Delving into the Role of Dietary Fiber in Gluten-Free Bread Formulations: Integrating Fundamental Rheological, Technological, Sensory, and Nutritional Aspects
Abstract
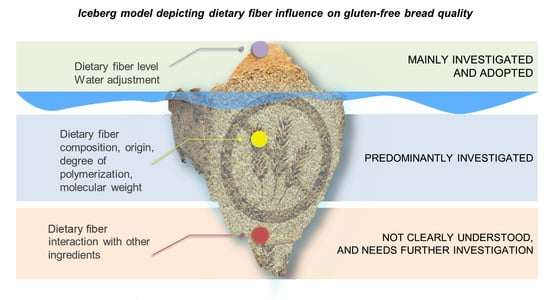
:1. Introduction
2. Definition, Classification and Sources of Dietary Fibers used in Gluten-Free Bread Production
- Soluble dietary fiber (SDF), which includes non-cellulosic polysaccharides, oligosaccharides, and hydrocolloids such as pectin, β-glucan, and gums;
- Insoluble dietary fiber (IDF), which includes cellulose, hemicellulose, lignin, resistant starch, and products of Maillard reactions;
- Prebiotic dietary fiber, which includes inulin, inulin-type fructans, trans-galactooligosaccharides, and fructooligosaccharides.
3. Role of Dietary Fibers in Gluten-Free Batter Rheology
3.1. Effect of Dietary Fibers on Gluten-Free Batter Flow Properties
3.2. Effect of Dietary Fibers on Gluten-Free Batter Viscoelastic Behavior
3.2.1. Dynamic Oscillatory Frequency Sweep Tests in Fiber-Enriched Gluten-Free Batter Formulations
3.2.2. Creep–Recovery Tests in Fiber-Enriched Gluten-Free Batter Formulations
4. Role of Dietary Fibers in Gluten-Free Bread’s Technological Quality
4.1. Effect of Dietary Fibers on Gluten-Free Bread Crust and Crumb Color
4.2. Effect of Dietary Fibers on Gluten-Free Bread’s Specific Volume
4.3. Effect of Dietary Fibers on Gluten-Free Bread’s Crumb Texture
5. Sensory Properties of Fiber-Enriched Gluten-Free Bread
6. Nutritional Quality of Fiber-Enriched Gluten-Free Bread
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Romano, A.; Toraldo, G.; Cavella, S.; Masi, P. Description of leavening of bread dough with mathematical modeling. J. Food Eng. 2007, 83, 142–148. [Google Scholar] [CrossRef]
- Scherf, K.A.; Koehler, P.; Deutsche, H.W. Gluten and wheat sensitivities—An overview. J. Cereal Sci. 2016, 67, 2–11. [Google Scholar] [CrossRef]
- Green, P.H.R.; Lebwohl, B.; Greywoode, R. Celiac disease. J. Allergy Clin. Immunol. 2015, 135, 1099–1106. [Google Scholar] [CrossRef]
- Scherf, K.A.; Poms, R.E. Recent developments in analytical methods for tracing gluten. J. Cereal Sci. 2016, 67, 112–122. [Google Scholar] [CrossRef]
- Houben, A.; Hochstotter, A.; Becker, T. Possibilities to increase the quality in gluten-free bread production: An overview. Eur. Food Res. Technol. 2012, 235, 195–208. [Google Scholar] [CrossRef]
- Gallagher, E.; Gormley, T.; Arendt, E. Recent advances in the formulation of gluten- free cereal-based products. Trends Food Sci. Technol. 2004, 15, 143–152. [Google Scholar] [CrossRef]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef]
- Thompson, T. Folate, iron, and dietary fiber contents of the gluten free diet. J. Am. Diet. Assoc. 2000, 100, S1389–S1396. [Google Scholar] [CrossRef]
- do Nascimento, A.; Fiates, G.; dos Anjos, A.; Teixeira, E. Analysis of ingredient lists of commercially available gluten-free and gluten-containing food products using the text mining technique. Int. J. Food Sci. Nutr. 2013, 64, 217–222. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Higgins, L.; Lee, A.; Sharrett, M. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef]
- Kinsey, L.; Burden, S.; Bannerman, E. A dietary survey to determine if patients with coeliac disease are meeting current healthy eating guidelines and how their diet compares to that of the British general population. Eur. J. Clin. Nutr. 2008, 62, 1333–1342. [Google Scholar] [CrossRef]
- Capriles, V.; dos Santos, F.; Arêas, J.A. Gluten-free breadmaking: Improving nutritional and bioactive compounds. J. Cereal Sci. 2016, 67, 83–91. [Google Scholar] [CrossRef]
- Tsatsaragkou, Κ.; Protonotariou, S.; Mandala, I. Structural role of fibre addition to increase knowledge of non-gluten bread. J. Cereal Sci. 2016, 67, 58–67. [Google Scholar] [CrossRef]
- Arslan, M.; Rakha, A.; Xiaobo, Z.; Mahmood, M.A. Complimenting gluten free bakery products with dietary fiber: Opportunities and constraints. Trends Food Sci. Technol. 2019, 83, 194–202. [Google Scholar] [CrossRef]
- Föste, M.; Verheyen, C.; Jekle, M.; Becker, T. Fibres of milling and fruit processing by-products in gluten-free bread making: A review of hydration properties, dough formation and quality-improving strategies. Food Chem. 2020, 306, 125451. [Google Scholar] [CrossRef]
- Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Dokić, L.J.; Djordjević, M.; Šereš, Z.; Šaranović, Ž. Rheology and bread-making performance of gluten-free formulations affected by different levels of sugar beet fibre, hydroxypropylmethylcellulose and water. Int. J. Food Sci. Technol. 2018, 53, 1832–1837. [Google Scholar] [CrossRef]
- Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Djordjević, M.; Šereš, Z.; Milašinović- Šeremešić, M. Sugar beet and apple fibres coupled with hydroxypropylmethylcellulose as functional ingredients in gluten-free formulations: Rheological, technological and sensory aspects. Food Chem. 2019, 295, 189–197. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, N.; Rößle, C.; Arendt, E.; Gallagher, E. Modelling the effects of orange pomace using response surface design for gluten- free bread baking. Food Chem. 2015, 166, 223–230. [Google Scholar] [CrossRef]
- Rocha Parra, A.F.; Ribotta, P.D.; Ferrero, C. Apple pomace in gluten-free formulations: Effect on rheology and product quality. Int. J. Food Sci. 2015, 50, 682–690. [Google Scholar] [CrossRef]
- Martínez, M.M.; Díaz, Á.; Gómez, M. Effect of different microstructural features of soluble and insoluble fibres on gluten-free dough rheology and bread-making. J. Food Eng. 2014, 142, 49–56. [Google Scholar] [CrossRef]
- Sabanis, D.; Lebesi, D.; Tzia, C. Effect of dietary fibre enrichment on selected properties of gluten-free bread. LWT-Food Sci. Technol. 2009, 42, 1380–1389. [Google Scholar] [CrossRef]
- Phimolsiripol, Y.; Mukprasirt, A.; Schoenlechner, R. Quality improvement of rice-based gluten-free bread using different dietary fibre fractions of rice bran. J. Cereal Sci. 2012, 56, 389–395. [Google Scholar] [CrossRef]
- Kaczmarczyk, M.M.; Miller, M.J.; Freund, G.G. The health benefits of dietary fiber: Beyond the usual suspects of type 2 diabetes mellitus, cardiovascular disease and colon cancer. Metab. Clin. Exp. 2012, 61, 1058–1066. [Google Scholar] [CrossRef] [Green Version]
- Kendall, C.W.C.; Esfahani, A.; Jenkins, D.J.A. The link between dietary fibre and human health. Food Hydrocoll. 2010, 24, 42–48. [Google Scholar] [CrossRef]
- Gill, S.K.; Rossi, M.; Bajka, B.; Whelan, K. Dietary fibre in gastrointestinal health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 101–116. [Google Scholar] [CrossRef]
- Food and Agriculture Organization/World Health Organization Codex Alimentarius Commission. Codex Alimentarius: Guidelines on Nutrition Labelling CAC/GL 2-1985; FAO: Rome, Italy, 2010. [Google Scholar]
- Gidley, M.J.; Yakubov, G.E. Functional categorisation of dietary fibre in foods: Beyond ‘soluble’ vs ‘insoluble’. Trends Food Sci. Technol. 2019, 86, 563–568. [Google Scholar] [CrossRef]
- Macagnan, F.T.; Picolli da Silva, L.; Hecktheuer, L.H. Dietary fibre: The scientific search for an ideal definition and methodology of analysis, and its physiological importance as a carrier of bioactive compounds. Food Res. Int. 2016, 85, 144–154. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, J.R.H.; Ferreri, S.; Knudtson, M.; Koraym, A. Health benefits of dietary fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Papathanasopoulos, A.; Camilleri, M. Dietary fiber supplements: Effects in obesity and metabolic syndrome and relationship to gastrointestinal functions. Gastroenterology 2010, 138, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Roberfroid, M.B. Prebiotics: Concept, Definition, Criteria, Methodologies, and Products. In Handbook of Prebiotics; Gibson, G.R., Roberfroid, M.B., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 39–68. [Google Scholar]
- Rezende, E.S.V.; Lima, G.C.; Naves, M.M.V. Dietary fibers as beneficial microbiota modulators: A proposed classification by prebiotic categories. Nutrition 2021, 89, 111217. [Google Scholar] [CrossRef]
- Westenbrink, S.; Brunt, K.; van der Kamp, J.-W. Dietary fibre: Challenges in production and use of food composition data. Food Chem. 2013, 140, 562–567. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Chen, B.; Cai, Y.; Liu, T.; Huang, L.; Deng, X.; Zhao, Q.; Zhao, M. Improvements in physicochemical and emulsifying properties of insoluble soybean fiber by physical-chemical treatments. Food Hydrocoll. 2019, 93, 167–175. [Google Scholar] [CrossRef]
- Meng, X.; Liu, F.; Xiao, Y.; Cao, J.; Wang, M.; Duan, X. Alterations in physicochemical and functional properties of buckwheat straw insoluble dietary fiber by alkaline hydrogen peroxide treatment. Food Chem. X 2019, 3, 100029. [Google Scholar] [CrossRef]
- Yan, L.; Li, T.; Liu, C.; Zheng, L. Effects of high hydrostatic pressure and superfine grinding treatment on physicochemical/functional properties of pear pomace and chemical composition of its soluble dietary fibre. LWT-Food Sci. Technol. 2019, 107, 171–177. [Google Scholar] [CrossRef]
- Makki, K.; Deehan, E.C.; Walter, J.; Bäckhed, F. The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 2018, 23, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary fibre in foods: A review. J. Food Sci. Technol. 2012, 49, 255–266. [Google Scholar] [CrossRef] [Green Version]
- Olive Li, Y.; Komarek, A.R. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017, 1, 47–59. [Google Scholar] [CrossRef]
- Ching, L.W.; Zulkipli, N.M.; Muhamad, I.I.; Marsin, A.M.; Khairc, Z.; Anis, S.N.S. Dietary management for healthier batter formulations. Trends Food Sci. Technol. 2021, 113, 411–422. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary fiber and fiber-rich by-products of food processing: Characterization, technological functionality and commercial applications: A review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Crepeau, M.-J.; Thibault, J.-F. Influence of ionic strength, pH and dielectric constant on hydration properties of native and modified fibres from sugar-beet and wheat bran. Ind. Crops Prod. 1994, 3, 75–84. [Google Scholar] [CrossRef]
- Sangnark, A.; Noomhorm, A. Effect of particle sizes on functional properties of dietary fibre prepared from sugarcane bagasse. Food Chem. 2003, 80, 221–229. [Google Scholar] [CrossRef]
- Bader Ul Ain, H.; Saeed, F.; Ahmed, A.; Khan, M.A.; Niaz, B.; Tufail, T. Improving the physicochemical properties of partially enhanced soluble dietary fiber through innovative techniques: A coherent review. J Food Process Preserv. 2019, 43, e13917. [Google Scholar] [CrossRef]
- Chau, C.-F.; Wang, Y.-T.; Wen, Y.-L. Different micronization methods significantly improve the functionality of carrot insoluble fibre. Food Chem. 2007, 100, 1402–1408. [Google Scholar] [CrossRef]
- Šoronja-Simović, D.; Šereš, Z.; Maravić, N.; Djordjević, M.; Djordjević, M.; Luković, J.; Tepić, A. Enhancement of physicochemical properties of sugar beet fibres affected by chemical modification and vacuum drying. Food Bioprod. Process. 2016, 100, 432–439. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. The impact of resistant starch on characteristics of gluten-free dough and bread. Food Hydrocoll. 2009, 23, 988–995. [Google Scholar] [CrossRef]
- Tsatsaragkou, K.; Gounaropoulos, G.; Mandala, I. Development of gluten free bread containing carob flour and resistant starch. LWT-Food Sci. Technol. 2014, 58, 124–129. [Google Scholar] [CrossRef]
- Sciarini, L.S.; Bustos, M.C.; Vignola, M.B.; Paesani, C.; Salinas, C.N.; Pérez, G.T. A study on fibre addition to gluten free bread: Its effects on bread quality and in vitro digestibility. J. Food Sci. Technol. 2017, 54, 244–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiumarsi, M.; Shahbazi, M.; Yeganehzad, S.; Majchrzak, D.; Lieleg, O.; Winkeljann, B. Relation between structural, mechanical and sensory properties of gluten-free bread as affected by modified dietary fibers. Food Chem. 2019, 277, 664–673. [Google Scholar] [CrossRef]
- Genevois, C.E.; Grenóvero, M.S.; de Escalada Pla, M.F. Use of different proportions of rice milling fractions as strategy for improving quality parameters and nutritional profile of gluten-free bread. J. Food Sci. Technol. 2021, 58, 3913–3923. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Donner, E.; Liu, Q. Development and characterisation of gluten-free potato bread. Int. J. Food Sci. Technol. 2021, 56, 3085–3098. [Google Scholar] [CrossRef]
- Čukelj Mustač, N.; Novotni, D.; Habuš, M.; Drakula, S.; Nanjara, L.; Voučko, B.; Benković, M.; Ćurić, D. Storage stability, micronisation, and application of nutrient-dense fraction of proso millet bran in gluten-free bread. J. Cereal Sci. 2020, 91, 102864. [Google Scholar] [CrossRef]
- Villanueva, M.; Abebe, W.; Collar, C.; Ronda, F. Tef [Eragrostis tef (Zucc.) Trotter] variety determines viscoelastic and thermal properties of gluten-free dough and bread quality. LWT-Food Sci. Technol. 2021, 135, 110065. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.; Gallagher, E. Nutritive value and chemical composition of pseudocereals as gluten-free ingredients. Int. J. Food Sci. Nutr. 2009, 60, 240–257. [Google Scholar] [CrossRef]
- Lazaridou, A.; Duta, D.; Papageorgiou, M.; Belc, N.; Biliaderis, C.G. Effects of hydrocolloids on dough rheology and bread quality parameters in gluten-free formulations. J. Food Eng. 2007, 79, 1033–1047. [Google Scholar] [CrossRef]
- Kittisuban, P.; Ritthiruangdej, P.; Suphantharik, M. Optimization of hydroxypropylmethylcellulose, yeast b-glucan, and whey protein levels based on physical properties of gluten-free rice bread using response surface methodology. LWT-Food Sci. Technol. 2014, 57, 738–748. [Google Scholar] [CrossRef]
- Hager, A.S.; Ryan, L.A.M.; Schwab, C.; Gänzle, M.G.; O’Doherty, J.V.; Arendt, E.K. Influence of the soluble fibres inulin and oat b-glucan on quality of dough and bread. Eur. Food Res. Technol. 2011, 232, 405–413. [Google Scholar] [CrossRef]
- Mariotti, M.; Pagani, M.A.; Lucisano, M. The role of buckwheat and HPMC on the breadmaking properties of some commercial gluten-free bread mixtures. Food Hydrocoll. 2013, 30, 393–400. [Google Scholar] [CrossRef]
- Wronkowska, M.; Haros, M.; Soral-Śmietana, M. Effect of starch substitution by buckwheat flour on gluten-free bread quality. Food Bioproc. Technol. 2013, 6, 1820–1827. [Google Scholar] [CrossRef] [Green Version]
- Ronda, F.; Perez-Quirce, S.; Angioloni, A.; Collar, C. Impact of viscous dietary fibres on the viscoelastic behaviour of gluten-free formulated rice doughs: A fundamental and empirical rheological approach. Food Hydrocoll. 2013, 32, 252–262. [Google Scholar] [CrossRef]
- Pérez-Quirce, S.; Collar, C.; Ronda, F. Significance of healthy viscous dietary fibres on the performance of gluten-free rice-based formulated breads. Int. J. Food Sci. Technol. 2014, 49, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Quirce, S.; Caballero, P.A.; Vela, A.J.; Villanueva, M.; Ronda, F. Impact of yeast and fungi (1―3)(1―6)-β-glucan concentrates on viscoelastic behavior and bread making performance of gluten-free rice-based doughs. Food Hydrocoll. 2018, 79, 382–390. [Google Scholar] [CrossRef]
- Elgeti, D.; Nordlohne, S.D.; Föste, M.; Besl, M.; Linden, M.H.; Heinz, V.; Jekl, M.; Becker, T. Volume and texture improvement of gluten-free bread using quinoa white flour. J. Cereal Sci. 2014, 59, 41–47. [Google Scholar] [CrossRef]
- Ronda, F.; Perez-Quirce, S.; Lazaridou, A.; Biliaderis, C.G. Effect of barley and oat β-glucan concentrates on gluten-free rice-based doughs and bread characteristics. Food Hydrocoll. 2015, 48, 197–207. [Google Scholar] [CrossRef]
- Turkut, G.M.; Cakmak, H.; Kumcuoglu, S.; Tavman, S. Effect of quinoa flour on gluten-free bread batter rheology and bread quality. J. Cereal Sci. 2016, 69, 174–181. [Google Scholar] [CrossRef]
- Pérez-Quirce, S.; Lazaridou, A.; Biliaderis, C.G.; Ronda, F. Effect of b-glucan molecular weight on rice flour dough rheology, quality parameters of breads and in vitro starch digestibility. LWT-Food Sci. Technol. 2017, 82, 446–453. [Google Scholar] [CrossRef] [Green Version]
- Sarawong, C.; Gutierrez, Z.; Berghofer, E.; Schoenlechner, R. Effect of green plantain flour addition to gluten-free bread on functional bread properties and resistant starch content. Int. J. Food Sci. 2014, 49, 1825–1833. [Google Scholar] [CrossRef]
- Korus, J.; Juszczak, L.; Ziobro, R.; Witczak, M.; Grzelak, K.; Sójka, M. Defatted strawberry and blackcurrant seeds as functional ingredients of gluten free bread. J. Texture Stud. 2012, 43, 29–39. [Google Scholar] [CrossRef]
- Cappa, C.; Lucisano, M.; Mariotti, M. Influence of Psyllium, sugar beet fibre and water on gluten-free dough properties and bread quality. Carbohydr. Polym. 2013, 98, 1657–1666. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, O.K.; Merta, B. The effects of microfluidization on rheological and textural properties of gluten-free corn breads. Food Res. Int. 2018, 105, 782–792. [Google Scholar] [CrossRef]
- Djeghim, F.; Bourekoua, H.; Rózyło, R.; Bieńczak, A.; Tanaś, W.; Zidoune, M.N. Effect of By-Products from Selected Fruits and Vegetables on Gluten-Free Dough Rheology and Bread Properties. Appl. Sci. 2021, 11, 4605. [Google Scholar] [CrossRef]
- Korus, J.; Juszczak, L.; Witczak, M.; Ziobro, R. Effect of Citrus Fiber on the Rheological Properties of Dough and Quality of the Gluten-Free Bread. Appl. Sci. 2020, 10, 6633. [Google Scholar] [CrossRef]
- Ren, Y.; Linter, B.R.; Foster, T.J. Starch replacement in gluten free bread by cellulose and fibrillated cellulose. Food Hydrocoll. 2020, 107, 105957. [Google Scholar] [CrossRef]
- Korus, J.; Grzelak, K.; Achremowicz, K.; Sabat, R. Influence of prebiotic additions on the quality of gluten-free bread and on the content of inulin and fructooligosaccharides. Food Sci. Technol. Int. 2006, 12, 489–495. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A.G. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 104–110. [Google Scholar] [CrossRef]
- Korus, J.; Witczak, M.; Ziobro, R.; Juszczak, L. The influence of acorn flour on rheological properties of gluten- free dough and physical characteristics of the bread. Eur. Food Res. Technol. 2015, 240, 1135–1143. [Google Scholar] [CrossRef] [Green Version]
- Skendi, A.; Mouselemidou, P.; Papageorgiou, M.; Papastergiadis, E. Effect of acorn meal-water combinations on technological properties and fine structure of gluten-free bread. Food Chem. 2018, 253, 119–126. [Google Scholar] [CrossRef]
- Juszczak, L.; Witczak, T.; Ziobro, R.; Korus, J.; Cieślik, E.; Witczak, M. Effect of inulin on rheological and thermal properties of gluten-free dough. Carbohydr. Polym. 2012, 90, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Steffolani, E.; de la Hera, E.; Pérez, G.; Gómez, M. Effect of Chia (Salvia hispanica L.) Addition on the Quality of Gluten-Free Bread. J. Food Qual. 2014, 37, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Ziobro, R.; Korus, J.; Juszczak, L.; Witczak, T. Influence of inulin on physical characteristics and staling rate of gluten-free bread. J. Food Eng. 2013, 116, 21–27. [Google Scholar] [CrossRef]
- Costantini, L.; Lukšič, L.; Molinari, R.; Kreft, I.; Bonafaccia, G.; Manzi, L.; Merendino, N. Development of gluten-free bread using tartary buckwheat and chia flour rich in flavonoids and omega-3 fatty acids as ingredients. Food Chem. 2014, 165, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Demirkesen, I.; Mert, B.; Sumnu, G.; Sahin, S. Utilization of chestnut flour in gluten-free bread formulations. J. Food Eng. 2010, 101, 329–336. [Google Scholar] [CrossRef]
- Demirkesen, I.; Sumnu, G.; Sahin, S. Image Analysis of gluten-free breads prepared with chestnut and rice flour and baked in different ovens. Food Bioproc. Technol. 2013, 6, 1749–1758. [Google Scholar] [CrossRef]
- Demirkesen, I.; Campanella, O.H.; Sumnu, G.; Sahin, S.; Hamaker, B.R. A study on staling characteristics of gluten-free breads prepared with chestnut and rice flours. Food Bioproc. Technol. 2014, 7, 806–820. [Google Scholar] [CrossRef]
- Morreale, F.; Benavent-Gil, Y.; Rosell, C.M. Inulin enrichment of gluten free breads: Interaction between inulin and yeast. Food Chem. 2019, 278, 545–551. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering, 2nd ed.; Freeman Press: East Lansing, MI, USA, 1996; pp. 45–60. [Google Scholar]
- Dobraszczyk, B.J. Development of a new dough inflation system to evaluate doughs. Cereal Foods World 1997, 42, 516–519. [Google Scholar]
- Walker, C.E.; Hazelton, J.L. Dough rheological tests. Cereal Foods World 1996, 41, 23–28. [Google Scholar]
- Dobraszczyk, B.J.; Morgenstern, M.P. Rheology and the breadmaking process. J. Cereal Sci. 2003, 38, 229–245. [Google Scholar] [CrossRef]
- Vergnes, B.; Della Valle, G.; Colonna, P. Rheological properties of biopolymers and applications to cereal processing. In Characterization of Cereals and Flours. Properties, Analysis and Applications; Kalentuç, G., Breslaner, K.J., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 209–257. [Google Scholar]
- Weipert, D. Descriptive and fundamental rheometry in a new light. Cereal Foods World 1992, 37, 15–24. [Google Scholar]
- Sahin, A.W.; Wiertz, J.; Arendt, E.K. Evaluation of a new method to determine the water addition level in gluten-free bread systems. J. Cereal Sci. 2020, 93, 102971. [Google Scholar] [CrossRef]
- Weipert, D. The Benefits of Basic Rheometry in Studying Dough Rheology. Cereal Chem. 1990, 67, 311–317. [Google Scholar]
- Ronda, F.; Pérez-Quirce, S.; Villanueva, M. Rheological Properties of Gluten-Free Bread Doughs: Relationship with Bread Quality. In Advances in Food Rheology and Its Applications; Woodhead Publishing: Sawston, UK, 2017; Ahmed, J., Ptaszek, P., Basu, S., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 297–334. [Google Scholar]
- Weipert, D. Fundamentals of rheology and spectrometry. In Future of Flour a Compendium of Flour Improvement; Popper, L., Schafer, W., Freund, W., Eds.; AgriMedia GmbH: Clenze, Germany, 2006; pp. 117–146. [Google Scholar]
- Song, K.; Kim, Y.S.; Chang, G.S. Rheology of concentrated xanthan gum solutions: Steady shear flow behavior. Fibers Polym. 2006, 7, 129–138. [Google Scholar] [CrossRef]
- Turabi, E.; Sumnu, G.; Sahin, S. Rheological properties and quality of rice cakes formulated with different gums and an emulsifier blend. Food Hydrocoll. 2008, 22, 305–312. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 4th ed.; Vincentz Netvork: Hannover, Germany, 2014; pp. 159–213. [Google Scholar]
- Létang, C.; Piau, M.; Verdier, C. Characterization of wheat flour-water doughs. Part I: Rheometry and microstructure. J. Food Eng. 1999, 41, 121–132. [Google Scholar] [CrossRef]
- Lazaridou, A.; Biliaderis, C.G. Gluten-free doughs: Rheological properties, testing procedures–methods and potential problems. In Gluten-Free Food SCIENCE and Technology; Gallagher, E., Ed.; Wiley-Blackwell: Oxford, UK, 2009; pp. 52–82. [Google Scholar]
- Ferry, J.D. Viscoelastic Properties of Polymers; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Barnes, H. A Handbook of Elementary Rheology; University of Wales: Aberystwyth, UK, 2000. [Google Scholar]
- Onyango, C.; Mutungi, C.; Unbehend, G.; Lindhauer, M.G. Rheological and baking characteristics of batter and bread prepared from pregelatinised cassava starch and sorghum and modified using microbial transglutaminase. J. Food Eng. 2010, 97, 465–470. [Google Scholar] [CrossRef]
- Rosell, C.M.; Santos, E.; Collar, C. Physico-chemical properties of commercial fibres from different sources: A comparative approach. Food Res. Int. 2009, 42, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Naqash, F.; Gani, A.; Gani, A.; Masoodi, F.A. Gluten-free baking: Combating the challenges—A review. Trends Food Sci. Technol. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Gallagher, E.; Gormley, T.R.; Arendt, E.K. Crust and crumb characteristics of gluten free breads. J. Food Eng. 2003, 56, 153–161. [Google Scholar] [CrossRef]
- Drabińska, N.; Zieliński, H.; Krupa-Kozak, U. Technological benefits of inulin-type fructans application in gluten-free products—A review. Trends Food Sci. Technol. 2016, 56, 149–157. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Auty, M.; Arendt, E.K.; Gallagher, E. Baking properties and microstructure of pseudocereal flours in gluten-free bread formulations. Eur. Food Res. Technol. 2010, 230, 437–445. [Google Scholar] [CrossRef]
- AACC International. Approved Methods of the American Association of Cereal Chemists, 10th ed.; The Association: St. Paul, MN, USA, 2000. [Google Scholar]
- Ahlborn, G.J.; Pike, O.A.; Hendrix, S.B.; Hess, W.M.; Huber, C.S. Sensory, mechanical, and microscopic evaluation of staling in low-protein and gluten- free breads. Cereal Chem. 2005, 82, 328–335. [Google Scholar] [CrossRef]
- Rubel, I.A.; Pérez, E.E.; Manrique, G.D.; Genovese, D.B. Fibre enrichment of wheat bread with Jerusalem artichoke inulin: Effect on dough rheology and bread quality. Food Struct. 2015, 3, 21–29. [Google Scholar] [CrossRef]
- Pareyt, B.; Finnie, S.M.; Putseys, J.A.; Delcour, J.A. Lipids in bread making: Sources, interactions, and impact on bread quality. J. Cereal Sci. 2011, 54, 266–279. [Google Scholar] [CrossRef]
- El Khoury, D.; Balfour-Ducharme, S.; Joye, I.J. A Review on the Gluten-Free Diet: Technological and Nutritional Challenges. Nutrients 2018, 10, 1410. [Google Scholar] [CrossRef] [Green Version]
- Vici, G.; Belli, L.; Biondi, M.; Polzonetti, V. Gluten free diet and nutrient deficiencies: A review. Clin. Nutr. 2016, 35, 1236–1241. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; Pontonio, E.; Filannino, P.; Rizzello, C.G.; De Angelis, M.; Di Cagno, R. How to improve the gluten-free diet: The state of the art from a food science perspective. Food Res. Int. 2018, 110, 22–32. [Google Scholar] [CrossRef]
- Pellegrini, N.; Agostoni, C. Nutritional aspects of gluten-free products. J. Sci. Food Agric. 2015, 95, 2380–2385. [Google Scholar] [CrossRef]
- Santos, F.G.; Aguiar, E.V.; Capriles, V.D. Analysis of ingredient and nutritional labeling of commercially available gluten-free bread in Brazil. Int. J. Food Sci. Nutr. 2019, 70, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Krupa-Kozak, U.; Świątecka, D.; Bączeka, N.; Brzóska, M.M. Inulin and fructooligosaccharide affect in vitro calcium uptake and absorption from calcium-enriched gluten-free bread. Food Funct. 2016, 7, 1950. [Google Scholar] [CrossRef]
- Laparra, J.M.; Tako, E.; Glahn, R.P.; Miller, D.D. Inulin Affects Iron Dialyzability from FeSO4 and FeEDTA Solutions but Does Not Alter Fe Uptake by Caco-2 Cells. J. Agric. Food Chem. 2008, 56, 2846–2851. [Google Scholar] [CrossRef]
- AOAC. Total, Soluble and Insoluble Dietary Fiber in Foods; AOAC Official Method 991.43; Association of Official Analytical Chemists: Arlington, TX, USA, 1991. [Google Scholar]
- Roman, L.; Belorio, M.; Gomez, M. Gluten-Free Breads: The Gap between Research and Commercial Reality. Compr. Rev. Food Sci. Food Saf. 2019, 18, 690–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos Segura, M.E.; Rosell, C.M. Chemical composition and starch digestibility of different gluten-free breads. Plant Foods Hum. Nutr. 2011, 66, 224–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, B.; Orfila, C. The availability and nutritional adequacy of gluten-free bread and pasta. Nutrients 2018, 10, 1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulai, T.; Rashid, M. Assessment of nutritional adequacy of packaged gluten-free food products. Can. J. Diet. Pract. Res. 2014, 75, 186–190. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, A.B.; Fiates, G.M.R.; dos Anjos, A.; Teixeira, E. Availability, cost and nutritional composition of gluten-free products. Br. Food J. 2014, 116, 1842–1852. [Google Scholar] [CrossRef]
- Capriles, V.D.; Arêas, J.A.G. Novel Approaches in Gluten-Free Breadmaking: Interface between Food Science, Nutrition, and Health. Compr. Rev. Food Sci. Food Saf. 2014, 13, 871–890. [Google Scholar] [CrossRef]
- EUR-Lex. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:En:PDF (accessed on 24 November 2021).

| Source | Addition Level (g/100 g) | Reference | Source | Addition Level (g/100 g) | Reference |
|---|---|---|---|---|---|
| Wholegrain cereal flours and brans | Resistant starch | 10, 15, 20 | [48] | ||
| 5, 10, 15 | [49] | ||||
| 5, 10 | [50] | ||||
| 7 | [51] | ||||
| Defatted rice bran | 10 | [22] | Isolated commercial fibers | ||
| Rice bran | 5, 20 | [52] | Bamboo | 10 | [20] |
| Wheat bran | 7 | [51] | Potato | 0.5, 1, 2 | [53] |
| Millet bran | 10 | [54] | Pea | 10 | [20] |
| Teff | 50, 70, 100 | [55] | Rice bran | 10 | [22] |
| Pseudocereals | Oat bran | 5, 10 | [50] | ||
| Amaranth | 50 | [56] | β-glucan | 1, 2 | [57,58] |
| Buckwheat | 50 | [56] | 5.6 | [59] | |
| 40 | [60,61] | 0.1–3.9 | [62,63] | ||
| Quinoa | 50 | [56] | 0.5, 1, 2 | [64] | |
| 40–100 | [65] | 1.8–11.8 | [66] | ||
| 12.5–50 | [67] | 1.3, 2.6, 3.9 | [68] | ||
| Fruit and vegetable byproducts | Cereal (oat, wheat, maize, barley fibers) | 3, 6, 9 | [21] | ||
| Green plantain | 30 | [69] | Nutriose®® | 10 | [20] |
| Defatted blackcurrant seed flour | 5, 10, 15 | [70] | Polydextrose | 10 | [20] |
| Strawberry seed flour | 5, 10, 15 | [70] | Sugar beet fiber | 0.5, 1.5 | [71] |
| Apple pomace | 5–20 | [19] | Citrus fibers | 5.4–6.8 | [72] |
| 2.5, 5, 7.5 | [73] | 10, 15, 20 | [74] | ||
| Orange pomace | 2, 4, 8 | [18] | Apple fibers | 3, 5, 7 | [17] |
| 2.5, 5, 7.5 | [73] | Fibrillated cellulose | 2, 4 | [75] | |
| Prickly pear peal | 2.5, 5, 7.5 | [73] | Cellulose | 10 | [20] |
| Prickly pear seed peal | 2.5, 5, 7.5 | [73] | 8, 10, 16, 18, 20 | [75] | |
| Tomato pomace | 2.5, 5, 7.5 | [73] | Prebiotic dietary fibers | ||
| Pepper pomace | 2.5, 5, 7.5 | [73] | Fructooligosaccharides | 3, 5, 8 | [76] |
| Sugar beet pulp | 3, 5, 7 | [16,17] | Inulin-type fructans | 8.6, 17.9, 22.7, 28 | [77] |
| Seed fibers | Inulin | 3, 5, 8 | [76] | ||
| Acorn | 20, 40, 60 | [78] | 9 | [59] | |
| 5, 15, 25 | [79] | 4, 8, 12 | [80] | ||
| Chia seed and flour | 15 | [81] | 4, 8, 12 | [82] | |
| 10 | [83] | 5, 10 | [50] | ||
| Chestnut | 10–50, 100 | [84,85,86] | 10 | [87] | |
| 7.5 | [51] |
| Source | Addition Level (g/100 g) | Specific vol. (cm3/g) | Hardness (N) | Reference |
|---|---|---|---|---|
| Predominantly insoluble fiber | ||||
| Millet bran | 10 | 2.02–2.04 | 13.4–13.7 | [54] |
| Rice bran | 5, 20 | 1.14–1.58 | 0.26–0.68 | [52] |
| 10 | 2.90–3.46 | 5.36–14.47 | [22] | |
| Oat bran | 5, 10 | 2.42–2.70 | 1.58–2.10 | [50] |
| Oat fiber | 10 | 2.86–3.89 | 11.62–42.71 | [20] |
| Potato fiber | 0.5, 1, 2 | 2.5–2.9 | / | [53] |
| 10 | 2.5 | / | [20] | |
| Pea fiber | 10 | 2.77 | 29.80 | [20] |
| Bamboo fiber | 10 | 2.75–3.82 | 10.19–52.75 | [20] |
| Resistant starch | 5, 10 | 2.36–2.50 | 1.80–2.54 | [50] |
| Insoluble β-glucan | 0.5, 1, 2 | 3.77–4.43 | 0.37–0.55 | [64] |
| Citrus fiber | 5.4–6.8 | 2.56–3.43 | / | [72] |
| Orange pomace | 2.5, 5, 7.5 | 2.00–2.46 | / | [73] |
| 2, 4, 8 | 2.3 | 1.34–8.60 | [18] | |
| Prickly pear peal | 2.5, 5, 7.5 | 1.83–2.50 | / | [73] |
| Prickly pear seed peal | 2.5, 5, 7.5 | 1.72–2.02 | / | [73] |
| Tomato pomace | 2.5, 5, 7.5 | 2.10–2.21 | / | [73] |
| Pepper pomace | 2.5, 5, 7.5 | 1.69–2.10 | / | [73] |
| Apple pomace | 2.5, 5, 7.5 | 2.02–2.16 | / | [73] |
| 5–20 | 1.6–3.2 | 0.93–4.15 | [19] | |
| Apple fiber | 3, 5, 7 | 1.72–3.97 | 2.10–13.93 | [17] |
| Sugar beet fiber | 3, 5, 7 | 1.52–2.44 | 2.29–22.74 | [17] |
| Predominantly soluble fiber | ||||
| Oat β-glucan | 1.8–11.8 | 1.93–2.75 | 1.30–2.04 | [66] |
| 1.3, 2.6, 3.9 | 2.04–3.17 | 1.21–6.53 | [68] | |
| Barley β-glucan | 1.8–11.8 | 2.40–2.56 | 0.93–4.15 | [66] |
| Yeast β-glucan | 0.5, 1, 2 | 3.57–3.83 | 0.37–0.60 | [64] |
| Inulin | 10 | 2.28–2.69 | 1.34–2.62 | [87] |
| Nutriose®® | 10 | 4.25 | 7.01 | [20] |
| Polydextrose | 10 | 5.09 | 5.18 | [20] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djordjević, M.; Djordjević, M.; Šoronja-Simović, D.; Nikolić, I.; Šereš, Z. Delving into the Role of Dietary Fiber in Gluten-Free Bread Formulations: Integrating Fundamental Rheological, Technological, Sensory, and Nutritional Aspects. Polysaccharides 2022, 3, 59-82. https://doi.org/10.3390/polysaccharides3010003
Djordjević M, Djordjević M, Šoronja-Simović D, Nikolić I, Šereš Z. Delving into the Role of Dietary Fiber in Gluten-Free Bread Formulations: Integrating Fundamental Rheological, Technological, Sensory, and Nutritional Aspects. Polysaccharides. 2022; 3(1):59-82. https://doi.org/10.3390/polysaccharides3010003
Chicago/Turabian StyleDjordjević, Marijana, Miljana Djordjević, Dragana Šoronja-Simović, Ivana Nikolić, and Zita Šereš. 2022. "Delving into the Role of Dietary Fiber in Gluten-Free Bread Formulations: Integrating Fundamental Rheological, Technological, Sensory, and Nutritional Aspects" Polysaccharides 3, no. 1: 59-82. https://doi.org/10.3390/polysaccharides3010003
APA StyleDjordjević, M., Djordjević, M., Šoronja-Simović, D., Nikolić, I., & Šereš, Z. (2022). Delving into the Role of Dietary Fiber in Gluten-Free Bread Formulations: Integrating Fundamental Rheological, Technological, Sensory, and Nutritional Aspects. Polysaccharides, 3(1), 59-82. https://doi.org/10.3390/polysaccharides3010003