Investigating Cellulose Nanocrystal and Polyvinyl Alcohol Composite Film in Moisture Sensing Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Composite Preparation
2.2. Zeta Potential
2.3. Scanning Electron Microscopy
2.4. Cross-Polarized Microscopy
2.5. UV-Vis Spectroscopy
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Sensitivity Analysis
2.8. Tensile Strength Determination
2.9. Film Thickness
3. Result and Discussion
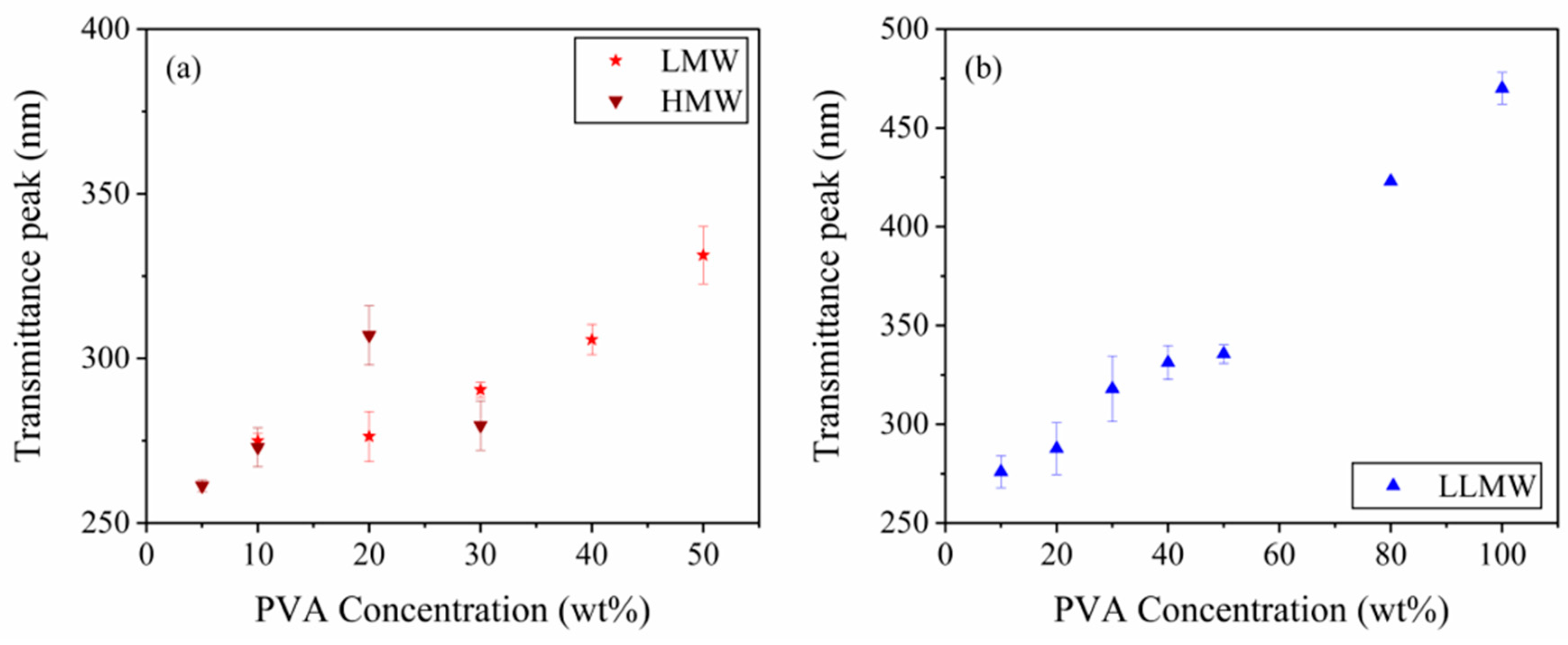
3.1. Effects of Different MW PVA on Optical Response of Composite Films
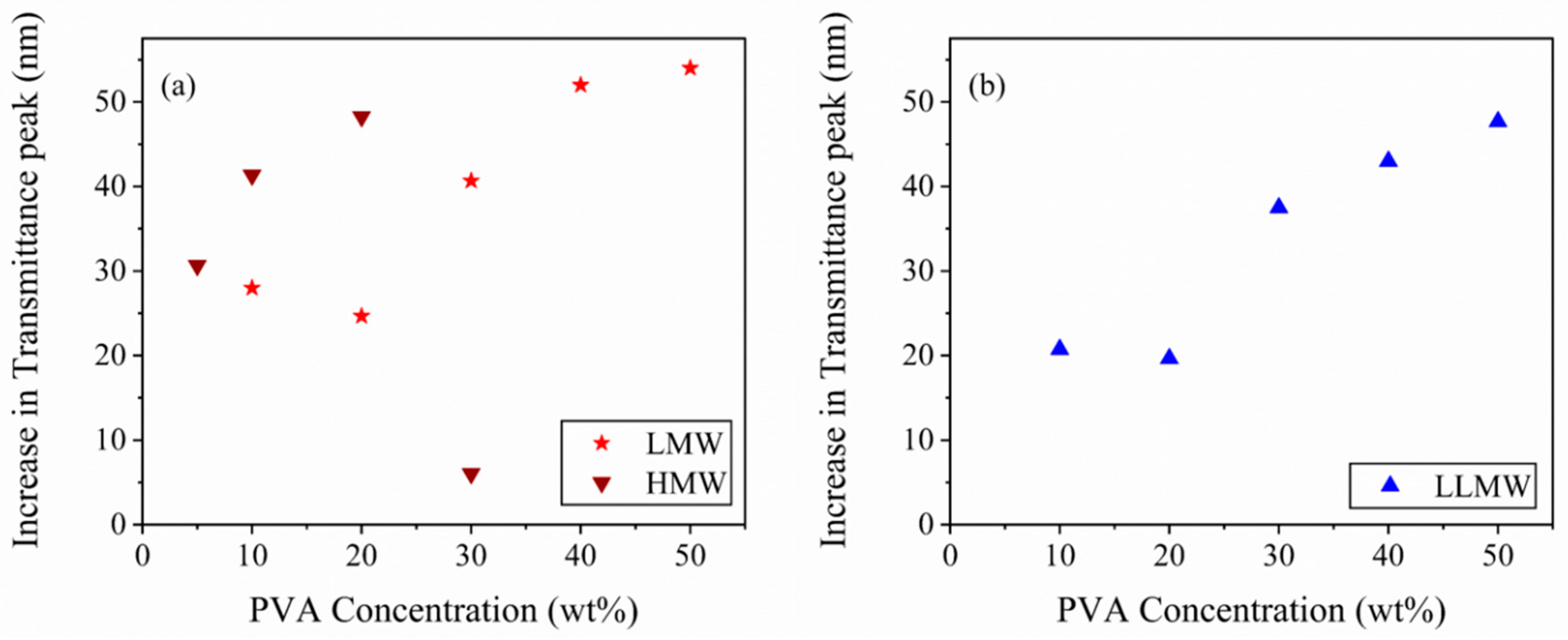
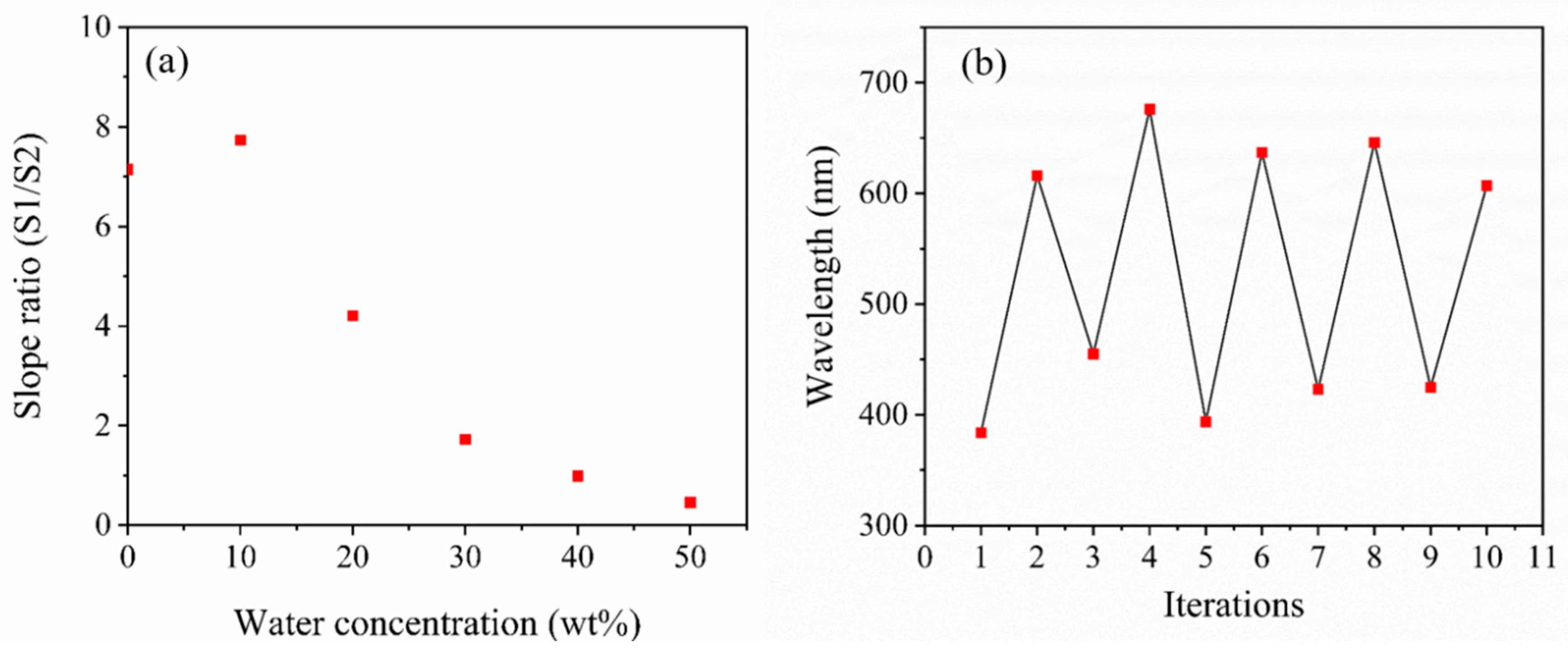
3.2. Moisture Sensitivity Analysis of Composite Films
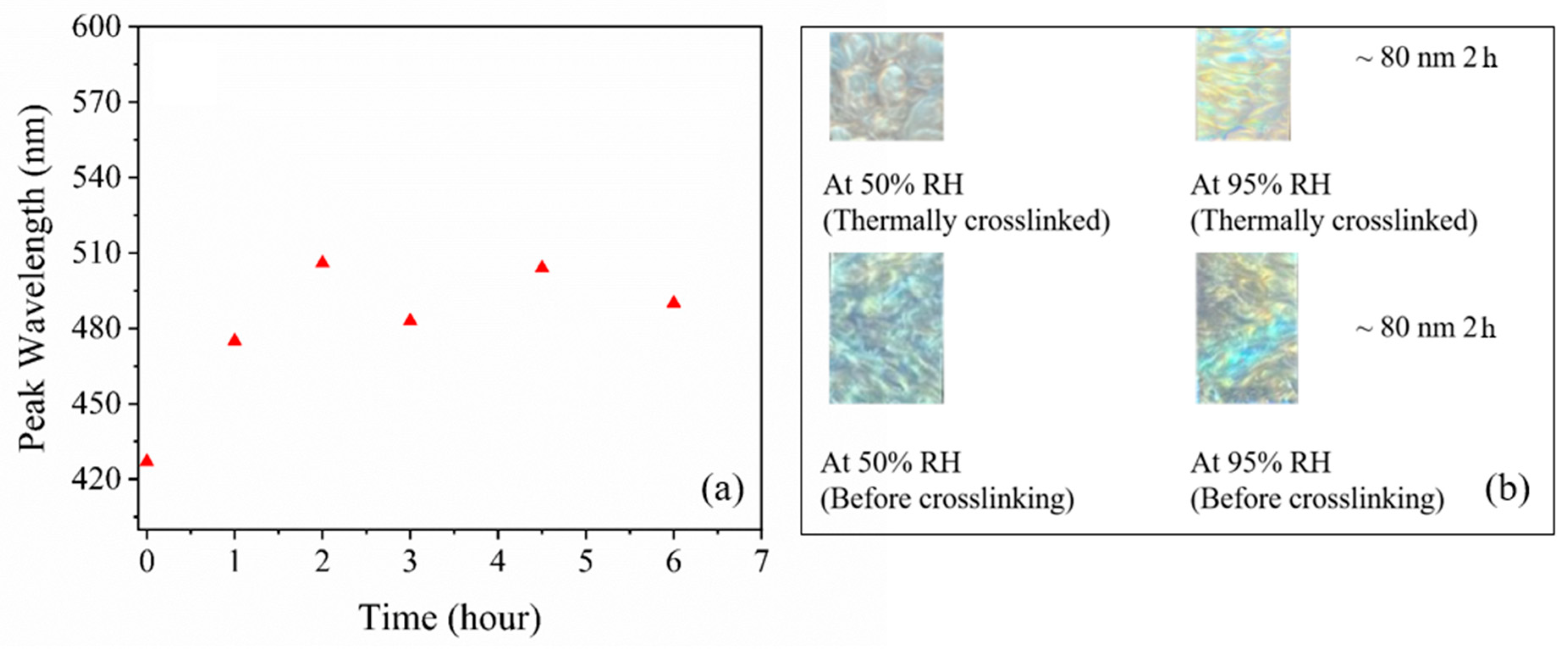
Effect of Thermal Crosslinking on the Transmittance Peak Minima
3.3. Humidity Detection
3.4. Effect of Polyvinyl Alcohol on Carboxylated CNC
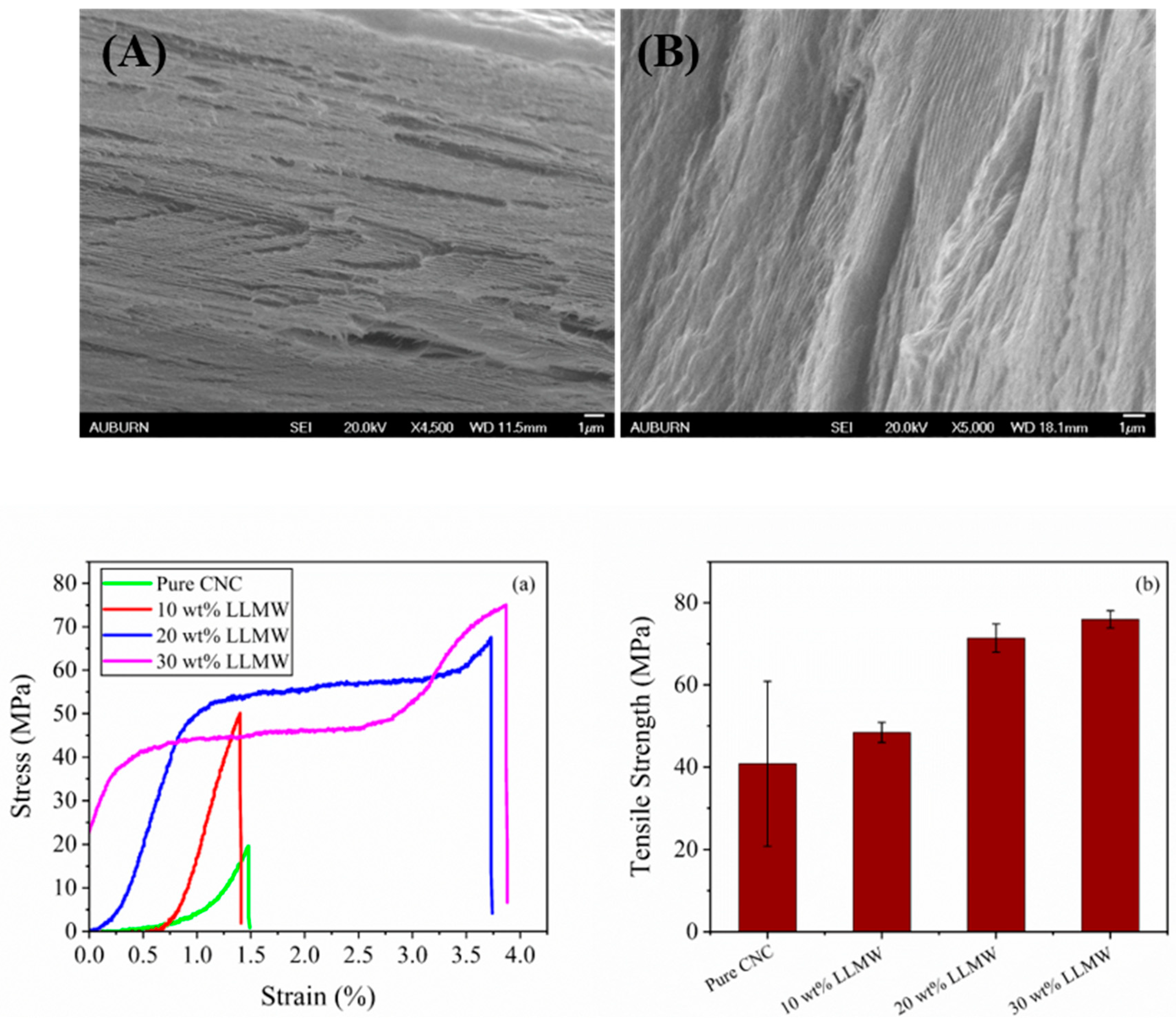
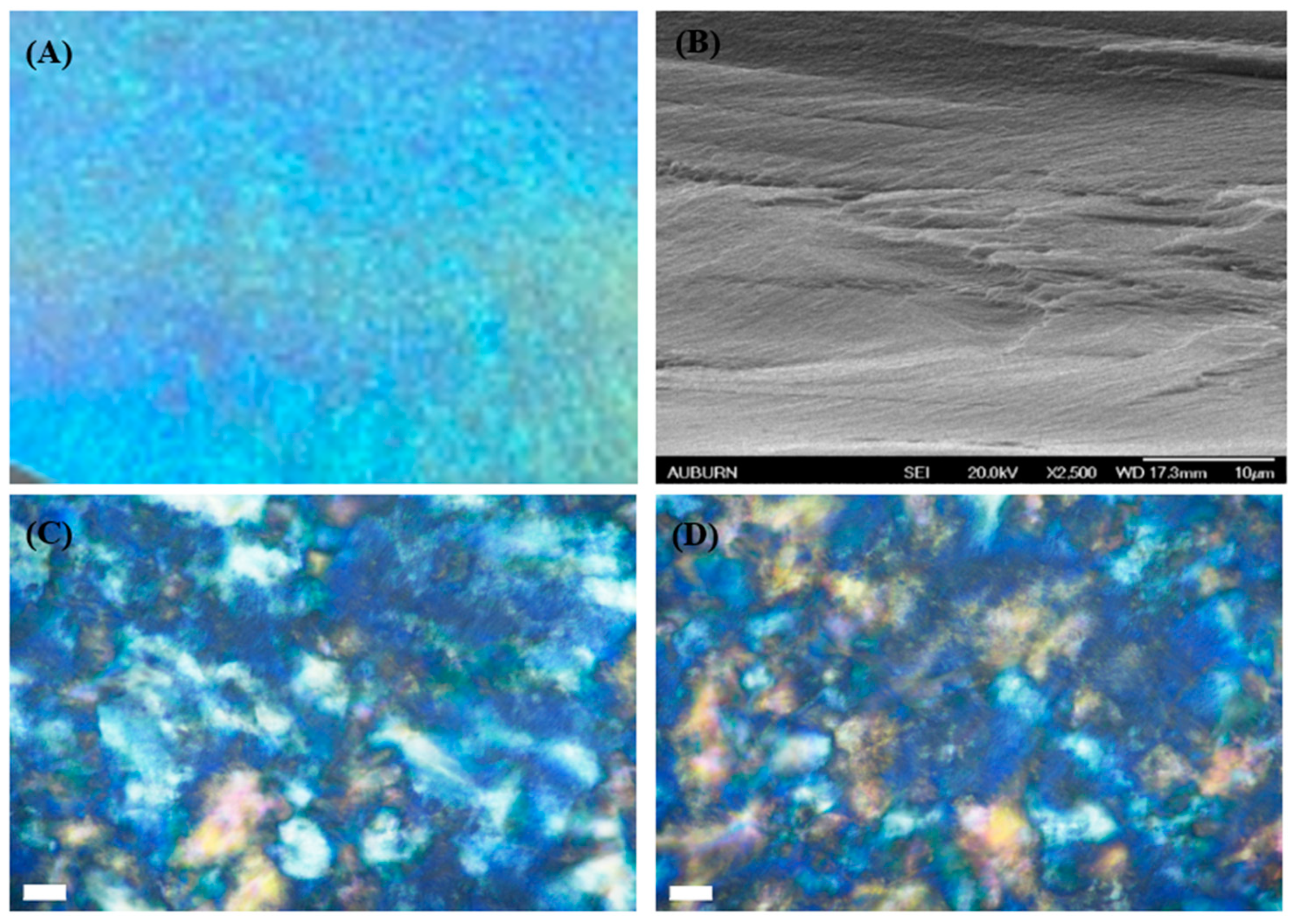
3.5. Morphology and Mechanical Property
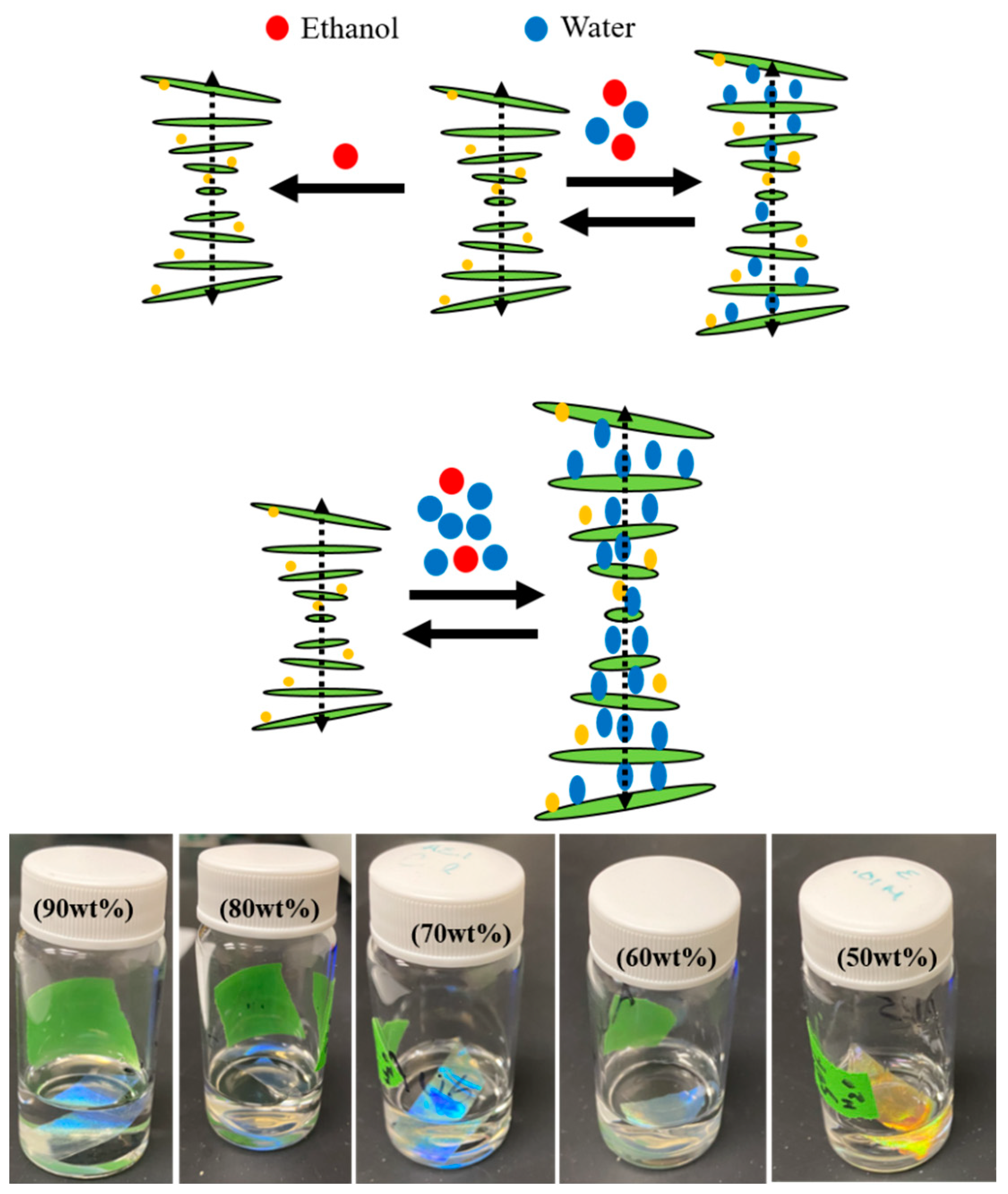
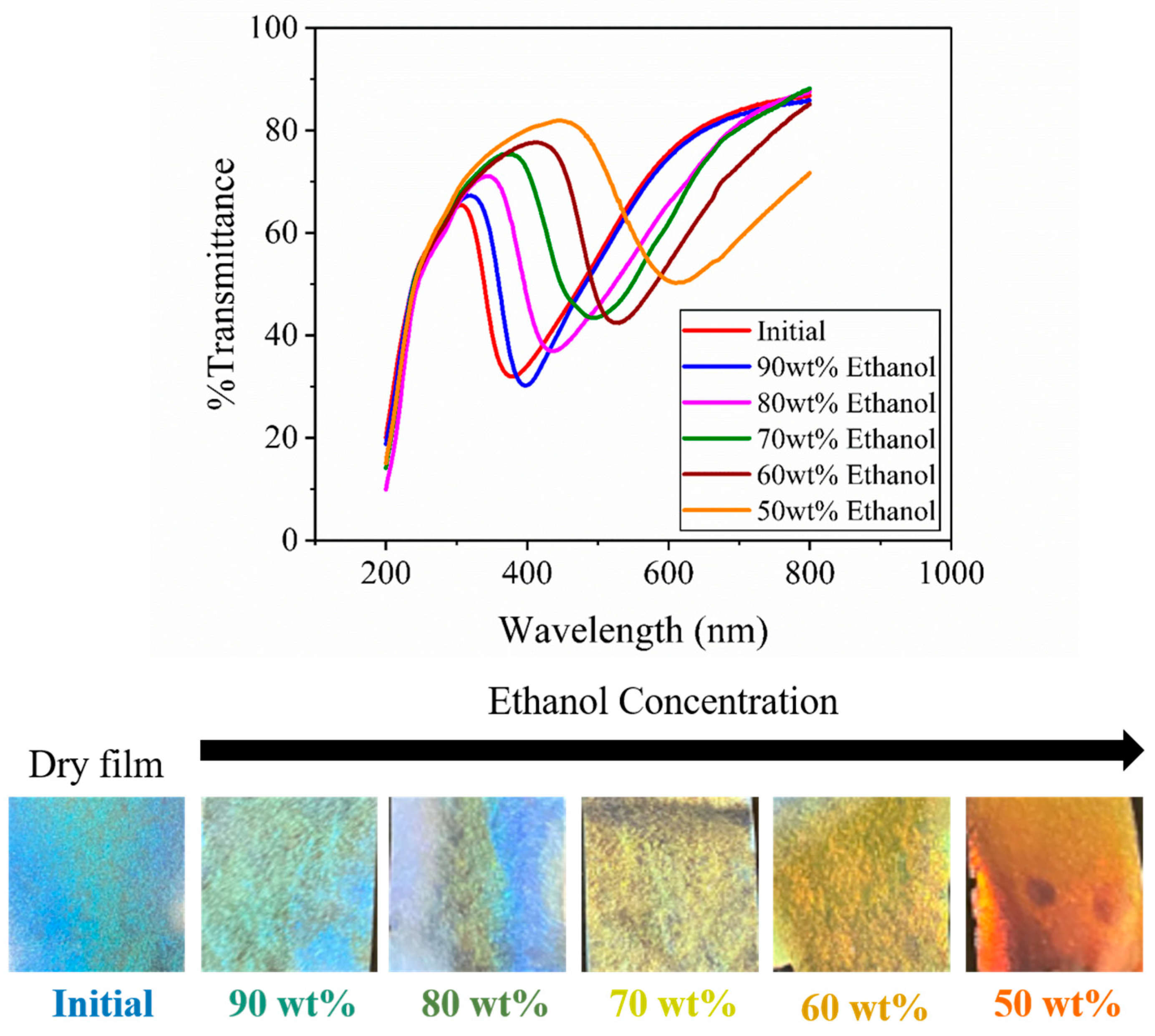
3.6. Ethanol–Water Concentration Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, T.; Pan, H.; Ma, J.; Li, Y.; Bokhari, S.W.; Jiang, X.; Zhu, S.; Zhang, D. Cellulose nanocrystals/polyacrylamide composites of high sensitivity and cycling performance to gauge humidity. ACS Appl. Mater. Interfaces 2017, 9, 18231–18237. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.; Meng, Q.; Bulone, V.; Zhou, Q. Flexible and responsive chiral nematic cellulose nanocrystal/poly (ethylene glycol) composite films with uniform and tunable structural color. Adv. Mater. 2017, 29, 1701323. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Kwon, S.; Lee, W.; Ko, S. Optical response of photonic cellulose nanocrystal film for a novel humidity indicator. Int. J. Biol. Macromol. 2019, 140, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, D.; Corral, J.; Quach, A.; Xian, X.; Forzani, E. Colorimetric humidity sensor based on liquid composite materials for the monitoring of food and pharmaceuticals. Langmuir 2014, 30, 10785–10791. [Google Scholar] [CrossRef]
- Neterebskaia, V.O.; Goncharenko, A.O.; Morozova, S.M.; Kolchanov, D.S.; Vinogradov, A.V. Inkjet printing humidity sensing pattern based on self-organizing polystyrene spheres. Nanomaterials 2020, 10, 1538. [Google Scholar] [CrossRef]
- Rao, X.; Zhao, L.; Xu, L.; Wang, Y.; Liu, K.; Wang, Y.; Chen, G.Y.; Liu, T.; Wang, Y. Review of optical humidity sensors. Sensors 2021, 21, 8049. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Chhajed, M.; Singh, S.; Sathwane, M.; Maji, P.K. Bioinspired structural color sensors based on self-assembled cellulose nanocrystal/citric acid to distinguish organic solvents. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130206. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Shojaeiarani, J.; Bajwa, D.S.; Chanda, S. Cellulose nanocrystal based composites: A review. Compos. Part C Open Access 2021, 5, 100164. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Imiete, I.E.; Giannini, L.; Tadiello, L.; Orlandi, M.; Zoia, L. The effect of sulfate half-ester groups on the mechanical performance of cellulose nanocrystal-natural rubber composites. Cellulose 2023, 30, 8929–8940. [Google Scholar] [CrossRef]
- Chowdhury, R.A.; Nuruddin, M.; Clarkson, C.; Montes, F.; Howarter, J.; Youngblood, J.P. Cellulose nanocrystal (CNC) coatings with controlled anisotropy as high-performance gas barrier films. ACS Appl. Mater. Interfaces 2018, 11, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Nuruddin, M.; Chowdhury, R.A.; Szeto, R.; Howarter, J.A.; Erk, K.A.; Szczepanski, C.R.; Youngblood, J.P. Structure–property relationship of cellulose nanocrystal–polyvinyl alcohol thin films for high barrier coating applications. ACS Appl. Mater. Interfaces 2021, 13, 12472–12482. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Shen, D.E.; Lu, Y.; Schueneman, G.T.; Shofner, M.L.; Meredith, J.C. Aqueous-Based Recycling of Cellulose Nanocrystal/Chitin Nanowhisker Barrier Coatings. ACS Sustain. Chem. Eng. 2023, 11, 10874–10883. [Google Scholar] [CrossRef]
- Csoka, L.; Hoeger, I.C.; Rojas, O.J.; Peszlen, I.; Pawlak, J.J.; Peralta, P.N. Piezoelectric effect of cellulose nanocrystals thin films. ACS Macro Lett. 2012, 1, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Carlos, C.; Zhang, Z.; Li, J.; Long, Y.; Yang, F.; Dong, Y.; Qiu, X.; Qian, Y.; Wang, X. Piezoelectric nanocellulose thin film with large-scale vertical crystal alignment. ACS Appl. Mater. Interfaces 2020, 12, 26399–26404. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ao, X.; Yang, L.; Ye, J.; Wang, C. Preparation and properties of cellulose nanocrystal-based ion-conductive hydrogels. RSC Adv. 2023, 13, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Butylina, S.; Geng, S.; Laatikainen, K.; Oksman, K. Cellulose nanocomposite hydrogels: From formulation to material properties. Front. Chem. 2020, 8, 655. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Muthoka, R.M.; Kim, J. Electroactive hydrogels made with polyvinyl alcohol/cellulose nanocrystals. Materials 2018, 11, 1615. [Google Scholar] [CrossRef]
- Bai, L.; Wang, Z.; He, Y.; Song, F.; Wang, X.; Wang, Y. Flexible photonic cellulose nanocrystal films as a platform with multisensing functions. ACS Sustain. Chem. Eng. 2020, 8, 18484–18491. [Google Scholar] [CrossRef]
- Kelly, J.A.; Shukaliak, A.M.; Cheung, C.C.Y.; Shopsowitz, K.E.; Hamad, W.Y.; MacLachlan, M.J. Responsive photonic hydrogels based on nanocrystalline cellulose. Angew. Chem. 2013, 34, 9080–9084. [Google Scholar] [CrossRef]
- Taokaew, S.; Kriangkrai, W. Recent progress in processing cellulose using ionic liquids as solvents. Polysaccharides 2022, 3, 671–691. [Google Scholar] [CrossRef]
- Sitnikov, P.; Legki, P.; Torlopov, M.; Druz, Y.; Mikhaylov, V.; Tarabukin, D.; Vaseneva, I.; Markarova, M.; Ushakov, N.; Udoratina, E. Efficient (Bio) emulsification/Degradation of Crude Oil Using Cellulose Nanocrystals. Polysaccharides 2023, 4, 402–420. [Google Scholar] [CrossRef]
- Abitbol, T.; Kam, D.; Levi-Kalisman, Y.; Gray, D.G.; Shoseyov, O. Surface charge influence on the phase separation and viscosity of cellulose nanocrystals. Langmuir 2018, 34, 3925–3933. [Google Scholar] [CrossRef] [PubMed]
- Davis, V.A. Liquid crystalline assembly of nanocylinders. J. Mater. Res. 2011, 26, 140–153. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, Z. Iridescent chiral nematic cellulose nanocrystal/polyvinylpyrrolidone nanocomposite films for distinguishing similar organic solvents. ACS Sustain. Chem. Eng. 2018, 6, 6192–6202. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, G.; Liu, D.; Tian, D.; Zhu, Y.; Chang, Y. Vapor sensing with color-tunable multilayered coatings of cellulose nanocrystals. Carbohydr. Polym. 2017, 174, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, K.; Lu, X. Flexible cellulose nanocrystal-based bionanocomposite film as a smart photonic material responsive to humidity. Int. J. Biol. Macromol. 2021, 188, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, W.; Ma, C.; Yu, H.; Wu, Y.; Wang, Y.; Chen, Z.; Li, J.; Liu, S. Multifunctional chiral nematic cellulose nanocrystals/glycerol structural colored nanocomposites for intelligent responsive films, photonic inks and iridescent coatings. J. Mater. Chem. C Mater. 2018, 6, 5391–5400. [Google Scholar] [CrossRef]
- Garg, M.; Apostolopoulou-Kalkavoura, V.; Linares, M.; Kaldéus, T.; Malmström, E.; Bergström, L.; Zozoulenko, I. Moisture uptake in nanocellulose: The effects of relative humidity, temperature and degree of crystallinity. Cellulose 2021, 28, 9007–9021. [Google Scholar] [CrossRef]
- He, J.; Bian, K.; Li, N.; Piao, G. Volatile acid responsiveness of chiral nematic luminescent cellulose nanocrystal/9, 10-Bis ((Z)-2-phenyl-2-(pyridin-2-yl) vinyl) anthracene composite films. ACS Sustain. Chem. Eng. 2019, 7, 12369–12375. [Google Scholar]
- Teodoro, K.B.R.; Migliorini, F.L.; Christinelli, W.A.; Correa, D.S. Detection of hydrogen peroxide (H2O2) using a colorimetric sensor based on cellulose nanowhiskers and silver nanoparticles. Carbohydr. Polym. 2019, 212, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.V.D.; Freitas, P.A.D.; Pola, C.C.; Terra, L.R.; Silva, J.O.D.; Badaró, A.T.; Junior, N.S.; Oliveira, M.M.D.; Silva, R.R.; Soares, N.D.F. The influence of intermolecular interactions between maleic anhydride, cellulose nanocrystal, and nisin-Z on the structural, thermal, and antimicrobial properties of starch-PVA plasticized matrix. Polysaccharides 2021, 2, 661–676. [Google Scholar] [CrossRef]
- Ejara, T.M.; Balakrishnan, S.; Kim, J.C. Nanocomposites of PVA/cellulose nanocrystals: Comparative and stretch drawn properties. SPE Polym. 2021, 2, 288–296. [Google Scholar] [CrossRef]
- Park, N.; Friest, M.A.; Liu, L. Enhancing the Properties of Polyvinyl Alcohol Films by Blending with Corn Stover-Derived Cellulose Nanocrystals and Beeswax. Polymers 2023, 15, 4321. [Google Scholar] [CrossRef]
- Wong, W.C.; Chan, C.C.; Chen, L.H.; Li, T.; Lee, K.X.; Leong, K.C. Polyvinyl alcohol coated photonic crystal optical fiber sensor for humidity measurement. Sens. Actuators B Chem. 2012, 174, 563–569. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, H.; Wang, L.; Zhang, J.; Deng, L.; Ma, T. An optical fiber sensor coated with electrospinning polyvinyl alcohol/carbon nanotubes composite film. Sensors 2020, 20, 6996. [Google Scholar] [CrossRef]
- Gastón, A.; Pérez, F.; Sevilla, J. Optical fiber relative-humidity sensor with polyvinyl alcohol film. Appl. Opt. 2004, 43, 4127–4132. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Shao, Y.; Liao, C.; Wang, Y. Highly sensitive surface plasmon resonance humidity sensor based on a polyvinyl-alcohol-coated polymer optical fiber. Biosensors 2021, 11, 461. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, H.-Y.; Tian, Y.; Yao, X.; Xue, B.; Xu, D.; Zou, F.; Mi, Q. Highly sensitive, self-healable, and robust polyvinyl alcohol/nanocellulose composite electronic skin by single-step Marangoni self-assembly for multipoint sensing. ACS Sustain. Chem. Eng. 2023, 11, 13149–13163. [Google Scholar] [CrossRef]
- Zhang, F.; Ge, W.; Wang, C.; Zheng, X.; Wang, D.; Zhang, X.; Wang, X.; Xue, X.; Qing, G. Highly strong and solvent-resistant cellulose nanocrystal photonic films for optical coatings. ACS Appl. Mater. Interfaces 2021, 13, 17118–17128. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Walther, A. Self-assembled, iridescent, crustacean-mimetic nanocomposites with tailored periodicity and layered cuticular structure. ACS Nano 2015, 9, 10637–10646. [Google Scholar] [CrossRef] [PubMed]
- Parit, M.; Jiang, Z. Cellulose nanocrystal films: Effect of electrolyte and lignin addition on self-assembly, optical, and mechanical properties. Cellulose 2023, 30, 9405–9423. [Google Scholar] [CrossRef]
- Sun, Q.; Lutz-Bueno, V.; Zhou, J.; Yuan, Y.; Fischer, P. Polymer induced liquid crystal phase behavior of cellulose nanocrystal dispersions. Nanoscale Adv. 2022, 4, 4863–4870. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Y.T.H.; Ly, T.B.; Bui, B.A.T.; Le, P.K. Biodegradable Polyvinyl Alcohol Composite Film Reinforced by Crystalline Nanocellulose from Rice Straw. Chem. Eng. Trans. 2023, 106, 493–498. [Google Scholar]
- Silvério, H.A.; Neto, W.P.F.; Pasquini, D. Effect of incorporating cellulose nanocrystals from corncob on the tensile, thermal and barrier properties of poly (vinyl alcohol) nanocomposites. J. Nanomater. 2013, 2013, 74. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Fardis, D.; Ntampou, X.; Tsivintzelis, I.; Panayiotou, C. Thermal Behavior of Poly (vinyl alcohol) in the Form of Physically Crosslinked Film. Polymers 2023, 15, 1843. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Liu, L.; Hu, Y.; Wu, Y. Water vapor sorption properties of TEMPO oxidized and sulfuric acid treated cellulose nanocrystal films. Carbohydr. Polym. 2018, 197, 524–530. [Google Scholar] [CrossRef]
- Meng, Y.; Long, Z.; He, Z.; Fu, X.; Dong, C. Chiral cellulose nanocrystal humidity-responsive iridescent films with glucan for tuned iridescence and reinforced mechanics. Biomacromolecules 2021, 22, 4479–4488. [Google Scholar] [CrossRef]
- Hop, T.T.T.; Mai, D.T.; Cong, T.D.; Nhi, T.T.Y.; Loi, V.D.; Huong, N.T.M.; Tung, N.T. A comprehensive study on preparation of nanocellulose from bleached wood pulps by TEMPO-mediated oxidation. Results Chem. 2022, 4, 100540. [Google Scholar]
- Zhou, L.; He, H.; Jiang, C.; Ma, L.I.; Yu, P. Cellulose nanocrystals from cotton stalk for reinforcement of poly (vinyl alcohol) composites. Cellul. Chem. Technol. 2017, 51, 109–119. [Google Scholar]





| Composite | PVA Concentration (wt%) | |
|---|---|---|
| Pure CNC | 0% | |
| CNC-PVA | CNC-LLMW | 10%, 20%, 30%, 40%, 50%, 100% |
| CNC-LMW | 10%, 20%, 30%, 40%, 50%, 100% | |
| CNC-HMW | 5%, 10%, 20%, 30% | |
| TEMPO-CNC | 0% | |
| TEMPO-CNC-PVA | TEMPO-CNC-LLMW | 30%, 50% |
| Sample | Avg. Zeta Potential, mV |
|---|---|
| Pure CNC | −49.60 ± 0.71 |
| TEMPO-CNC | −47.00 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghosh, A.; Parit, M.; Jiang, Z. Investigating Cellulose Nanocrystal and Polyvinyl Alcohol Composite Film in Moisture Sensing Application. Polysaccharides 2024, 5, 288-304. https://doi.org/10.3390/polysaccharides5030019
Ghosh A, Parit M, Jiang Z. Investigating Cellulose Nanocrystal and Polyvinyl Alcohol Composite Film in Moisture Sensing Application. Polysaccharides. 2024; 5(3):288-304. https://doi.org/10.3390/polysaccharides5030019
Chicago/Turabian StyleGhosh, Ananya, Mahesh Parit, and Zhihua Jiang. 2024. "Investigating Cellulose Nanocrystal and Polyvinyl Alcohol Composite Film in Moisture Sensing Application" Polysaccharides 5, no. 3: 288-304. https://doi.org/10.3390/polysaccharides5030019






