Beyond Proteins—Edible Insects as a Source of Dietary Fiber
Abstract
1. Introduction
2. Edible Insects
| Insect Type | Protein (%) | Fat (%) | Chitin (%) | References |
|---|---|---|---|---|
| Mealworms | 16–45 | 20–43 | 1–3 | [15,16,17,18] |
| Crickets | 15–47 | 7–29 | 1–12 | [16,19,20] |
| Grasshopper | 50–70 | 6–11 | 5–15 | [16,21] |
| Black soldier fly | 12–55 | 11–29 | 3–15 | [17,18,22] |
2.1. Limitation of the Possible Widespread Use of Insects
2.2. Possible Limitations of Insect Use within the European Union
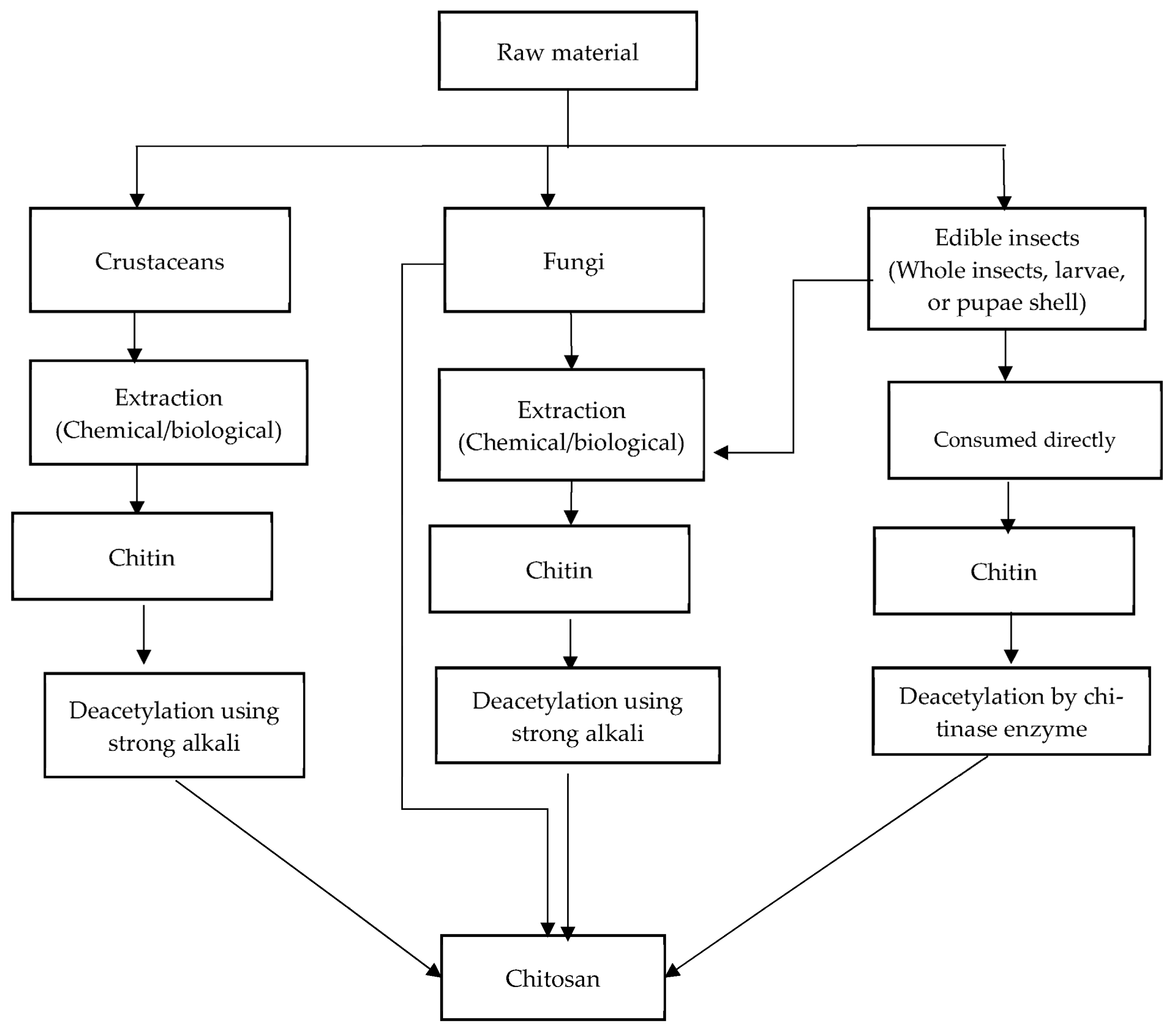
3. Chitin and Chitosan as a Dietary Fiber
| Source | Advantages | Disadvantages | Reference |
|---|---|---|---|
| Crustaceans Content 16–49% | The highest content of chitin; hence, it is viable for industrial extraction | Limited to seasonal and regional variations High mineral content requiring aggressive extraction Highly susceptible to heavy metal contaminants | [47,48] |
| Fungi Content 1–29% | Possibility of the direct production of chitosan by some species No seasonal variations Can be produced by solid phase fermentation that can control for desired chitin/chitosan production Important as a vegan source of chitin and chitosan Consistent physiological and chemical properties | Limited availability and low content Industrial production not widely established | [47,49,57,58,59] |
| Edible insects Content 6–36% | Can be consumed directly Can be produced all year round Can be produced by bacterial fermentation With industrial insect farming, chitin can be available as a cheap byproduct | Commercial production has not been established The risk of allergic reactions has not been fully described | [47,58,60,61] |
Insect Chitin as a Prebiotic
| Study | Methods | Main Findings | Recommendations | References |
|---|---|---|---|---|
| Critical review on the effects of chitin and derived polysaccharides on human gut microbiota | Review | Whole insect meal is beneficial in the modulation of gut microbiota. Chitin-derived chitosan has the potential to be prebiotic when ingested with low protein diets, as a high protein content may counteract the benefits of chitin. Chitosan promotes the growth of beneficial bacteria, suppresses potentially pathogenic bacteria, has anti-inflammatory and immune stimulating properties, and may also help to treat obesity and diabetes | There is a need for more research to promote the use of chitin and chitosan as human food. | [65] |
| Effects of consuming whole cricket powder on gut microbiota; n = 20 | Stool analysis | Enhanced growth of Bifidobacterium animalis and reduced TNF-α | Need to understand underlying mechanisms | [87] |
| The potential of the edible insect as a prebiotic | In vitro bacterial composition of human fecal microbiome | Changes in microbial composition induced by undigested insect material in the batch culture—particularly, an increase in Faecalibacterium spp., previously associated with anti-inflammation | There is a need to test this in vivo | [88] |
| In vitro gastrointestinal digestion of cricket protein hydrolysates | Sequential fractionation | Protein peptides from edible crickets have positive effects on inflammation and hypersensitivity | Further research on the underlying mechanisms of the anti-inflammation effects of cricket peptides | [89] |
| Adding insects to the diet | Review | Reduced nutrient deficiencies and beneficial effects of insect bioactive compounds in diseases such as coronary heart disease, inflammation, and cancer | Bioactive compounds derived from insects should be used to formulate diets for better health | [90] |
| Benefits of eating insects and shrimps | Review | Insect extracts have antioxidant properties and the potential to be used in low-sodium diets | There is a need for further in vivo and clinical studies | [91] |
| Environmental and health benefits of edible insects | Review | Improved gastrointestinal health, reduced infection, and improved immunity. This could be due to reduced inflammation and a high protein content that is important in building muscles | Need for well-designed clinical studies | [92] |
| Consumption of insect-derived protein | Double-blind controlled trial | Increased muscle synthesis at rest and during exercise | Increase the use of insects to provide high-quality proteins | [93] |
| The antioxidant ability of insect products | Review | Edible insect-derived products can help the oxidative stress-mediated infection | Research to develop oxidative molecules from edible insects | [94] |
| Edible insects in complementary food | Randomized control trial | Improved micronutrient status in infants | More research on nutrient bioavailability; there is also a need to use edible insect products to save humanitarian situations, especially in malnourished children | [95] |
| Effects of edible insects on gut health | Review | Improved gut health and microbial diversity, and increased secretion of short-chain fatty acids | Study of digestion and bioavailability of chitin in humans | [86] |
| Prebiotic potential of insect chitosan | In vitro study | Inhibition of pathogenic bacteria | Need for in vivo studies to test its use as a prebiotic. | [96] |
| Feeding bugs to bugs | Batch culture inoculation | Modulation of gut microbiota | Need for in vivo studies to gain insight into the required dosage | [88] |
4. Conclusions and Outlook
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rumpold, B.A.; Schluter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef] [PubMed]
- Van Huis, A.; Rumpold, B.; van der Fels-Klerx, H.; Tomberlin, J. Advancing edible insects as food and feed in a circular economy. J. Insects Food Feed 2021, 7, 935–948. [Google Scholar] [CrossRef]
- Sogari, G.; Riccioli, F.; Moruzzo, R.; Menozzi, D.; Sosa, D.A.T.; Li, J.; Liu, A.J.; Mancini, S. Engaging in entomophagy: The role of food neophobia and disgust between insect and non-insect eaters. Food Qual. Prefer. 2023, 104, 104764. [Google Scholar] [CrossRef]
- European Commission. Authorising the placing on the market of frozen, dried and powder forms of Acheta domesticus as a novel food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2022, 7. [Google Scholar]
- Gasco, L.; Jozefiak, A.; Henry, M. Beyond the protein concept: Health aspects of using edible insects on animals. J. Insects Food Feed 2021, 7, 715–741. [Google Scholar] [CrossRef]
- Aguilar-Toala, J.E.; Cruz-Monterrosa, R.G.; Liceaga, A.M. Beyond Human Nutrition of Edible Insects: Health Benefits and Safety Aspects. Insects 2022, 13, 1007. [Google Scholar] [CrossRef] [PubMed]
- van Huis, A. Edible insects are the future? Proc. Nutr. Soc. 2016, 75, 294–305. [Google Scholar] [CrossRef]
- van Huis, A.; Rumpold, B.; Maya, C.; Roos, N. Nutritional Qualities and Enhancement of Edible Insects. Annu. Rev. Nutr. 2021, 41, 551–576. [Google Scholar] [CrossRef]
- Lumanlan, J.C.; Williams, M.; Jayasena, V. Edible insects: Environmentally friendly sustainable future food source. Int. J. Food Sci. Technol. 2022, 57, 6317–6325. [Google Scholar] [CrossRef]
- Wade, M.; Hoelle, J. A review of edible insect industrialization: Scales of production and implications for sustainability. Environ. Res. Lett. 2020, 15, 123013. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B.; Gahukar, R.T.; Ghosh, S.; Jung, C. Chemical Composition, Nutrient Quality and Acceptability of Edible Insects Are Affected by Species, Developmental Stage, Gender, Diet, and Processing Method. Foods 2021, 10, 1036. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Bueno, R.P.; Gonzalez-Fernandez, M.J.; Sanchez-Muros-Lozano, M.J.; Garcia-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- Hematyar, N.; Rustad, T.; Sampels, S.; Dalsgaard, T.K. Relationship between lipid and protein oxidation in fish. Aquac. Res. 2019, 50, 1393–1403. [Google Scholar] [CrossRef]
- Slocinska, M.; Marciniak, P.; Rosinski, G. Insects antiviral and anticancer peptides: New leads for the future? Protein Pept. Lett. 2008, 15, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will yellow mealworm become a source of safe proteins for Europe? Agriculture 2020, 10, 233. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo Biol. 2015, 34, 554–564. [Google Scholar] [CrossRef]
- Payne, C.L.R.; Scarborough, P.; Rayner, M.; Nonaka, K. Are edible insects more or less ‘healthy’ than commonly consumed meats? A comparison using two nutrient profiling models developed to combat over- and undernutrition. Eur. J. Clin. Nutr. 2016, 70, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Thrastardottir, R.; Olafsdottir, H.T.; Thorarinsdottir, R.I. Yellow Mealworm and Black Soldier Fly Larvae for Feed and Food Production in Europe, with Emphasis on Iceland. Foods 2021, 10, 2744. [Google Scholar] [CrossRef]
- Pastell, H.; Mellberg, S.; Ritvanen, T.; Raatikainen, M.; Mykkanen, S.; Niemi, J.; Latomaki, I.; Wirtanen, G. How Does Locally Produced Feed Affect the Chemical Composition of Reared House Crickets (Acheta domesticus)? ACS Food Sci. Technol. 2021, 1, 625–635. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Siejak, P.; Jarzębski, M.; Jakubowicz, J.; Jeżowski, P.; Walkowiak, K.; Smarzyński, K.; Ostrowska-Ligęza, E.; Baranowska, H. Comparison of technological and physicochemical properties of cricket powders of different origin. J. Insects Food Feed 2022, 1–10. [Google Scholar] [CrossRef]
- Rodríguez-Miranda, J.; Alcántar-Vázquez, J.P.; Zúñiga-Marroquín, T.; Juárez-Barrientos, J.M. Insects as an alternative source of protein: A review of the potential use of grasshopper (Sphenarium purpurascens Ch.) as a food ingredient. Eur. Food Res. Technol. 2019, 245, 2613–2620. [Google Scholar] [CrossRef]
- Magalhães, R.; Sánchez-López, A.; Leal, R.S.; Martínez-Llorens, S.; Oliva-Teles, A.; Peres, H. Black soldier fly (Hermetia illucens) pre-pupae meal as a fish meal replacement in diets for European seabass (Dicentrarchus labrax). Aquaculture 2017, 476, 79–85. [Google Scholar] [CrossRef]
- Singh, A.; Gairola, K.; Upadhyay, V.; Kumar, J. Chitosan: An elicitor and antimicrobial Bio-resource in plant protection. Agric. Rev. 2018, 39, 163–168. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Zhang, H.Y.; He, Y.H.; He, L. Identification of a chitin deacetylase producing bacteria isolated from soil and its fermentation optimization. Afr. J. Microbiol. Res. 2010, 4, 2597–2603. [Google Scholar]
- Xu, Y.; Mao, H.; Yang, C.; Du, H.; Wang, H.; Tu, J. Effects of chitosan nanoparticle supplementation on growth performance, humoral immunity, gut microbiota and immune responses after lipopolysaccharide challenge in weaned pigs. J. Anim. Physiol. Anim. Nutr. 2020, 104, 597–605. [Google Scholar] [CrossRef]
- Kröncke, N.; Baur, A.; Böschen, V.; Demtröder, S.; Benning, R.; Delgado, A. Automation of insect mass rearing and processing technologies of mealworms (Tenebrio molitor). In African Edible Insects as Alternative Source of Food, Oil, Protein and Bioactive Components; Springer: Berlin/Heidelberg, Germany, 2020; pp. 123–139. [Google Scholar]
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millan, R.; Raposo, A. Traditional consumption of and rearing edible insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef]
- Han, R.; Shin, J.T.; Kim, J.; Choi, Y.S.; Kim, Y.W. An overview of the South Korean edible insect food industry: Challenges and future pricing/promotion strategies. Entomol. Res. 2017, 47, 141–151. [Google Scholar] [CrossRef]
- Kelemu, S.; Niassy, S.; Torto, B.; Fiaboe, K.; Affognon, H.; Tonnang, H.; Maniania, N.K.; Ekesi, S. African edible insects for food and feed: Inventory, diversity, commonalities and contribution to food security. J. Insects Food and Feed 2015, 1, 103–119. [Google Scholar] [CrossRef]
- Garino, C.; Mielke, H.; Knuppel, S.; Selhorst, T.; Broll, H.; Braeuning, A. Quantitative allergenicity risk assessment of food products containing yellow mealworm (Tenebrio molitor). Food Chem. Toxicol. 2020, 142, 111460. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, X.M.; Zhao, M.; He, Z.; Sun, L.; Wang, C.Y.; Ding, W.F. Edible insects in China: Utilization and prospects. Insect Sci. 2018, 25, 184–198. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Das, C. Foodborne infections and food safety. East. J. Med. Sci. 2022, 60–63. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodríguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jäger, H. Edible insects: Cross-recognition of IgE from crustacean-and house dust mite allergic patients, and reduction of allergenicity by food processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef] [PubMed]
- Murefu, T.R.; Macheka, L.; Musundire, R.; Manditsera, F.A. Safety of wild harvested and reared edible insects: A review. Food Control 2019, 101, 209–224. [Google Scholar] [CrossRef]
- Zuk-Golaszewska, K.; Galecki, R.; Obremski, K.; Smetana, S.; Figiel, S.; Golaszewski, J. Edible Insect Farming in the Context of the EU Regulations and Marketing-An Overview. Insects 2022, 13, 446. [Google Scholar] [CrossRef]
- Meyer, A.M.; Meijer, N.; Hoek-van den Hil, E.F.; Van der Fels-Klerx, H.J. Chemical food safety hazards of insects reared for food and feed. J. Insects Food Feed 2021, 7, 823–831. [Google Scholar] [CrossRef]
- European Commision. Council Directive 98/58/EC of 20 July 1998 Concerning the Protection of Animals Kept for Farming Purposes. European Commision, Ed.; European Commission, European Union. 1998. Available online: http://data.europa.eu/eli/dir/1998/58/oj (accessed on 10 March 2023).
- Delgado, L.; Garino, C.; Moreno, F.J.; Zagon, J.; Broll, H. Sustainable Food Systems: EU Regulatory Framework and Contribution of Insects to the Farm-To-Fork Strategy. Food Rev. Int. 2022, 1–22. [Google Scholar] [CrossRef]
- Smetana, S.; Palanisamy, M.; Mathys, A.; Heinz, V. Sustainability of insect use for feed and food: Life Cycle Assessment perspective. J. Clean. Prod. 2016, 137, 741–751. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Ristow, B.; Rahayu, T.; Putra, N.S.; Yuwono, N.W.; Nisa, K.; Mategeko, B.; Smetana, S.; Saki, M.; Nawaz, A.; et al. Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Manag. 2022, 140, 1–13. [Google Scholar] [CrossRef]
- Lalander, C.; Diener, S.; Zurbrugg, C.; Vinneras, B. Effects of feedstock on larval development and process efficiency in waste treatment with black soldier fly (Hermetia illucens). J. Clean. Prod. 2019, 208, 211–219. [Google Scholar] [CrossRef]
- Tokwaro, R.; Semiyaga, S.; Niwagaba, C.B.; Nakagiri, A.; Sempewo, J.I.; Muoghalu, C.C.; Manga, M. Application of black soldier fly larvae in decentralized treatment of faecal sludge from pit latrines in informal settlements in Kampala City. Front. Environ. Sci. 2023, 11, 138. [Google Scholar] [CrossRef]
- Chirere, T.E.S.; Khalil, S.; Lalander, C. Fertiliser effect on Swiss chard of black soldier fly larvae-frass compost made from food waste and faeces. J. Insects Food Feed 2021, 7, 457–469. [Google Scholar] [CrossRef]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.J. Food applications of chitin and chitosans. Trends Food Sci. Tech. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Amiri, H.; Aghbashlo, M.; Sharma, M.; Gaffey, J.; Manning, L.; Basri, S.M.M.; Kennedy, J.F.; Gupta, V.K.; Tabatabaei, M. Chitin and chitosan derived from crustacean waste valorization streams can support food systems and the UN Sustainable Development Goals. Nat. Food 2022, 3, 822–828. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A Review of Crustacean and Fungal Chitin in Wound Treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef]
- Ma, J.; Faqir, Y.; Tan, C.; Khaliq, G. Terrestrial insects as a promising source of chitosan and recent developments in its application for various industries. Food Chem. 2022, 373, 131407. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; Paul, A.; Salvia, R.; Falabella, P.; Zibek, S. Current state of chitin purification and chitosan production from insects. J. Chem. Technol. Biotechnol. 2020, 95, 2775–2795. [Google Scholar] [CrossRef]
- Kipkoech, C.; Kinyuru, J.; Imathiu, S.; Roos, N. Use of house cricket to address food security in Kenya: Nutrient and chitin composition of farmed crickets as influenced by age. Afr. J. Agric. Res. 2017, 12, 3189–3197. [Google Scholar] [CrossRef]
- Derrien, C.; Boccuni, A. Current status of the insect producing industry in Europe. In Edible Insects in Sustainable Food Systems; Springer: Cham, Switzerland, 2018; pp. 471–479. [Google Scholar]
- European Commission. Approval of chitosan hydrochloride as basic substance in accordance with Regulation (EC) No 1107/2009; European Commission, Ed.; European Commission, European Union: Washington, DC, USA, 2014; pp. 5–7. [Google Scholar]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; de Lima, M.A.B.; Franco, L.D.; de Campos-Takaki, G.M. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef]
- Kemboi, V.J.; Kipkoech, C.; Njire, M.; Were, S.; Lagat, M.K.; Ndwiga, F.; Wesonga, J.M.; Tanga, C.M. Biocontrol Potential of Chitin and Chitosan Extracted from Black Soldier Fly Pupal Exuviae against Bacterial Wilt of Tomato. Microorganisms 2022, 10, 165. [Google Scholar] [CrossRef]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A.; Danmaigoro, A.; Nicholas, N.P. Gut health and serum growth hormone levels of broiler chickens fed dietary chitin and chitosan from cricket and shrimp. Poult. Sci. 2019, 98, 745–752. [Google Scholar] [CrossRef]
- Kamilya, D.; Khan, M.I.R. Chitin and chitosan as promising immunostimulant for aquaculture. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 761–771. [Google Scholar]
- Zhang, J.; Wan, J.; Wu, G.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; He, J. Low-molecular-weight chitosan relieves enterotoxigenic Escherichia coli-induced growth retardation in weaned pigs. Int. Immunopharmacol. 2020, 78, 105798. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Araújo, D.; Ferreira, I.C.; Torres, C.A.; Neves, L.; Freitas, F. Chitinous polymers: Extraction from fungal sources, characterization and processing towards value-added applications. J. Chem. Technol. Biot. 2020, 95, 1277–1289. [Google Scholar] [CrossRef]
- Lagat, M.K.; Were, S.; Ndwigah, F.; Kemboi, V.J.; Kipkoech, C.; Tanga, C.M. Antimicrobial Activity of Chemically and Biologically Treated Chitosan Prepared from Black Soldier Fly (Hermetia illucens) Pupal Shell Waste. Microorganisms 2021, 9, 2417. [Google Scholar] [CrossRef]
- Zainol Abidin, N.A.; Kormin, F.; Zainol Abidin, N.A.; Mohamed Anuar, N.A.F.; Abu Bakar, M.F. The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- Gong, T.; Zhou, Y.; Zhang, L.; Wang, H.; Zhang, M.; Liu, X. Capsaicin combined with dietary fiber prevents high-fat diet associated aberrant lipid metabolism by improving the structure of intestinal flora. Food Sci. Nutr. 2023, 11, 114–125. [Google Scholar] [CrossRef]
- Abbott, A. Scientists bust myth that our bodies have more bacteria than human cells. Nat. News 2016. [Google Scholar] [CrossRef]
- Lopez-Santamarina, A.; Mondragon, A.D.C.; Lamas, A.; Miranda, J.M.; Franco, C.M.; Cepeda, A. Animal-Origin Prebiotics Based on Chitin: An Alternative for the Future? A Critical Review. Foods 2020, 9, 782. [Google Scholar] [CrossRef]
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.B.; Alexeev, D.G.; Boytsov, S.A. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 2016, 32, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Maukonen, J.; Saarela, M. Human gut microbiota: Does diet matter? Proc. Nutr. Soc 2015, 74, 23–36. [Google Scholar] [CrossRef]
- Merra, G.; Noce, A.; Marrone, G.; Cintoni, M.; Tarsitano, M.G.; Capacci, A.; De Lorenzo, A. Influence of mediterranean diet on human gut microbiota. Nutrients 2021, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Rio-Aige, K.; Azagra-Boronat, I.; Massot-Cladera, M.; Selma-Royo, M.; Parra-Llorca, A.; Gonzalez, S.; Garcia-Mantrana, I.; Castell, M.; Rodriguez-Lagunas, M.J.; Collado, M.C.; et al. Association of Maternal Microbiota and Diet in Cord Blood Cytokine and Immunoglobulin Profiles. Int. J. Mol. Sci. 2021, 22, 1778. [Google Scholar] [CrossRef] [PubMed]
- Teng, N.M.Y.; Price, C.A.; McKee, A.M.; Hall, L.J.; Robinson, S.D. Exploring the impact of gut microbiota and diet on breast cancer risk and progression. Int. J. Cancer 2021, 149, 494–504. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota-Gut-Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef] [PubMed]
- Lankelma, J.M.; Nieuwdorp, M.; de Vos, W.M.; Wiersinga, W.J. The gut microbiota in internal medicine: Implications for health and disease. Neth. J. Med. 2015, 73, 61–68. [Google Scholar] [PubMed]
- Lee, Y.K. Effects of diet on gut microbiota profile and the implications for health and disease. Biosci. Microbiota Food Health 2013, 32, 1–12. [Google Scholar] [CrossRef]
- Walker, W. Dysbiosis. In The Microbiota in Gastrointestinal Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 227–232. [Google Scholar]
- Carding, S.; Verbeke, K.; Vipond, D.T.; Corfe, B.M.; Owen, L.J. Dysbiosis of the gut microbiota in disease. Microb. Ecol. Health Dis. 2015, 26, 26191. [Google Scholar] [CrossRef]
- Levy, M.; Kolodziejczyk, A.A.; Thaiss, C.A.; Elinav, E. Dysbiosis and the immune system. Nat. Rev. Immunol. 2017, 17, 219–232. [Google Scholar] [CrossRef]
- Bibbo, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ju, Z.; Zuo, T. Time for food: The impact of diet on gut microbiota and human health. Nutrition 2018, 51–52, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, T.K.; Jeong, C.H.; Yong, H.I.; Cha, J.Y.; Kim, B.K.; Choi, Y.S. Biological activity and processing technologies of edible insects: A review. Food Sci. Biotechnol. 2021, 30, 1003–1023. [Google Scholar] [CrossRef]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Ushakova, N.; Nekrasov, R.; Pravdin, I.; Sverchkova, N.; Kolomiyets, E.; Pavlov, D. Mechanisms of the effects of probiotics on symbiotic digestion. Biol. Bull. 2015, 42, 394–400. [Google Scholar] [CrossRef]
- Anusha, S.; Negi, P.S. Edible insects and gut health. In Nutrition and Functional Foods in Boosting Digestion, Metabolism and Immune Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 523–539. [Google Scholar]
- Stull, V.J.; Finer, E.; Bergmans, R.S.; Febvre, H.P.; Longhurst, C.; Manter, D.K.; Patz, J.A.; Weir, T.L. Impact of Edible Cricket Consumption on Gut Microbiota in Healthy Adults, a Double-blind, Randomized Crossover Trial. Sci. Rep. 2018, 8, 10762. [Google Scholar] [CrossRef]
- Young, W.; Arojju, S.K.; McNeill, M.R.; Rettedal, E.; Gathercole, J.; Bell, N.; Payne, P. Feeding Bugs to Bugs: Edible Insects Modify the Human Gut Microbiome in an in vitro Fermentation Model. Front. Microbiol. 2020, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and Characterization of Edible Cricket Peptides on Hypertensive and Glycemic In Vitro Inhibition and Their Anti-Inflammatory Activity on RAW 264.7 Macrophage Cells. Nutrients 2020, 12, 3588. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Estrada, B.A.; Reyes, A.; Rosell, C.M.; Rodrigo, D.; Ibarra-Herrera, C.C. Benefits and Challenges in the Incorporation of Insects in Food Products. Front. Nutr. 2021, 8, 687712. [Google Scholar] [CrossRef] [PubMed]
- Mishyna, M.; Glumac, M. So different, yet so alike Pancrustacea: Health benefits of insects and shrimps. J. Funct. Foods 2021, 76, 104316. [Google Scholar] [CrossRef]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential health benefits of edible insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef]
- Hermans, W.J.H.; Senden, J.M.; Churchward-Venne, T.A.; Paulussen, K.J.M.; Fuchs, C.J.; Smeets, J.S.J.; van Loon, J.J.A.; Verdijk, L.B.; van Loon, L.J.C. Insects are a viable protein source for human consumption: From insect protein digestion to postprandial muscle protein synthesis in vivo in humans: A double-blind randomized trial. Am. J. Clin. Nutr. 2021, 114, 934–944. [Google Scholar] [CrossRef]
- Oghenesuvwe, E.E.; Paul, C. Edible insects bio-actives as anti-oxidants: Current status and perspectives. J. Complement. Med. Res. 2019, 10, 89–102. [Google Scholar] [CrossRef]
- Konyole, S.O. Effect of Improved Complementary Foods on Growth and Iron Status of Kenyan Infants; University of Nairobi: Nairobi, Kenya, 2014. [Google Scholar]
- Kipkoech, C.; Kinyuru, J.N.; Imathiu, S.; Meyer-Rochow, V.B.; Roos, N. In Vitro Study of Cricket Chitosan’s Potential as a Prebiotic and a Promoter of Probiotic Microorganisms to Control Pathogenic Bacteria in the Human Gut. Foods 2021, 10, 2310. [Google Scholar] [CrossRef]
- Hong, K.B.; Kim, J.H.; Kwon, H.K.; Han, S.H.; Park, Y.; Suh, H.J. Evaluation of prebiotic effects of high-purity galactooligosaccharides in vitro and in vivo. Food Technol. Biotechnol. 2016, 54, 156. [Google Scholar] [CrossRef]
- Zaman, S.A.; Sarbini, S.R. The potential of resistant starch as a prebiotic. Crit. Rev. Biotechnol. 2016, 36, 578–584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kipkoech, C. Beyond Proteins—Edible Insects as a Source of Dietary Fiber. Polysaccharides 2023, 4, 116-128. https://doi.org/10.3390/polysaccharides4020009
Kipkoech C. Beyond Proteins—Edible Insects as a Source of Dietary Fiber. Polysaccharides. 2023; 4(2):116-128. https://doi.org/10.3390/polysaccharides4020009
Chicago/Turabian StyleKipkoech, Carolyne. 2023. "Beyond Proteins—Edible Insects as a Source of Dietary Fiber" Polysaccharides 4, no. 2: 116-128. https://doi.org/10.3390/polysaccharides4020009
APA StyleKipkoech, C. (2023). Beyond Proteins—Edible Insects as a Source of Dietary Fiber. Polysaccharides, 4(2), 116-128. https://doi.org/10.3390/polysaccharides4020009





