Chitosan and Collagen-Based Materials Enriched with Curcumin (Curcuma longa): Rheological and Morphological Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Chitosan Obtention
2.2.2. Collagen Obtention
2.2.3. Curcumin Purification
2.2.4. Preparation of Chitosan/Collagen/Curcumin Scaffolds
2.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.6. Rheological Assays
2.2.7. Thermogravimetric Analysis (TGA)
2.2.8. Scanning Electron Microscopy (SEM)
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Rheological Assays
3.2.1. Strain Sweep Measurements
3.2.2. Temperature Sweep Measurements
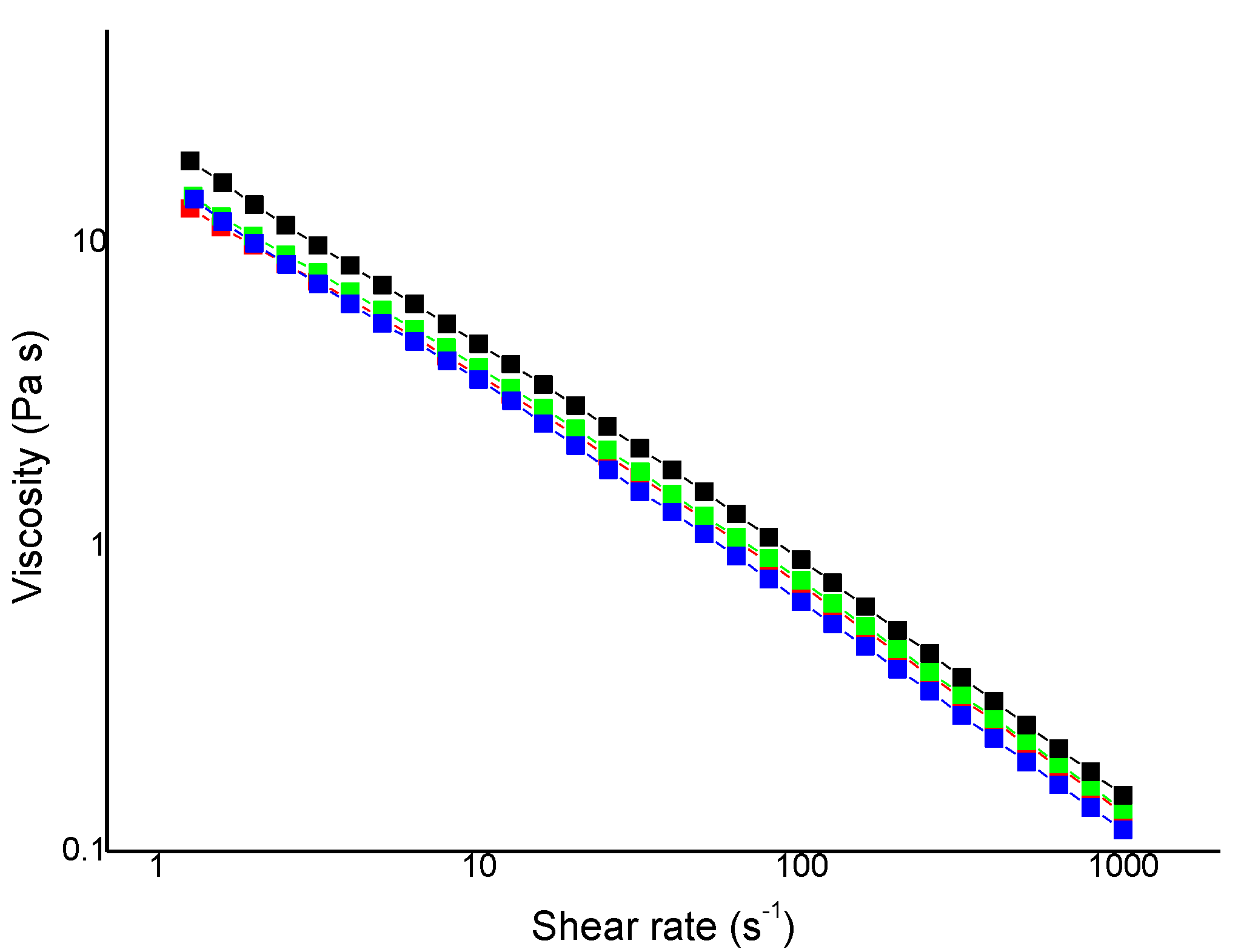
3.2.3. Flow Measurements
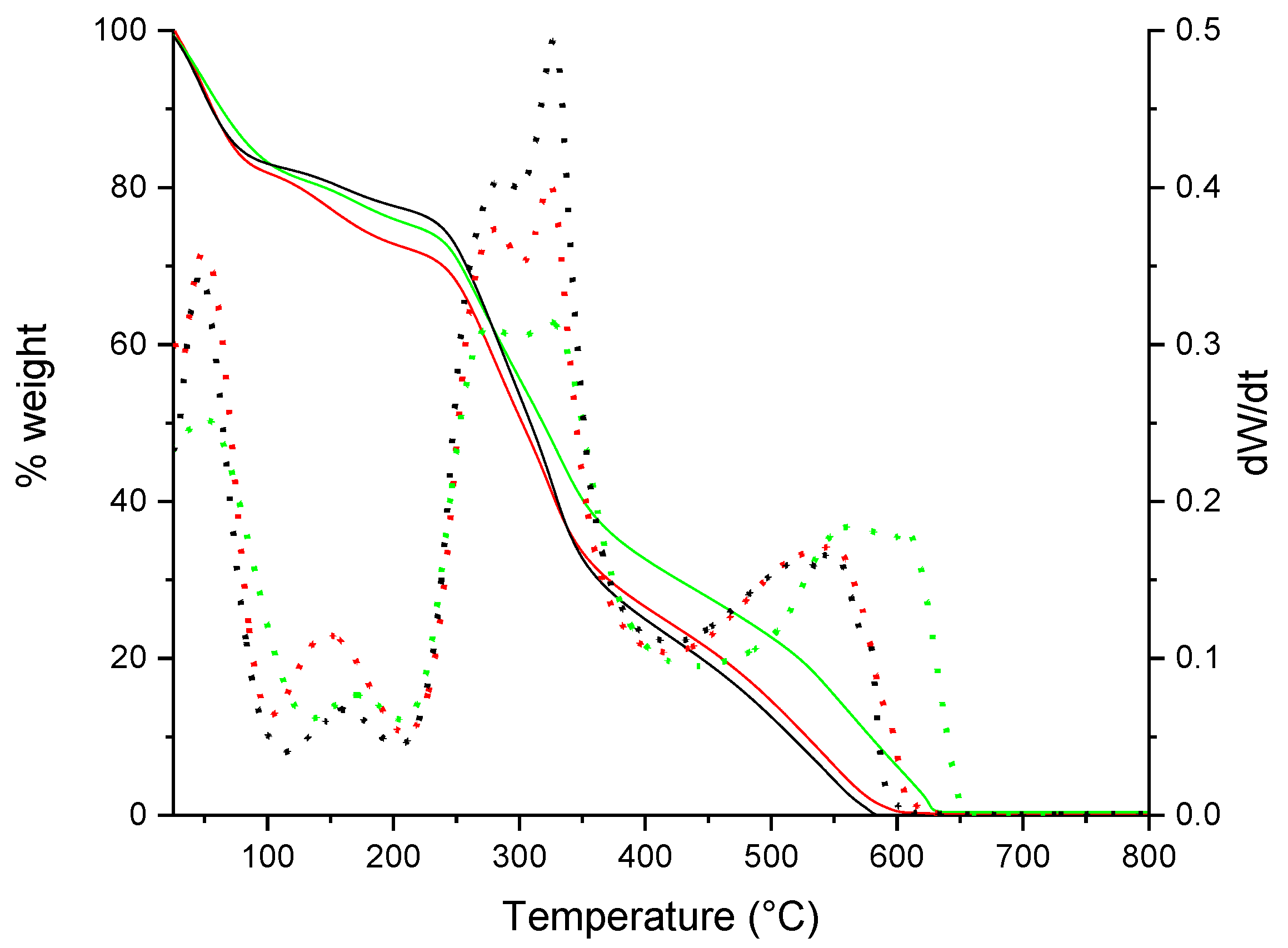
3.3. Thermogravimetric Analysis (TGA)
3.4. Scanning Electron Microscopy (SEM)
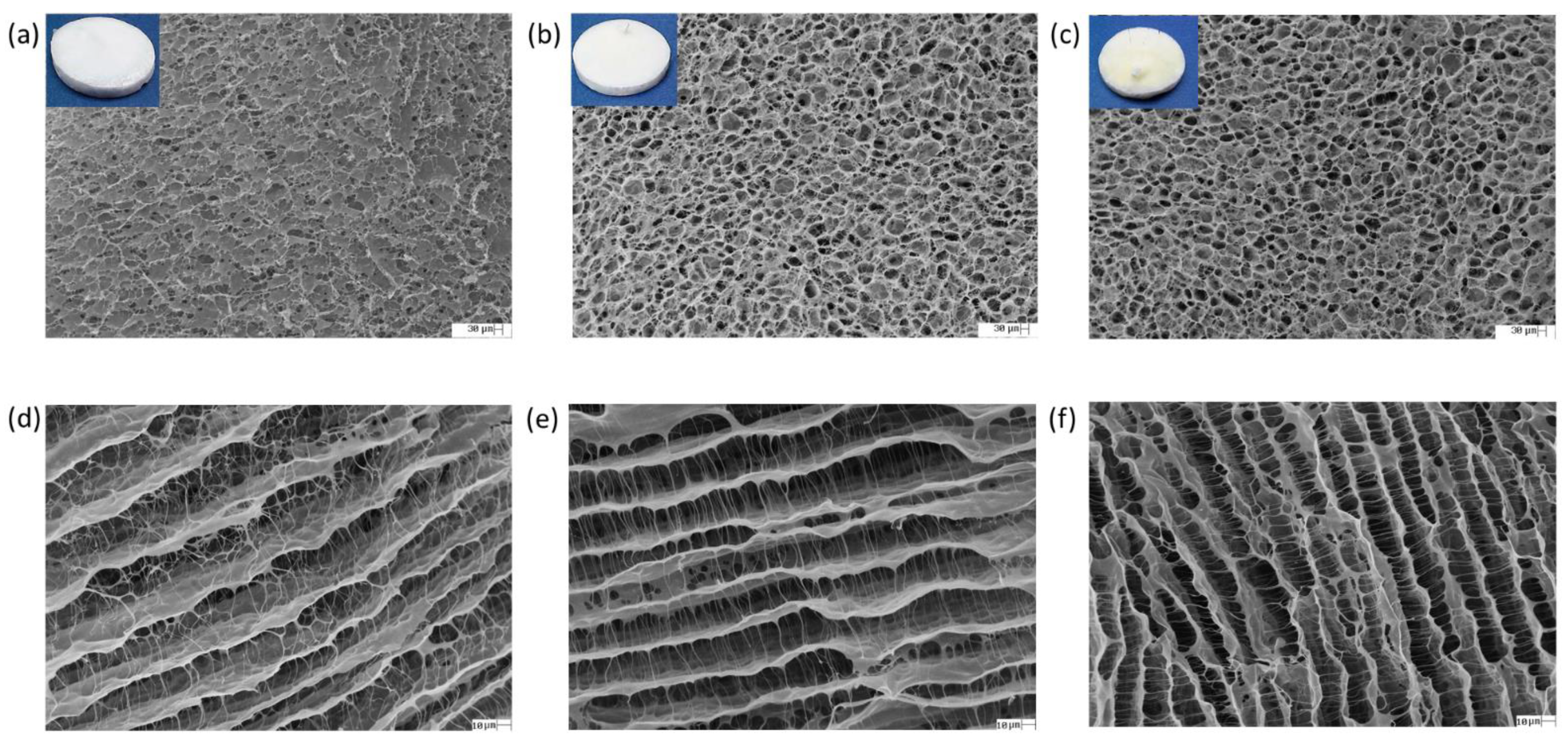
3.4.1. Surface and Cross-Sectional Surface Images
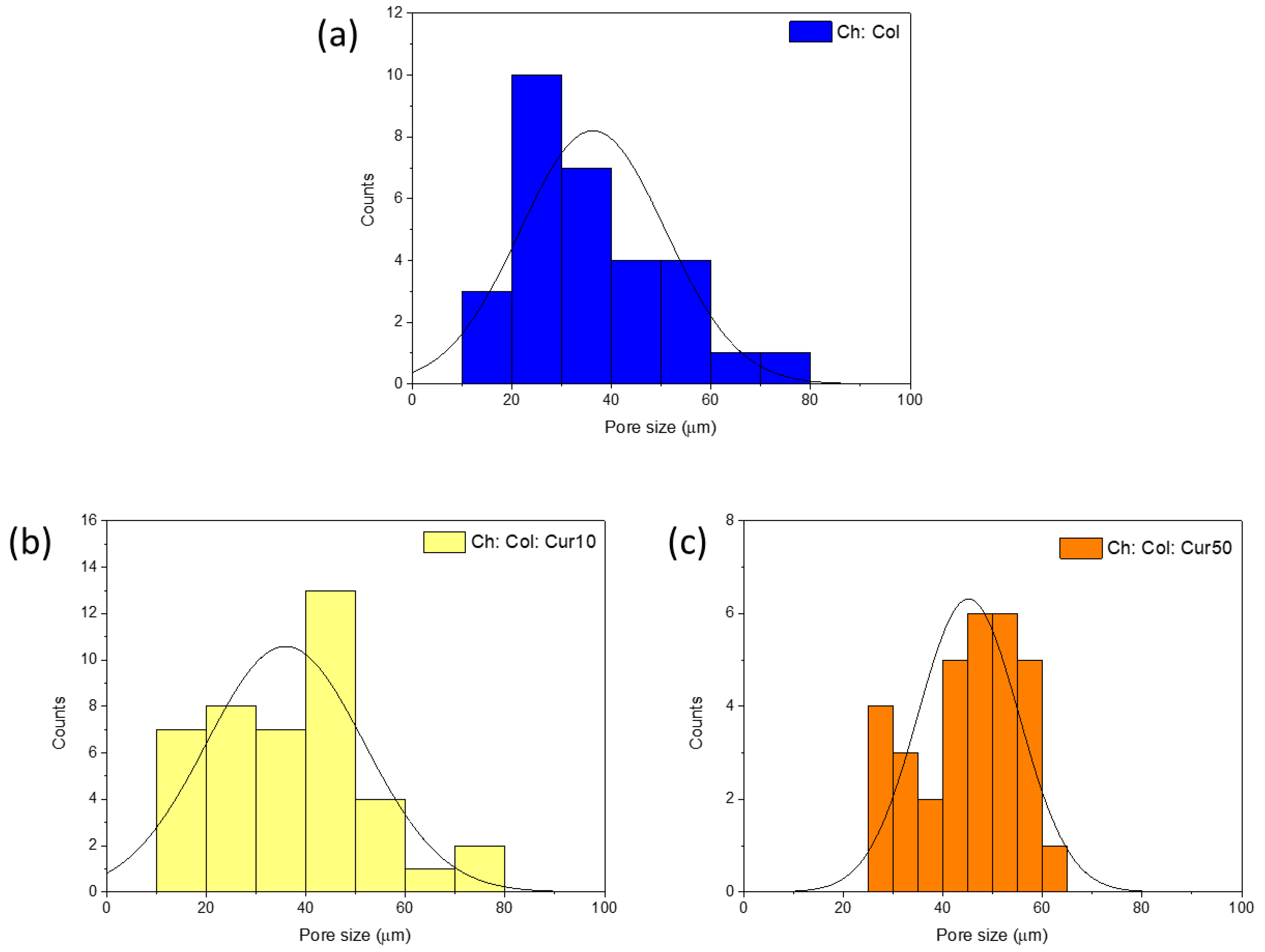
3.4.2. Pore and Channel Size
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pattanayak, S.P.; Sunita, P. Wound healing, anti-microbial and antioxidant potential of Dendrophthoe falcata (L.f) Ettingsh. J. Ethnopharmacol. 2008, 120, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Zhang, H.; Guo, B. Conductive biomaterials as bioactive wound dressing for wound healing and skin tissue engineering. Nano-Micro Lett. 2022, 14, 751. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Lu, Z.; Wu, K.; Nam, C.; Zhang, L.; Guo, J. Recent advances in the development of nitric oxide-releasing biomaterials and their application potentials in chronic wound healing. J. Mater. Chem. B 2021, 9, 7063–7075. [Google Scholar] [CrossRef] [PubMed]
- Dharunya, G.; Duraipandy, N.; Lakra, R.; Korapatti, P.S.; Jayavel, R.; Kiran, M.S. Curcumin cross-linked collagen aerogels with controlled anti-proteolytic and pro-angiogenic efficacy. Biomed. Mater. 2016, 11, 045011. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef]
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri Darban, S.; Fazly Bazzaz, B.S.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-loaded chitosan-PEO nanofibers for local antibiotic delivery and wound healing. Int. J. Biol. Macromol. 2020, 162, 645. [Google Scholar] [CrossRef]
- Bayat, S.; Amiri, N.; Pishavar, E.; Kalalinia, F.; Movaffagh, J.; Hashemi, M. Bromelain-loaded chitosan nanofibers prepared by electrospinning method for burn wound healing in animal models. Life Sci. 2019, 229, 57–66. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Raines, R.T. Collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Lee, K.; Yang, Y.; Kawazoe, N.; Chen, G. PLGAcollagen-ECM hybrid meshes mimicking stepwise osteogenesis and their influence on the osteogenic differentiation of hMSCs. Biofabrication 2020, 12, 025027. [Google Scholar] [CrossRef]
- Elhendawi, H.; Felfel, R.M.; El-Hady, A.; Bothaina, M.; Reicha, F.M. Effect of synthesis temperature on the crystallization and growth of in situ prepared nanohydroxyapatite in chitosan matrix. Biomaterials 2014, 2014, 897468. [Google Scholar] [CrossRef] [Green Version]
- Bertolo, M.R.V.; Martins, V.C.A.; de Guzzi Plepis, A.M. Effects of calcium phosphates incorporation on structural, thermal and drug-delivery properties of collagen:chitosan scaffolds. Int. J. Adv. Med. Biotechnol.-IJAMB 2020, 2, 25–35. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the development of chitosan-based biomaterials for tissue engineering and regenerative medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, K.; Zhang, D.; Macedo, M.H.; Cui, W.; Sarmento, B.; Shen, G. Advanced collagen-based biomaterials for regenerative biomedicine. Adv. Funct. Mater. 2019, 29, 1804943. [Google Scholar] [CrossRef]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Abbas, M.; Hussain, T.; Arshad, M.; Ansari, A.R.; Irshad, A.; Nisar, J.; Hussain, F.; Masood, N.; Nazir, A.; Iqbal, M. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 140, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Fathima, N.N.; Devi, R.S.; Rekha, K.B.; Dhathathreyan, A. Collagen–curcumin interaction—A physico-chemical study. J. Chem. Sci. 2009, 121, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Rezaei, M.; Oryan, S.; Nourani, M.R.; Mofid, M.; Mozafari, M. Curcumin nanoparticle-incorporated collagen/chitosan scaffolds for enhanced wound healing. Bioinspired Biomim. Nanobiomater. 2018, 7, 159–166. [Google Scholar] [CrossRef]
- Rezaei, M.; Oryan, S.; Javeri, A. Curcumin nanoparticles incorporated collagen-chitosan scaffold promotes cutaneous wound healing through regulation of TGF-β1/Smad7 gene expression. Mater. Sci. Eng. C 2019, 98, 347–357. [Google Scholar] [CrossRef]
- Jirofti, N.; Golandi, M.; Movaffagh, J.; Ahmadi, F.S.; Kalalinia, F. Improvement of the wound-healing process by curcumin-loaded chitosan/collagen blend electrospun nanofibers: In vitro and in vivo studies. ACS Biomater. Sci. Eng. 2021, 7, 3886–3897. [Google Scholar] [CrossRef]
- Horn, M.M.; Martins, V.C.A.; Plepis, A.M.G. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.N.; Berrada, M.; Rodrigues, A.; Wang, D.; Buschmann, M.D.; Gupta, A. A validated 1H NMR method for the determination of the degree of deacetylation of chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Cai, L.; Shi, H.; Cao, A.; Jia, J. Characterization of gelatin/chitosan ploymer films integrated with docosahexaenoic acids fabricated by different methods. Sci. Rep. 2019, 9, 8375. [Google Scholar] [CrossRef] [PubMed]
- Batista, T.M.; Martins, V.C.A.; Plepis, A.M.G. Thermal behavior of in vitro mineralized anionic collagen matrices. J. Therm. Anal. Calorim. 2009, 95, 945–949. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The stability, sustained release and cellular antioxidant activity of curcumin nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [Green Version]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.; Gómez-Guillén, M.C. Structural properties of films and rheology of film-forming solutions based on chitosan and chitosan-starch blend enriched with murta leaf extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Razmkhah, S.; Mohammad, S.; Razavi, A. Dilute solution, flow behavior, thixotropy and viscoelastic characterization of cress seed (Lepidium sativum) gum fractions. Food Hydrocoll. 2017, 63, 404–413. [Google Scholar] [CrossRef]
- Romanelli Vicente Bertolo, M.; Leme, R.; da Conceição Amaro Martins, V.; de Guzzi Plepis, A.M.; Bogusz Junior, S. Rheological characterization of the influence of pomegranate peel extract addition and concentration in chitosan and gelatin coatings. Polysaccharides 2021, 2, 648–660. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Mazur, O. Collagen-based materials modified by phenolic acids—A review. Materials 2020, 13, 3641. [Google Scholar] [CrossRef]
- Mandala, I.; Savvas, T.; Kostaropoulos, A. Xanthan and locust bean gum influence on the rheology and structure of a white model-sauce. J. Food Eng. 2004, 64, 335–342. [Google Scholar] [CrossRef]
- Almeida, C.M.R.; Magalhães, J.M.C.S.; Souza, H.K.S.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466. [Google Scholar] [CrossRef]
- Leong, K.F.; Chua, C.K.; Sudarmadji, N.; Yeong, W.Y. Engineering functionally graded tissue engineering scaffolds. J. Mech. Behav. Biomed. Mater. 2008, 1, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for wound healing applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, M.R.V.; Martins, V.C.A.; Horn, M.M.; Brenelli, L.B.B.; Plepis, A.M.G. Rheological and antioxidant properties of chitosan/gelatin-based materials functionalized by pomegranate peel extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–330. [Google Scholar] [CrossRef]
- Bruzauskaite, I.; Bironaite, D.; Bagdonas, E.; Bernotiene, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [Green Version]


| Wavenumber (cm−1) | Chitosan | Collagen | Curcumin |
|---|---|---|---|
| 3700–3100 | O-H and N-H deformation | ||
| 3300 | O-H and N-H deformation | ||
| 3450–3360 | O-H phenol deformation | ||
| 2930–2880 | C-H axial deformation | C-H axial deformation | |
| 1660 | C=O carbonyl and keto-enol tautomerism | ||
| 1655 (sh) * | Amide I | ||
| 1653 (sh) * | Amide I | ||
| 1640 | O-H water bond | ||
| 1560 | Amide II | ||
| 1558 | Amide II | ||
| 1456 | C-H pyrrolidine rings deformation | ||
| 1413 | CH2 axial deformation | ||
| 1385 | CH2 out-of-plane deformation | ||
| 1400-1350 | C-O alcohol and phenol deformation | ||
| 1240 | O-H axial deformation | ||
| 1238 | Amide III | ||
| 1190–960 | C-O axial deformation | ||
| 1000 | O-H and N-H deformation | C-H alkene deformation |
| Parameter | Ch: Col | Ch: Col: Cur10 | Ch: Col: Cur20 | Ch: Col: Cur50 |
|---|---|---|---|---|
| γLVR (%) | 17.9 | 18.6 | 17.8 | 18.5 |
| G’LVR (Pa) | 77.6 | 44.0 | 46.9 | 57.5 |
| tanδ | 0.5 | 0.7 | 0.7 | 0.6 |
| G’-G″ | 35.7 | 13.7 | 18.5 | 24.0 |
| Tcrossover1 (°C) | 33.8 | 32.9 | 34.7 | 35.3 |
| G’croosver1 (Pa) | 14.5 | 15.5 | 13.8 | 13.2 |
| Tcrossover2 (°C) | 59.8 | 53.9 | 51.6 | 55 |
| G’croosver2 (Pa) | 7.4 | 4.1 | 2.7 | 3.5 |
| η0 (Pa s) | 35.3 | 42.8 | 57.5 | 61.3 |
| Scaffold | Weight Loss (%) | Tonset (°C) | |||
|---|---|---|---|---|---|
| 25–100 °C | 100–200 °C | 200–410 °C | 410–750 °C | ||
| Ch: Col | 18.09 | 9.08 | 47.37 | 25.18 | 254.38 |
| Ch: Col: Cur10 | 18.51 | 5.49 | 46.8 | 28.8 | 259.53 |
| Ch: Col: Cur50 | 16.96 | 5.39 | 53.86 | 24.19 | 259.89 |
| Scaffold | Pore Size (µm) | Channel Size (µm) |
|---|---|---|
| Ch: Col | 35.15 ± 10.86 a | 36.47 ± 7.30 a |
| Ch: Col: Cur10 | 35.14 ± 12.64 a | 28.62 ± 2.36 b |
| Ch: Col: Cur50 | 45.31 ± 8.75 a | 18.48 ± 2.30 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milan, E.P.; Bertolo, M.R.V.; Martins, V.C.A.; Bogusz Junior, S.; Plepis, A.M.G. Chitosan and Collagen-Based Materials Enriched with Curcumin (Curcuma longa): Rheological and Morphological Characterization. Polysaccharides 2022, 3, 236-249. https://doi.org/10.3390/polysaccharides3010013
Milan EP, Bertolo MRV, Martins VCA, Bogusz Junior S, Plepis AMG. Chitosan and Collagen-Based Materials Enriched with Curcumin (Curcuma longa): Rheological and Morphological Characterization. Polysaccharides. 2022; 3(1):236-249. https://doi.org/10.3390/polysaccharides3010013
Chicago/Turabian StyleMilan, Eduardo P., Mirella Romanelli V. Bertolo, Virginia C. A. Martins, Stanislau Bogusz Junior, and Ana Maria G. Plepis. 2022. "Chitosan and Collagen-Based Materials Enriched with Curcumin (Curcuma longa): Rheological and Morphological Characterization" Polysaccharides 3, no. 1: 236-249. https://doi.org/10.3390/polysaccharides3010013
APA StyleMilan, E. P., Bertolo, M. R. V., Martins, V. C. A., Bogusz Junior, S., & Plepis, A. M. G. (2022). Chitosan and Collagen-Based Materials Enriched with Curcumin (Curcuma longa): Rheological and Morphological Characterization. Polysaccharides, 3(1), 236-249. https://doi.org/10.3390/polysaccharides3010013








