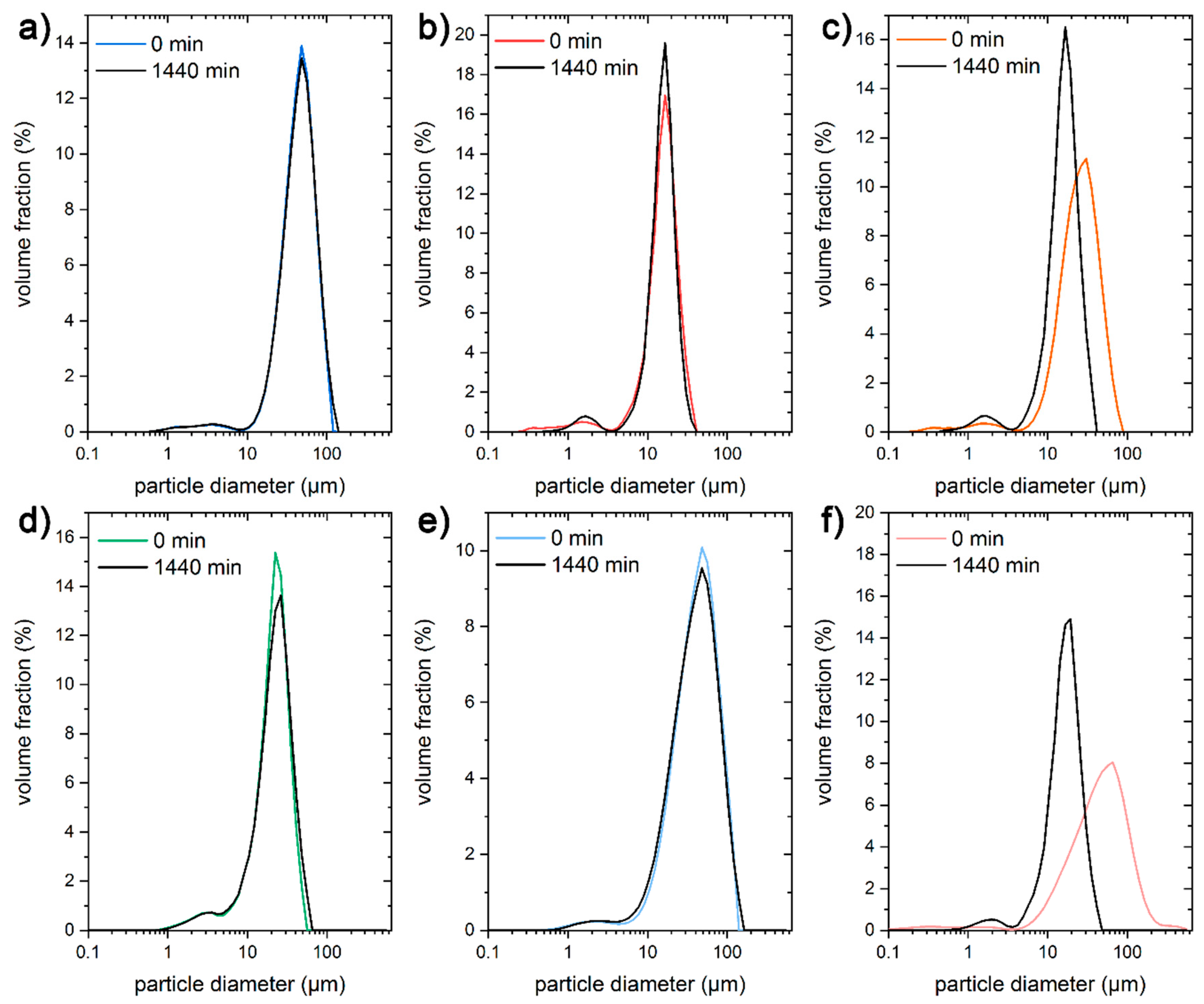
3.1. Characterisation of Starch
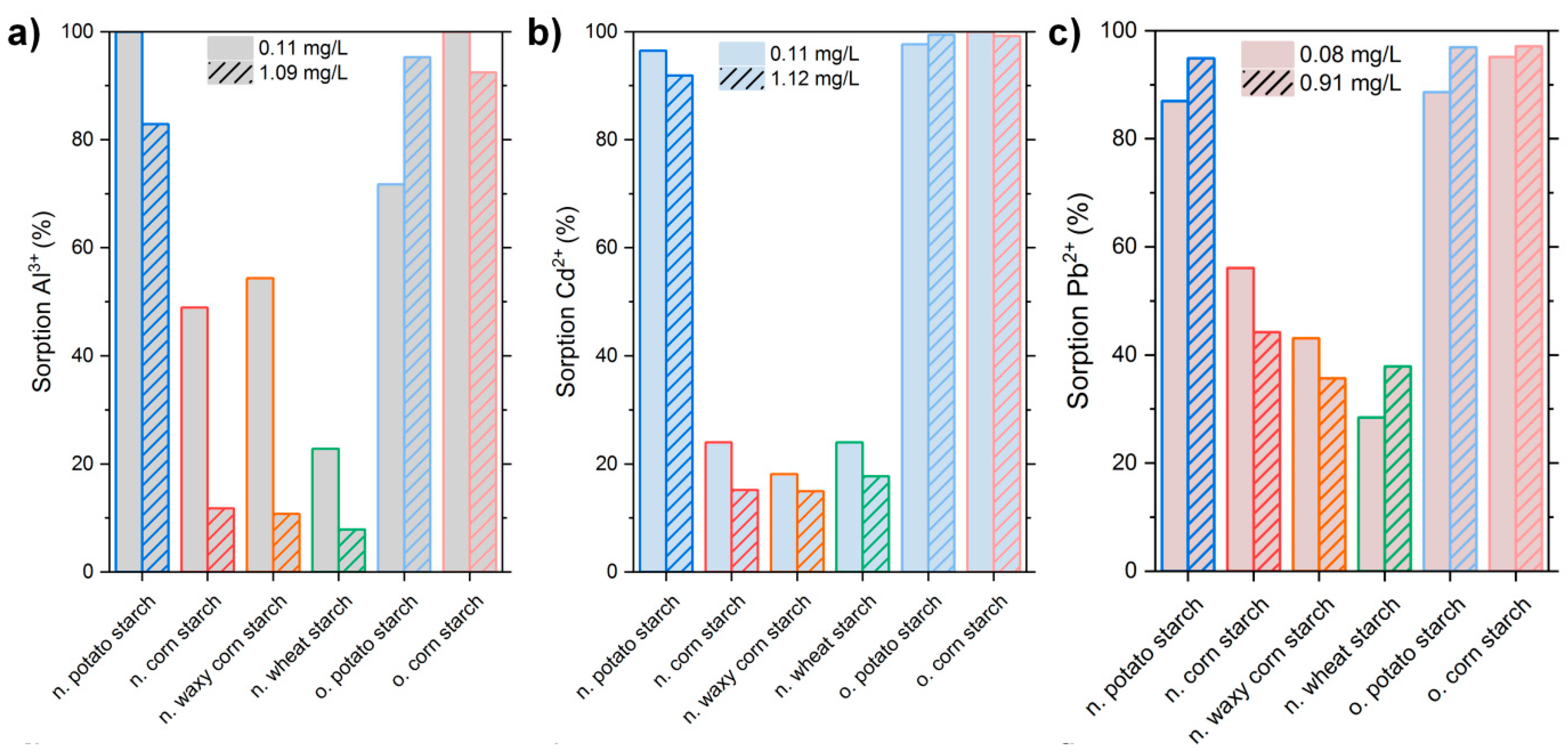
3.2. Low-Concentration Sorption Experiments
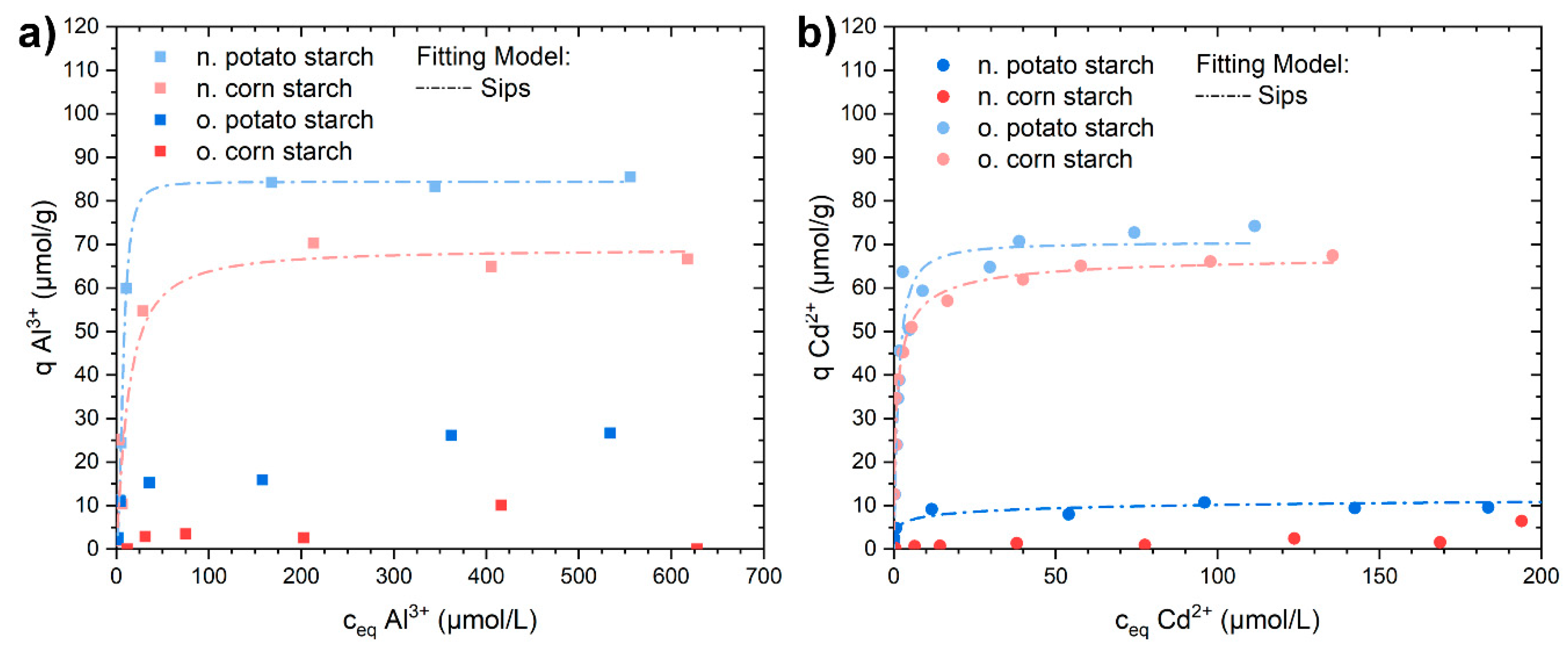
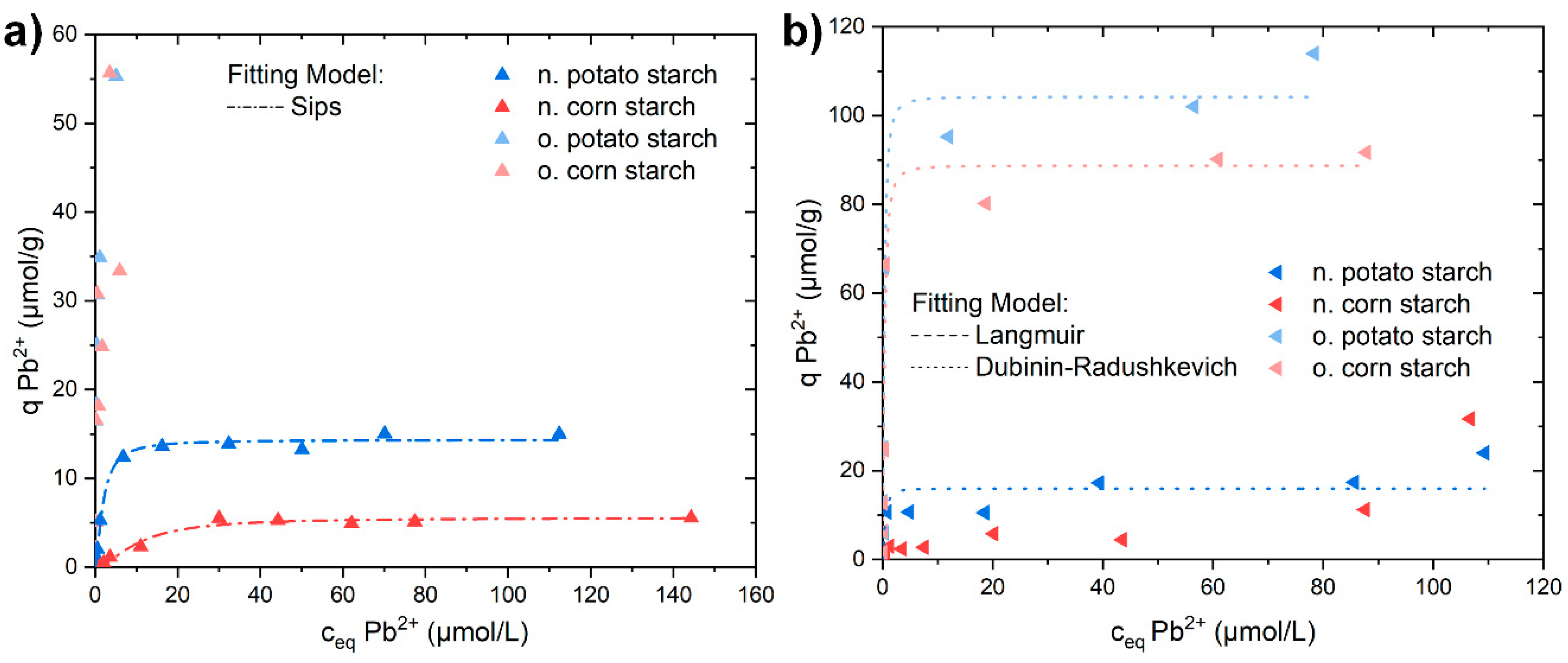
3.3. Sorption Isotherm Experiments with Al3+, Pb2+, and Cd2+
3.4. Pb2+ Batch Adsorption Experiments with Decreased Adsorbent Dose
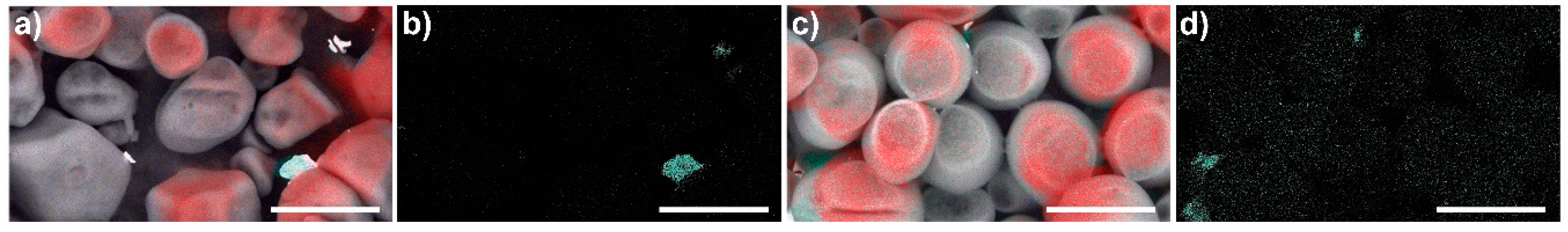
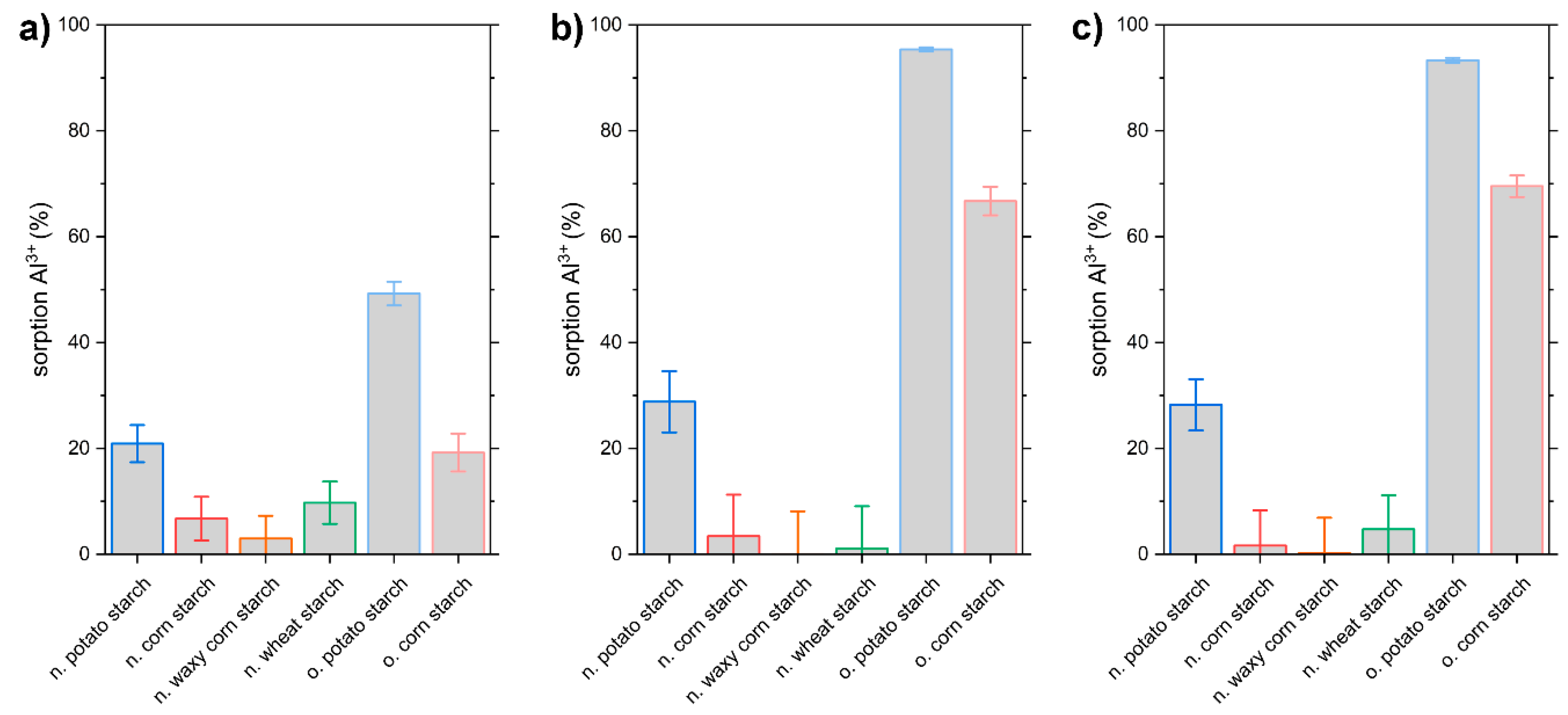
3.5. Al3+ Sorption Experiments with Real Water Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Yang, N.; Li, Y.; Ren, B.; Ding, X.; Bian, H.; Yao, X. Total concentrations and sources of heavy metal pollution in global river and lake water bodies from 1972 to 2017. Glob. Ecol. Conserv. 2020, 22, e00925. [Google Scholar] [CrossRef]
- Soto, D.; Urdaneta, J.; Pernía, K.; León, O.; Muñoz-Bonilla, A.; Fernandez-García, M. Removal of heavy metal ions in water by starch esters. Starch Stärke 2015, 68, 37–46. [Google Scholar] [CrossRef]
- Anghel, N.; Marius, N.; Spiridon, I. Heavy metal adsorption ability of a new composite material based on starch strengthened with chemically modified cellulose. Polym. Adv. Technol. 2019, 30, 1453–1460. [Google Scholar] [CrossRef]
- El-Hamshary, H.; Fouda, M.M.G.; Moydeen, M.; Al-Deyab, S.S. Removal of heavy metal using poly (N-vinylimidazole)-grafted-carboxymethylated starch. Int. J. Biol. Macromol. 2014, 66, 289–294. [Google Scholar] [CrossRef]
- Garcia, M.A.V.T.; Garcia, C.F.; Faraco, A.A.G. Pharmaceutical and Biomedical Applications of Native and Modified Starch: A Review. Starch Stärke 2020, 72, 1900270. [Google Scholar] [CrossRef]
- Gui, Q.; Ouyang, Q.; Xu, C.; Ding, H.; Shi, S.; Chen, X. Facile and Safe Synthesis of Novel Self-Pored Amine-Functionalized Polystyrene with Nanoscale Bicontinuous Morphology. Int. J. Mol. Sci. 2020, 21, 9404. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 9, 10–40. [Google Scholar] [CrossRef] [Green Version]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2020, 251, 116986. [Google Scholar] [CrossRef]
- Weißpflog, J.; Gündel, A.; Vehlow, D.; Steinbach, C.; Müller, M.; Boldt, R.; Schwarz, S.; Schwarz, D. Solubility and Selectivity Effects of the Anion on the Adsorption of Different Heavy Metal Ions onto Chitosan. Molecules 2020, 25, 2482. [Google Scholar] [CrossRef] [PubMed]
- Ghiorghita, C.-A.; Borchert, K.B.L.; Vasiliu, A.-L.; Zaharia, M.-M.; Schwarz, D.; Mihai, M. Porous thiourea-grafted-chitosan hydrogels: Synthesis and sorption of toxic metal ions from contaminated waters. Colloids Surfaces A Physicochem. Eng. Asp. 2020, 607, 125504. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef] [Green Version]
- Borchert, K.B.L.; Steinbach, C.; Schwarz, S.; Schwarz, D. A Comparative Study on the Flocculation of Silica and China Clay with Chitosan and Synthetic Polyelectrolytes. Mar. Drugs 2021, 19, 102. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, B.; Ren, J.; Li, A.; Yang, H. Coagulation/flocculation in dewatering of sludge: A review. Water Res. 2018, 143, 608–631. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of Chitin/Chitosan and Their Derivatives in the Papermaking Industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef] [Green Version]
- van den Broek, L.A.M.; Boeriu, C.G.; Stevens, C.V. (Eds.) Chitin and Chitosan: Properties and Applications; Wiley: Hoboken, NJ, USA, 2020; ISBN 978-1-119-45043-6. [Google Scholar]
- Da Silva, S.B.; Batista, G.L.; Santin, C.K. Chitosan for Sensors and Electrochemical Applications. In Chitin and Chitosan: Properties and Applications; van den Broek, L.A.M., Boeriu, C.G., Stevens, C.V., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 461–476. ISBN 978-1-119-45043-6. [Google Scholar]
- Suginta, W.; Khunkaewla, P.; Schulte, A. Electrochemical Biosensor Applications of Polysaccharides Chitin and Chitosan. Chem. Rev. 2013, 113, 5458–5479. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Zhang, B.; Wang, J.; Xu, J.; Manzoor, K.; Ahmad, S.; Ikram, S. New method for hydrogel synthesis from diphenylcarbazide chitosan for selective copper removal. Int. J. Biol. Macromol. 2019, 136, 189–198. [Google Scholar] [CrossRef]
- Manzoor, K.; Ahmad, M.; Ahmad, S.; Ikram, S. Synthesis, Characterization, Kinetics, and Thermodynamics of EDTA-Modified Chitosan-Carboxymethyl Cellulose as Cu(II) Ion Adsorbent. ACS Omega 2019, 4, 17425–17437. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Bratskaya, S.Y.; Genest, S.; Petzold-Welcke, K.; Heinze, T.; Schwarz, S. Flocculation Efficiency of Novel Amphiphilic Starch Derivatives: A Comparative Study. Macromol. Mater. Eng. 2013, 299, 722–728. [Google Scholar] [CrossRef]
- Krentz, D.-O.; Lohmann, C.; Schwarz, S.; Bratskaya, S.; Liebert, T.; Laube, J.; Heinze, T.; Kulicke, W.-M. Properties and Flocculation Efficiency of Highly Cationized Starch Derivatives. Starch Stärke 2006, 58, 161–169. [Google Scholar] [CrossRef]
- Aly, A.A. Preparation, Characterization and Evaluation of Anionic Starch Derivatives as Flocculants and for Metal Removal. Starch Stärke 2006, 58, 391–400. [Google Scholar] [CrossRef]
- Pant, B.R.; Jeon, H.-J.; Song, H.H. Radiation cross-linked carboxymethylated starch and iron removal capacity in aqueous solution. Macromol. Res. 2011, 19, 307–312. [Google Scholar] [CrossRef]
- Ekebafe, L.O. Removal of heavy metals from aqueous media using native cassava starch hydrogel. Afr. J. Environ. Sci. Technol. 2012, 6, 6. [Google Scholar] [CrossRef]
- Soto, D.; Urdaneta, J.; Pernía, K.; León, O.; Muñoz-Bonilla, A.; Fernández-García, M. Heavy metal (Cd2+, Ni2+, Pb2+and Ni2+) adsorption in aqueous solutions by oxidized starches. Polym. Adv. Technol. 2014, 26, 147–152. [Google Scholar] [CrossRef]
- United Nations Environment Programme, Chemical Branch, DTIE. Final Review of Scientific Information on Lead-Version of December 2010. 2010. Available online: https://wedocs.unep.org/handle/20.500.11822/27635 (accessed on 15 February 2021).
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar] [CrossRef]
- Ahmed, M.J.K.; Ahmaruzzaman, M. A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions. J. Water Process. Eng. 2016, 10, 39–47. [Google Scholar] [CrossRef]
- Das, A.; Bar, N.; Das, S.K. Pb(II) adsorption from aqueous solution by nutshells, green adsorbent: Adsorption studies, regeneration studies, scale-up design, its effect on biological indicator and MLR modeling. J. Colloid Interface Sci. 2020, 580, 245–255. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E. Bioaccumulation of non-essential hazardous heavy metals and metalloids in freshwater fish. Risk to human health. Environ. Chem. Lett. 2018, 16, 903–917. [Google Scholar] [CrossRef]
- Church, B.G.; Van Sprang, P.A.; Chowdhury, M.J.; Deforest, D.K. Updated species sensitivity distribution evaluations for acute and chronic lead toxicity to saltwater aquatic life. Environ. Toxicol. Chem. 2017, 36, 2974–2980. [Google Scholar] [CrossRef]
- Air Quality Guidelines for Europe, 2nd ed.; WHO: Geneva, Switzerland; Regional Office for Europe: Copenhagen, Denmark, 2000; ISBN 978-9-28-901358-1.
- Caravati, E.M. Medical Toxicology, 3rd ed.; Lippincott, Williams & Wilkins: Philadelphia, PA, USA, 2004; ISBN 978-0-7817-2845-4. [Google Scholar]
- Cleveland, L.M.; Minter, M.L.; Cobb, K.A.; Scott, A.A.; German, V.F. Lead hazards for pregnant women and children: Part 1: Immigrants and the poor shoulder most of the burden of lead exposure in this country. Part 1 of a two-part article details how exposure happens, whom it affects, and the harm it can do. Am. J. Nurs. 2008, 108, 40–49. [Google Scholar] [CrossRef]
- Moiseenko, T.I.; Gashkina, N.A. Biogeochemistry of Cadmium: Anthropogenic Dispersion, Bioaccumulation, and Ecotoxicity. Geochem. Int. 2018, 56, 798–811. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Huang, C.-Y.; Chuang, Y.-H.; Chen, H.-W.; Chan, Y.-T.; Teah, H.-Y.; Chen, T.-Y.; Chang, C.-F.; Liu, Y.-T.; Tzou, Y.-M. Accumulation of heavy metals and trace elements in fluvial sediments received effluents from traditional and semiconductor industries. Sci. Rep. 2016, 6, 34250. [Google Scholar] [CrossRef]
- Hutton, M. Sources of cadmium in the environment. Ecotoxicol. Environ. Saf. 1983, 7, 9–24. [Google Scholar] [CrossRef]
- Pavlaki, M.D.; Araújo, M.J.; Cardoso, D.N.; Silva, A.R.R.; Cruz, A.; Mendo, S.; Soares, A.M.V.M.; Calado, R.; Loureiro, S. Ecotoxicity and genotoxicity of cadmium in different marine trophic levels. Environ. Pollut. 2016, 215, 203–212. [Google Scholar] [CrossRef]
- Hartwig, A. Cadmium and Cancer. Metal Ions Life Sci. 2012, 11, 491–507. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [Green Version]
- Mathy, P. (Ed.) Air Pollution and Ecosystems; Springer: Dordrecht, The Netherlands, 1988; ISBN 978-94-010-8276-1. [Google Scholar]
- Rosseland, B.O.; Eldhuset, T.D.; Staurnes, M. Environmental effects of aluminium. Environ. Geochem. Health 1990, 12, 17–27. [Google Scholar] [CrossRef]
- Shinzato, M.C.; Hypolito, R. Effect of disposal of aluminum recycling waste in soil and water bodies. Environ. Earth Sci. 2016, 75, 1–10. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Sekine, Y.; Otoshi, T. Atmospheric aluminum from human activities. Atmos. Environ. Part B Urban Atmos. 1992, 26, 295–300. [Google Scholar] [CrossRef]
- Igbokwe, I.O.; Igwenagu, E.; Igbokwe, N.A. Aluminium toxicosis: A review of toxic actions and effects. Interdiscip. Toxicol. 2019, 12, 45–70. [Google Scholar] [CrossRef] [Green Version]
- Gensemer, R.W.; Playle, R.C. The Bioavailability and Toxicity of Aluminum in Aquatic Environments. Crit. Rev. Environ. Sci. Technol. 1999, 29, 315–450. [Google Scholar] [CrossRef]
- Choksi, P.M.; Joshi, V.Y. Adsorption kinetic study for the removal of nickel (II) and aluminum (III) from an aqueous solution by natural adsorbents. Desalination 2007, 208, 216–231. [Google Scholar] [CrossRef]
- Gonzalez, M.H.; Souza, G.B.; Oliveira, R.V.; Forato, L.A.; Nóbrega, J.A.; Nogueira, A.R.A. Microwave-assisted digestion procedures for biological samples with diluted nitric acid: Identification of reaction products. Talanta 2009, 79, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.S.; Gorton, L.; Bergquist, K.-E.; Nilsson, U.J. Determination of the Degree of Branching in Normal and Amylopectin Type Potato Starch with1H-NMR Spectroscopy Improved resolution and two-dimensional spectroscopy. Starch Stärke 1996, 48, 352–357. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef] [Green Version]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Dubinin, M.M. The Equation of the Characteristic Curve of Activated Charcoal. Proceedings of the USSR Academy of Sciences 1947, 55, 327–329. [Google Scholar]
- Kamga, F.T. Modeling adsorption mechanism of paraquat onto Ayous (Triplochiton scleroxylon) wood sawdust. Appl. Water Sci. 2018, 9, 1. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Cael, J.J.; Koenig, J.L.; Blackwell, J. Infrared and Raman spectroscopy of carbohydrates. Part VI: Normal coordinate analysis of V-amylose. Biopolymers 1975, 14, 1885–1903. [Google Scholar] [CrossRef]
- Cael, S.J.; Koenig, J.L.; Blackwell, J. Infrared and raman spectroscopy of carbohydrates. Carbohydr. Res. 1973, 29, 123–134. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of Irradiated Starches by Using FT-Raman and FTIR Spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Kuakpetoon, D.; Wang, Y.-J. Locations of hypochlorite oxidation in corn starches varying in amylose content. Carbohydr. Res. 2008, 343, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-R.; Wang, X.-L.; Zhao, G.-M.; Wang, Y.-Z. Preparation and properties of oxidized starch with high degree of oxidation. Carbohydr. Polym. 2012, 87, 2554–2562. [Google Scholar] [CrossRef]
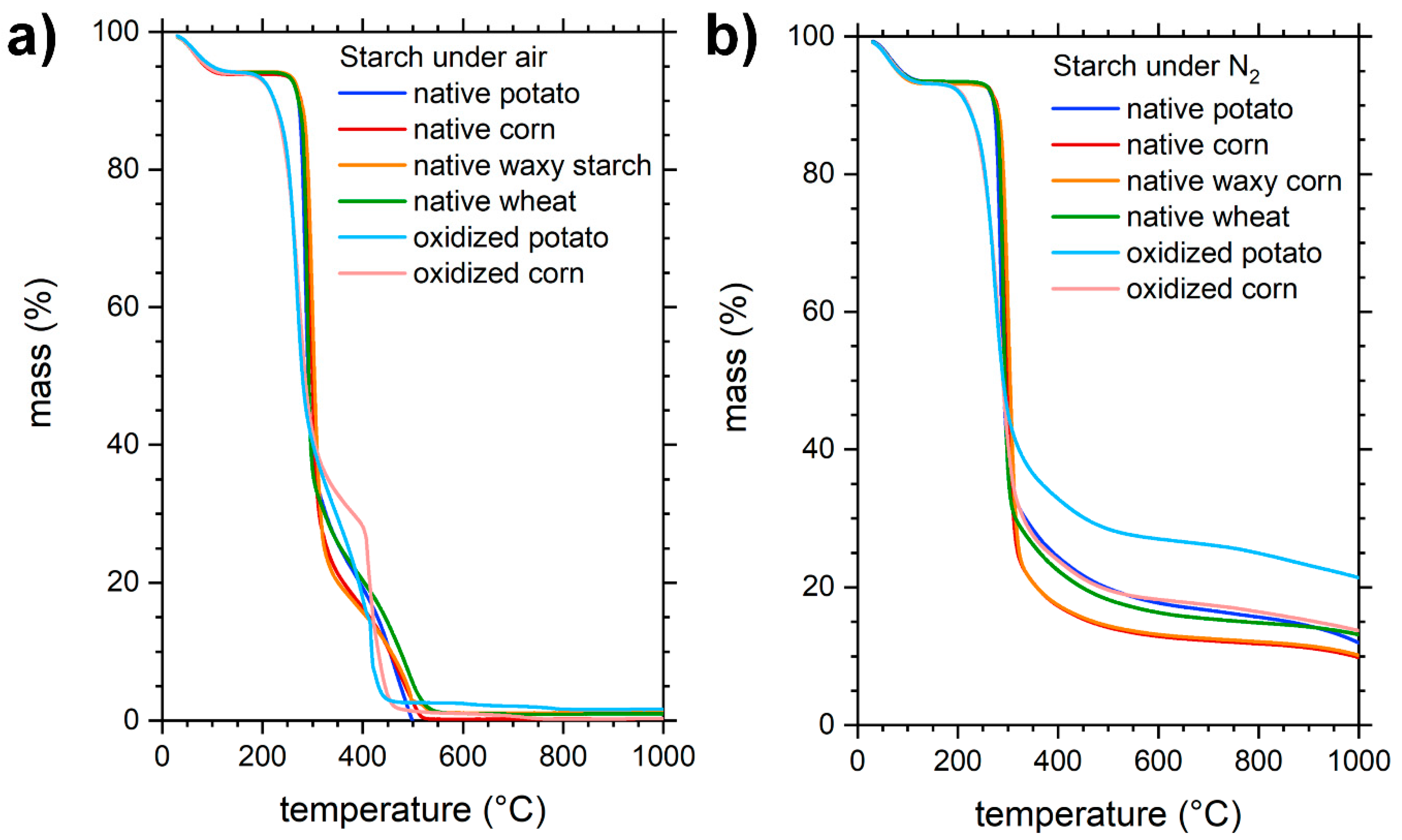
- Liu, Y.; Yang, L.; Ma, C.; Zhang, Y. Thermal Behavior of Sweet Potato Starch by Non-Isothermal Thermogravimetric Analysis. Materials 2019, 12, 699. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, Y.; Yu, L.; Tong, Z.; Chen, L.; Liu, H.; Li, X. Thermal degradation and stability of starch under different processing conditions. Starch Stärke 2012, 65, 48–60. [Google Scholar] [CrossRef]
- Liu, X.; Yu, L.; Xie, F.; Li, M.; Chen, L.; Li, X. Kinetics and mechanism of thermal decomposition of cornstarches with different amylose/amylopectin ratios. Starch Stärke 2010, 62, 139–146. [Google Scholar] [CrossRef]
- Soliman, A.A.A.; El-Shinnawy, N.A.; Mobarak, F. Thermal behaviour of starch and oxidized starch. Thermochim. Acta 1997, 296, 149–153. [Google Scholar] [CrossRef]
- Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2017; ISBN 978-92-4-154995-0.
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerin der Justiz und für Verbraucherschutz-Trinkwasserverordnung, Anlage 2 (zu § 6 Absatz 2): TrinkwV, 2019.




| Sample | C (wt %) | H (wt %) | N (wt %) | Residue (wt %) |
|---|---|---|---|---|
| Native potato starch | 37.7 | 6.0 | 0 | 56.3 |
| Native corn starch | 39.8 | 6.3 | 0 | 54.0 |
| Native waxy corn starch | 39.6 | 6.1 | 0 | 54.3 |
| Native wheat starch | 39.7 | 6.3 | 0 | 54.1 |
| Oxidized potato starch | 39.3 | 6.2 | 0 | 54.6 |
| Oxidized corn starch | 37.4 | 5.8 | 0 | 56.9 |
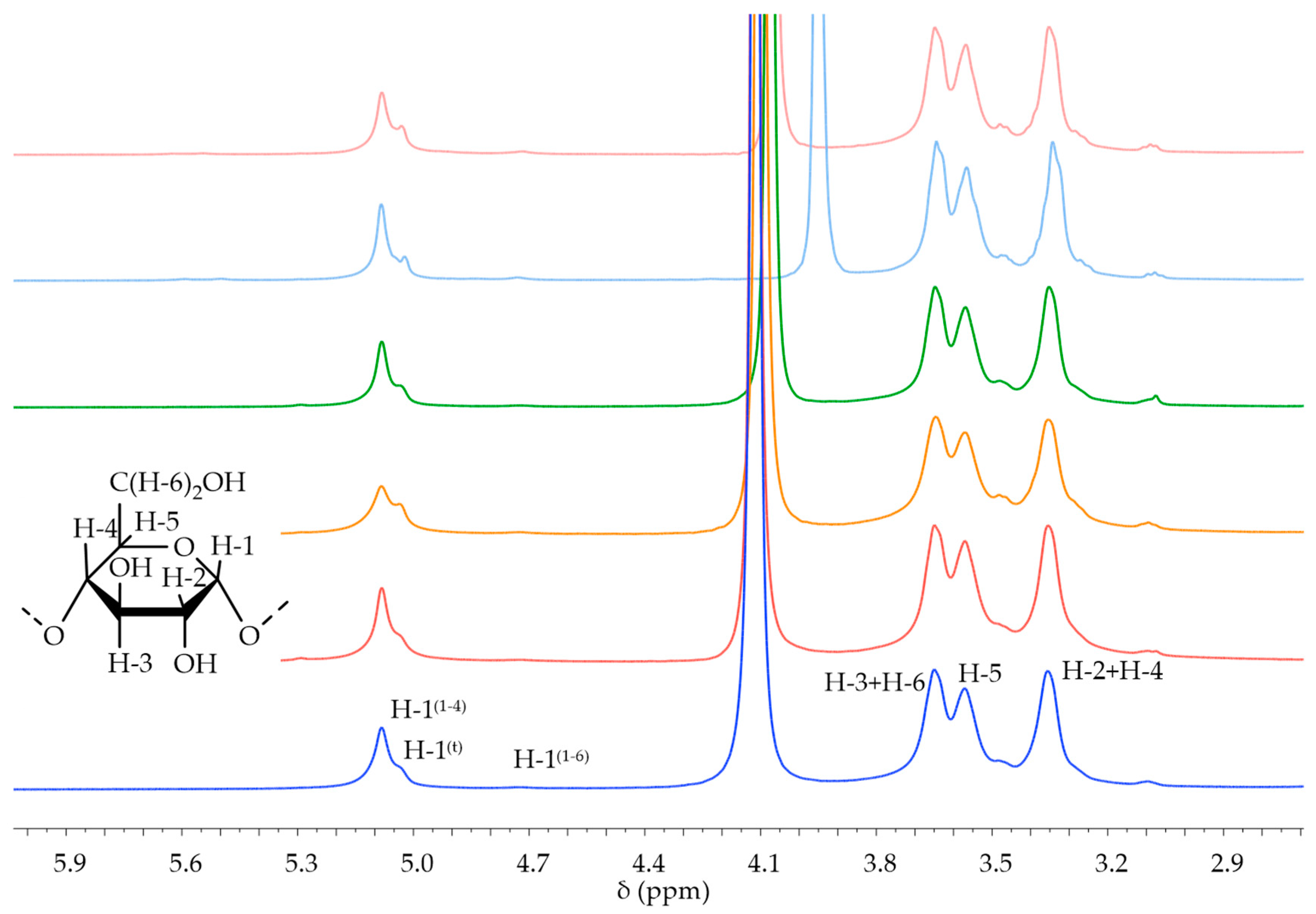
| Starch | Integral H1 α-1,4 + H-1 (t) 5.40 ppm–4.90 ppm | Integral H-1 α-1,6 4.80 ppm–4.65 ppm | BD |
|---|---|---|---|
| Native potato | 1.0000 | 0.0414 | 4.0% |
| Native corn | 1.0000 | 0.0428 | 4.1% |
| Native waxy corn | 1.0000 | 0.0564 | 5.3% |
| Native wheat | 1.0000 | 0.0567 | 5.4% |
| Oxidized potato | 1.0000 | 0.0578 | 5.5% |
| Oxidized corn | 1.0000 | 0.0333 | 3.2% |
| Sample | S (mg/g) | P (mg/g) | Si (mg/g) | Na (mg/g) | K (mg/g) | Ca (mg/g) | Sum (mg/g) |
|---|---|---|---|---|---|---|---|
| Native potato starch | 0.6 | 0.7 | 2.4 | 2.0 | 2.7 | 0.3 | 8.8 |
| Native corn starch | 0.4 | 0.1 | 0.6 | 1.2 | 0.8 | 0.1 | 3.3 |
| Native waxy corn starch | 0.3 | 0.1 | 0.3 | 1.1 | 0.6 | 0.1 | 2.5 |
| Native wheat starch | 0.2 | 0.5 | 0.2 | 0.9 | 0.5 | 0.1 | 2.3 |
| Oxidized potato starch | 0.1 * | 0.8 | 0.2 | 9.4 | 0.7 | 0.1 | 11.3 |
| Oxidized corn starch | 0.1 | 0.1 | 0.1 | 6.1 | 0.3 | 0.2 | 7.1 |
| Sample | Model | Qm µmol/g | K L/µmol | mol2/J2 | n | Eads,DR kJ/mol | R2 (COD) |
|---|---|---|---|---|---|---|---|
| Native potato starch | Langmuir | 23.1 ± 2.5 | 0.12 ± 0.08 | -- | -- | -- | 0.850 |
| Dubinin-Radushkevich | 21.0 ± 2.5 | -- | 2.16 ∙ 10−6 ± 1.50 ∙ 10−6 | -- | 0.48 ± 0.17 | 0.791 | |
| Oxidized potato starch | Langmuir | 88.5 ± 6.0 | 0.09 ± 0.02 | -- | -- | -- | 0.944 |
| Sips | 84.4 ± 0.7 | 0.01 ± 0.00 | -- | 2.30 ± 0.1 | -- | 0.999 | |
| Dubinin-Radushkevich | 83.6 ± 1.9 | -- | 5.91 ∙ 10−6 ± 5.8 ∙ 10−7 | -- | 0.29 ± 0.01 | 0.993 | |
| Oxidized corn starch | Langmuir | 70.5 ± 6.0 | 0.07 ± 0.03 | -- | -- | -- | 0.913 |
| Sips | 68.9 ± 7.2 | 0.05 ± 0.06 | -- | 1.2 ± 0.5 | -- | 0.916 | |
| Dubinin-Radushkevich | 66.1 ± 6.1 | -- | 2.16 ∙ 10−5 ± 1.50 ∙ 10−5 | -- | 0.48 ± 0.17 | 0.864 |
| Sample | Model | Qm µmol/g | K L/µmol | mol2/J2 | n | Eads,DR kJ/mol | R2 (COD) |
|---|---|---|---|---|---|---|---|
| Native potato starch | Langmuir | 10.4 ± 0.7 | 1.45 ± 0.70 | -- | -- | -- | 0.831 |
| Sips | 14.6 ± 6.5 | 0.48 ± 0.41 | -- | 0.3 ± 0.2 | -- | 0.876 | |
| Dubinin-Radushkevich | 10.3 ± 0.7 | -- | 1.49 ∙ 10−7 ± 8.4 ∙ 10−8 | -- | 1.83 ± 0.52 | 0.809 | |
| Oxidized potato starch | Langmuir | 72.7 ± 2.7 | 0.72 ± 0.11 | -- | -- | -- | 0.967 |
| Sips | 70.5 ± 2.8 | 0.72 ± 0.12 | -- | 1.2 ± 0.2 | -- | 0.970 | |
| Dubinin-Radushkevich | 67.4 ± 2.5 | -- | 3.40 ∙ 10−7 ± 5.3 ∙ 10−8 | -- | 1.21 ± 0.94 | 0.955 | |
| Oxidized corn starch | Langmuir | 63.6 ± 1.7 | 1.23 ± 0.18 | -- | -- | -- | 0.980 |
| Sips | 68.1 ± 2.2 | 1.01 ± 0.11 | -- | 0.7 ± 0.1 | -- | 0.991 | |
| Dubinin-Radushkevich | 60.0 ± 2.5 | -- | 1.49 ∙ 10−7 ± 8.4 ∙ 10−8 | -- | 1.84 ± 0.20 | 0.941 |
| Sample | Model | Qm µmol/g | K L/µmol | mol2/J2 | n | Eads,DR kJ/mol | R2 (COD) |
|---|---|---|---|---|---|---|---|
| Native potato starch | Langmuir | 6.4 ± 0.6 | 0.07 ± 0.02 | -- | -- | -- | 0.947 |
| Sips | 5.6 ± 0.4 | 0.02 ± 0.02 | -- | 1.7 ± 0.5 | -- | 0.971 | |
| Dubinin-Radushkevich | 5.5 ± 0.3 | -- | 1.68 ∙ 10−5 ± 5.0 ∙ 10−6 | -- | 0.17 ± 0.03 | 0.950 | |
| Native corn starch | Langmuir | 15.3 ± 0.5 | 0.39 ± 0.07 | -- | -- | -- | 0.982 |
| Sips | 14.3 ± 0.4 | 0.38 ± 0.06 | -- | 1.5 ± 0.2 | -- | 0.992 | |
| Dubinin-Radushkevich | 14.0 ± 0.3 | -- | 4.04 ∙ 10−7 ± 5.16 ∙ 10−8 | -- | 1.11 ± 0.07 | 0.987 |
| Sample | Model | Qm µmol/g | K L/µmol | mol2/J2 | Eads,DR kJ/mol | R2 (COD) |
|---|---|---|---|---|---|---|
| Native potato starch | Langmuir | 17.1 ± 2.0 | 1.31 ± 0.96 | -- | -- | 0.770 |
| Dubinin-Radushkevich | 16.0 ± 1. 9 | -- | 7.79 ∙ 10−8 ± 4.27 ∙ 10−8 | 2.53 ± 0.69 | 0.740 | |
| Oxidized potato starch | Langmuir | 105.5 ± 9.7 | 2.78 ± 1.25 | -- | -- | 0.896 |
| Dubinin-Radushkevich | 104.2 ± 9.4 | -- | 4.91 ∙ 10−8 ± 1.30 ∙ 10−8 | 3.19 ± 0.42 | 0.898 | |
| Oxidized corn starch | Langmuir | 89.5 ± 6.1 | 2.52 ± 0.78 | -- | -- | 0.941 |
| Dubinin-Radushkevich | 88.7 ± 4.5 | -- | 5.86 ∙ 10−8 ± 9.2 ∙ 10−9 | 2.92 ± 0.23 | 0.966 |
| Sample | SO42− (mg/L) | Cd2+ (mg/L) | Al3+ (mg/L) | Pb2+ (mg/L) | pH0 |
|---|---|---|---|---|---|
| Water 1 | 446.95 | 0.01 | 2.16 | 0.06 | 3.4 |
| Water 2 | 374.80 | 0.00 | 1.68 | 0.02 | 3.7 |
| Water 3 | 367.43 | 0.00 | 1.87 | 0.02 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borchert, K.B.L.; Boughanmi, R.; Reis, B.; Zimmermann, P.; Steinbach, C.; Graichen, P.; Svirepa, A.; Schwarz, J.; Boldt, R.; Schwarz, S.; et al. Removal of Lead, Cadmium, and Aluminum Sulfate from Simulated and Real Water with Native and Oxidized Starches. Polysaccharides 2021, 2, 429-453. https://doi.org/10.3390/polysaccharides2020027
Borchert KBL, Boughanmi R, Reis B, Zimmermann P, Steinbach C, Graichen P, Svirepa A, Schwarz J, Boldt R, Schwarz S, et al. Removal of Lead, Cadmium, and Aluminum Sulfate from Simulated and Real Water with Native and Oxidized Starches. Polysaccharides. 2021; 2(2):429-453. https://doi.org/10.3390/polysaccharides2020027
Chicago/Turabian StyleBorchert, Konstantin B. L., Rahma Boughanmi, Berthold Reis, Philipp Zimmermann, Christine Steinbach, Peter Graichen, Anastasiya Svirepa, Johannes Schwarz, Regine Boldt, Simona Schwarz, and et al. 2021. "Removal of Lead, Cadmium, and Aluminum Sulfate from Simulated and Real Water with Native and Oxidized Starches" Polysaccharides 2, no. 2: 429-453. https://doi.org/10.3390/polysaccharides2020027
APA StyleBorchert, K. B. L., Boughanmi, R., Reis, B., Zimmermann, P., Steinbach, C., Graichen, P., Svirepa, A., Schwarz, J., Boldt, R., Schwarz, S., Mertig, M., & Schwarz, D. (2021). Removal of Lead, Cadmium, and Aluminum Sulfate from Simulated and Real Water with Native and Oxidized Starches. Polysaccharides, 2(2), 429-453. https://doi.org/10.3390/polysaccharides2020027