Polysaccharide as a Separation Medium for Gel Electrophoresis
Abstract
:1. Introduction
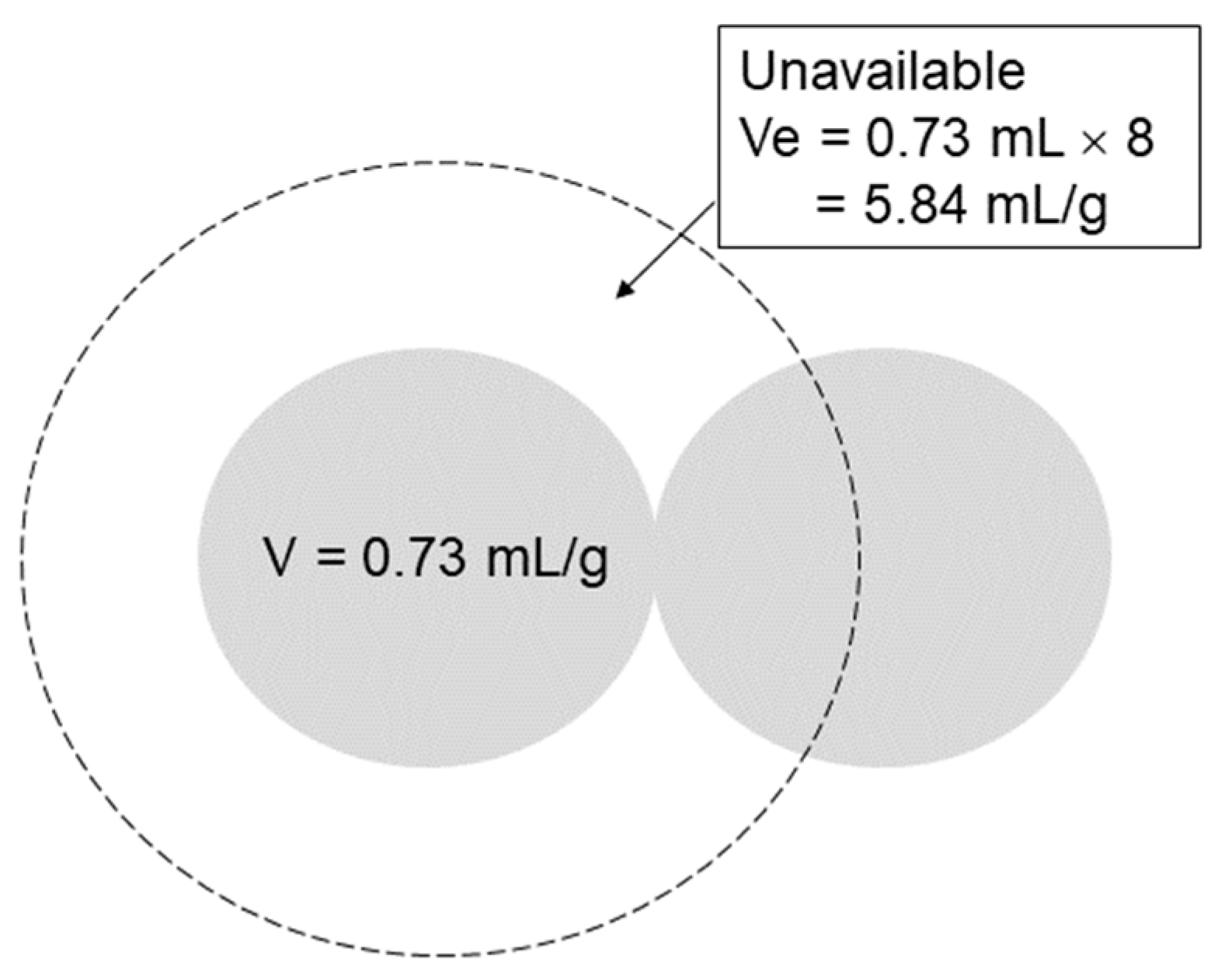
2. Mechanism of Molecular Sieving
3. Polysaccharide vs. Polyacrylamide
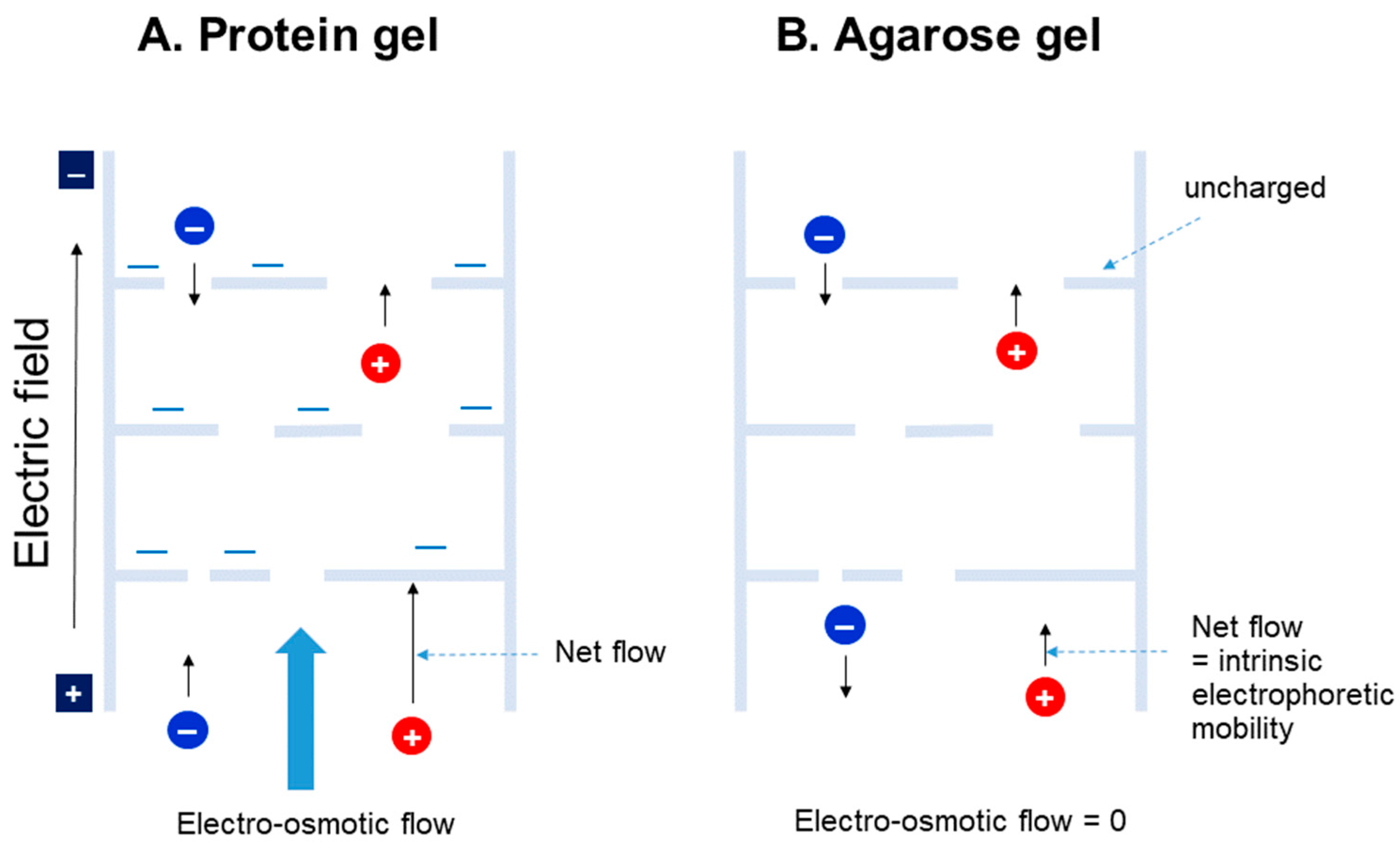
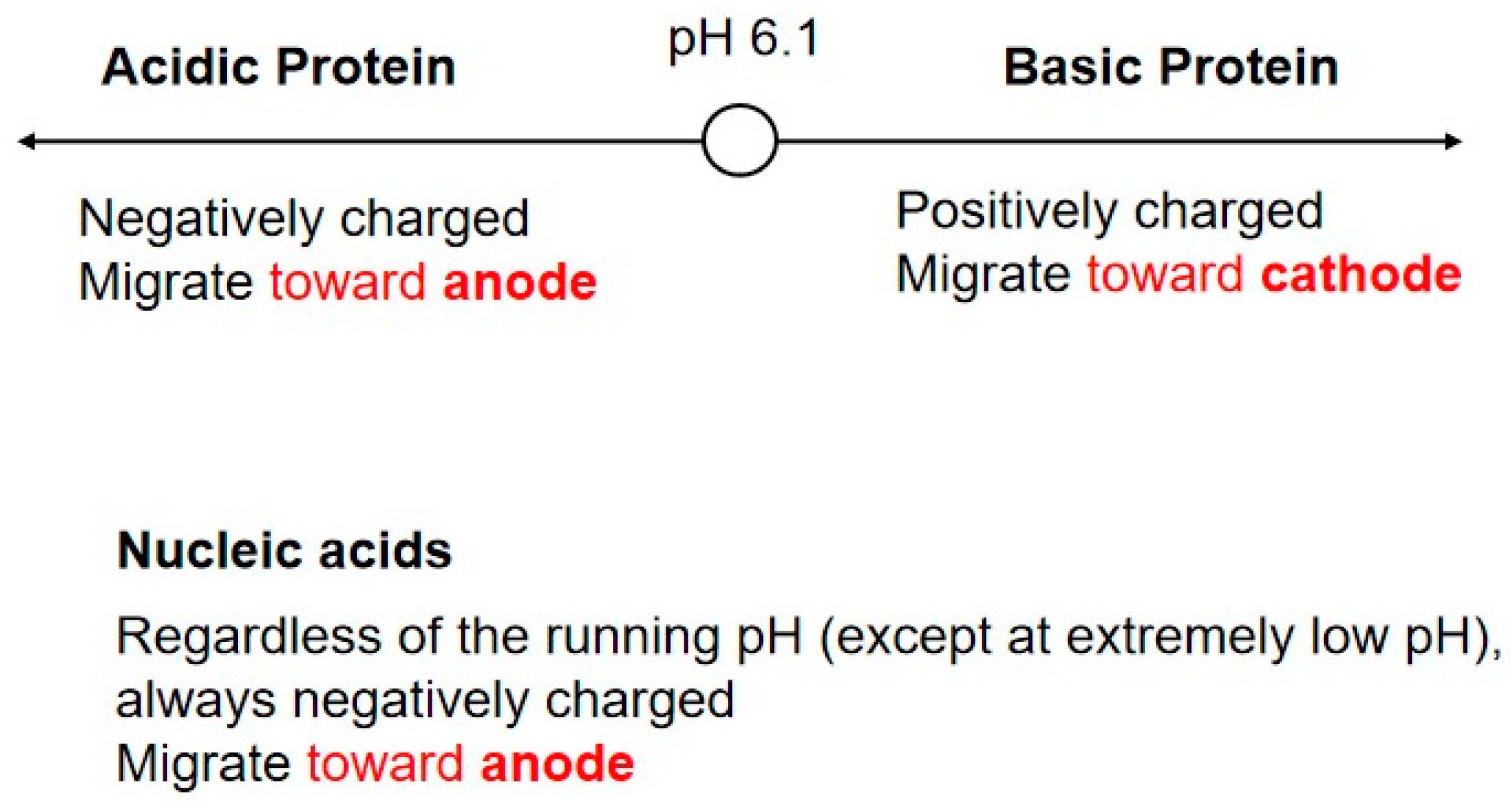
4. Agarose Gel vs. Protein Gel
5. Denaturing vs. Native Gel Electrophoresis
6. Effect of Small Pore Structure
7. Application of Agarose Gel Electrophoresis
7.1. Nucleic Acids
7.1.1. Gel Shift Assay
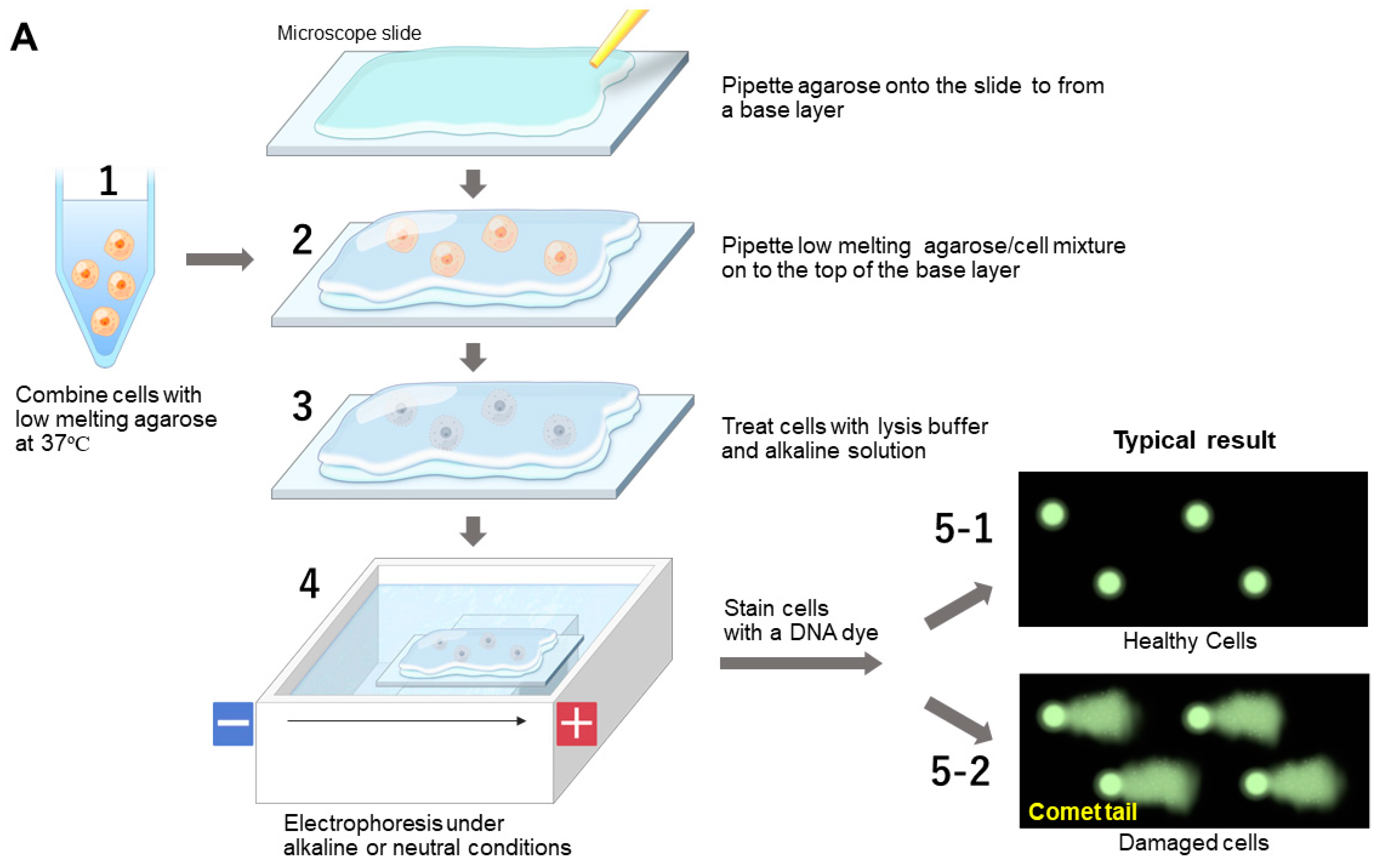
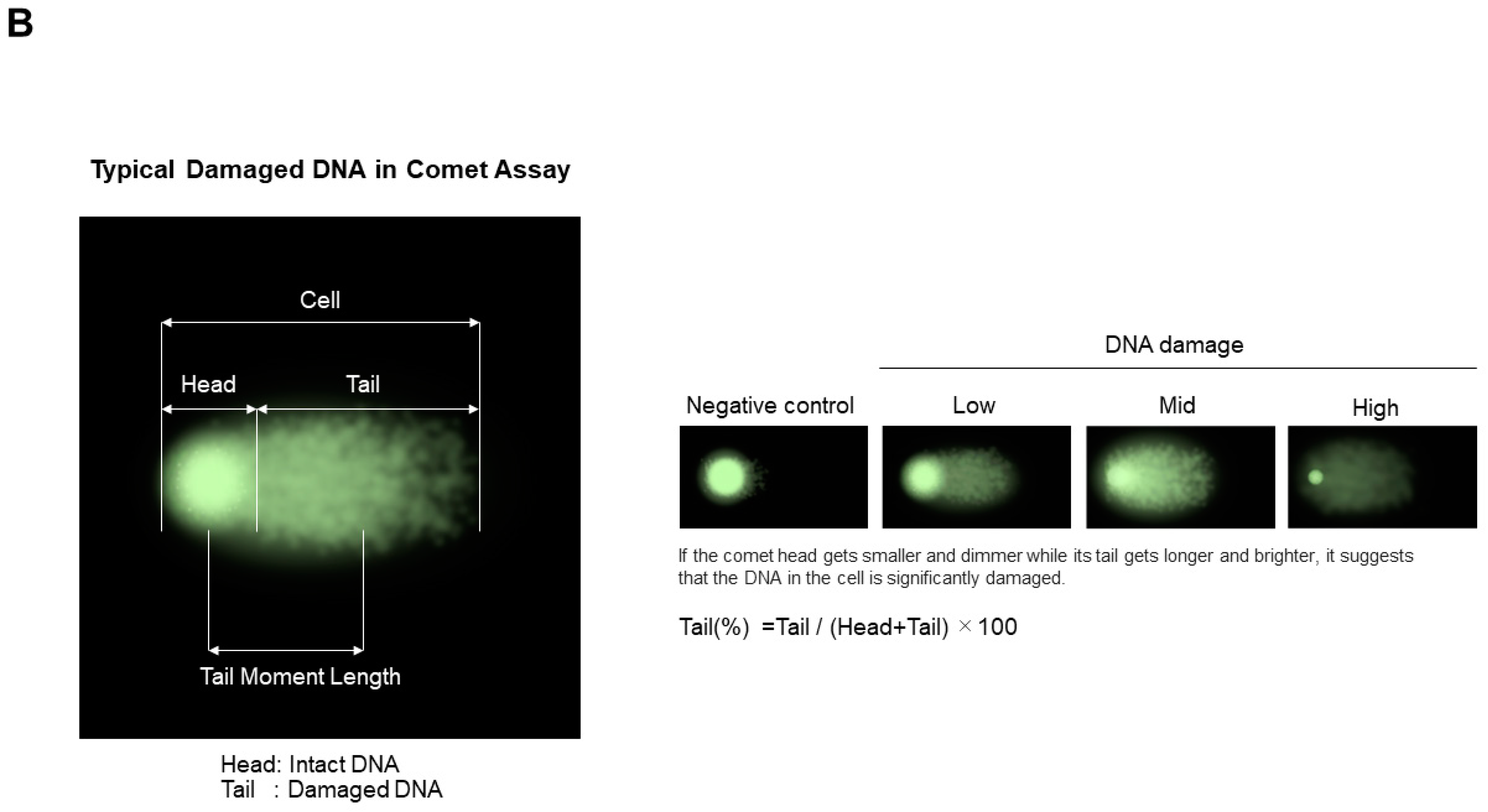
7.1.2. Comet Assay
7.2. Proteins
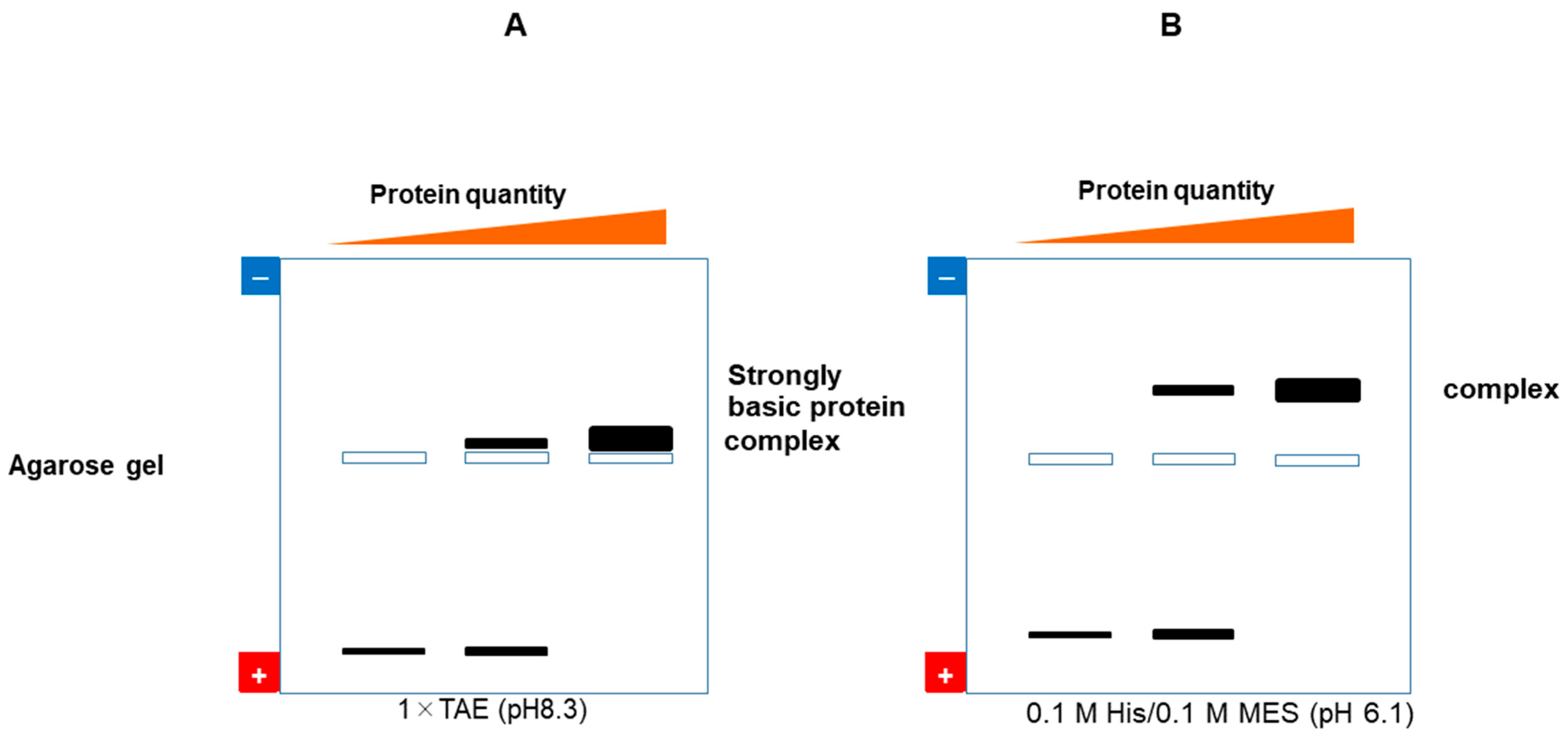
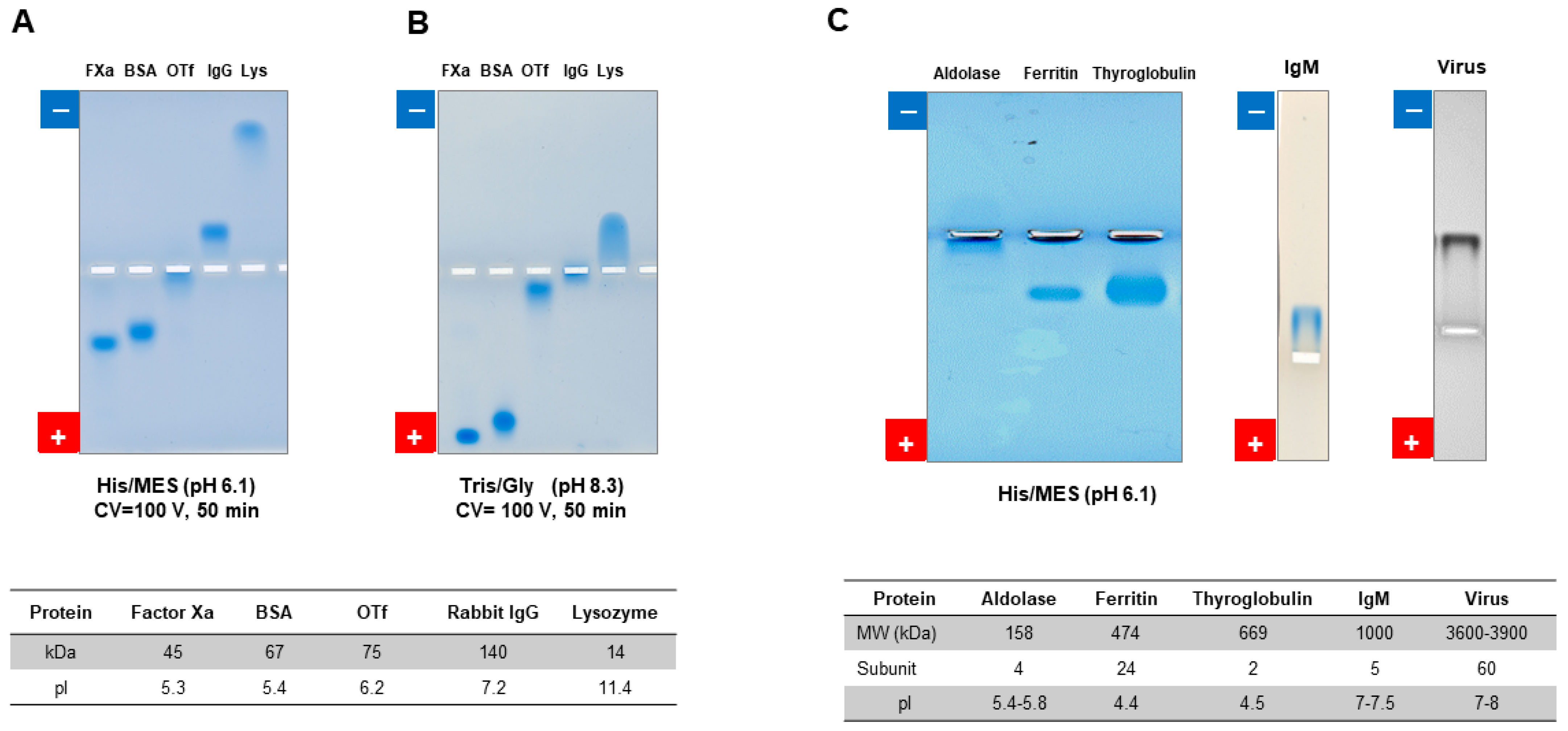
7.2.1. Native Gel Electrophoresis
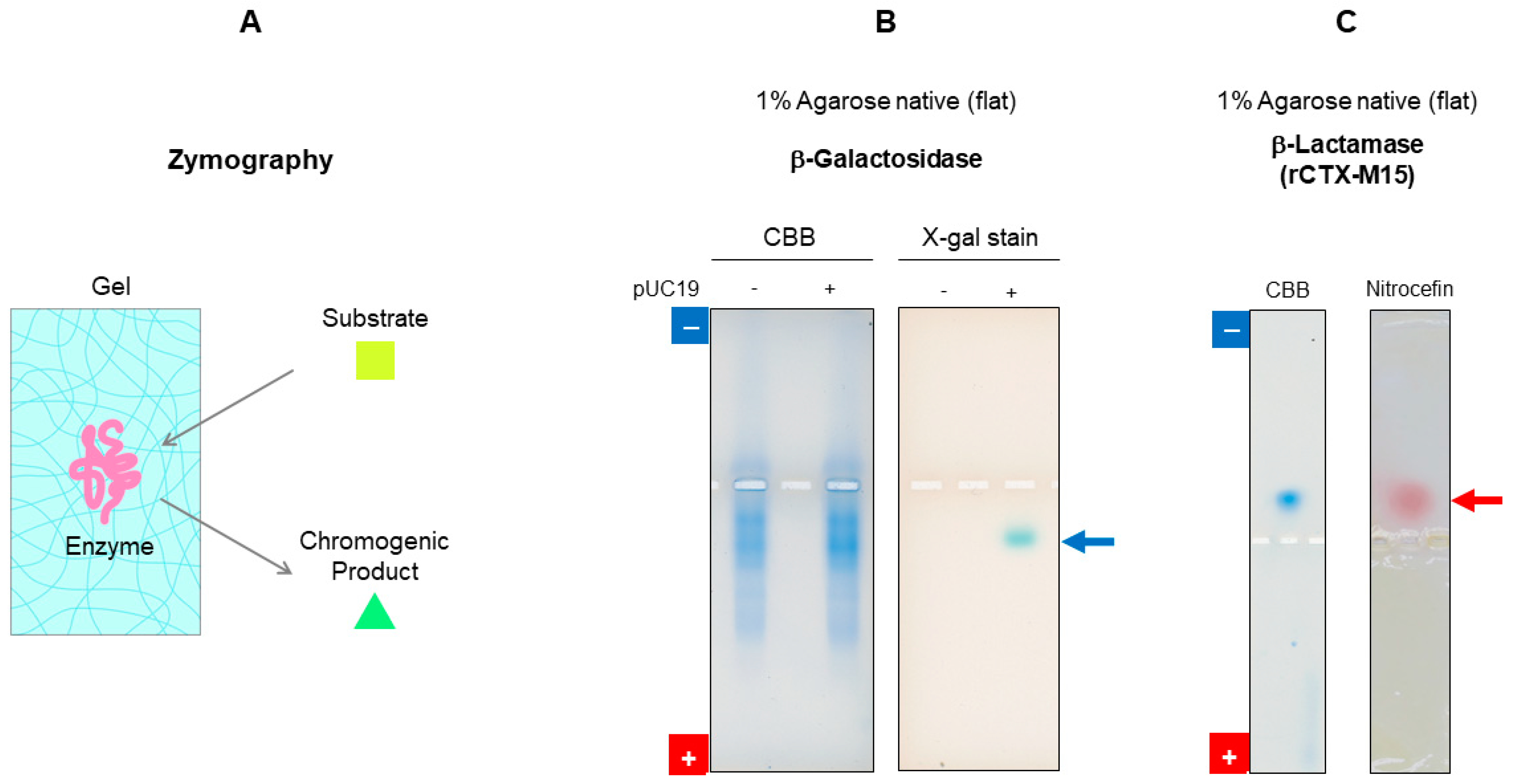
7.2.2. Activity Staining
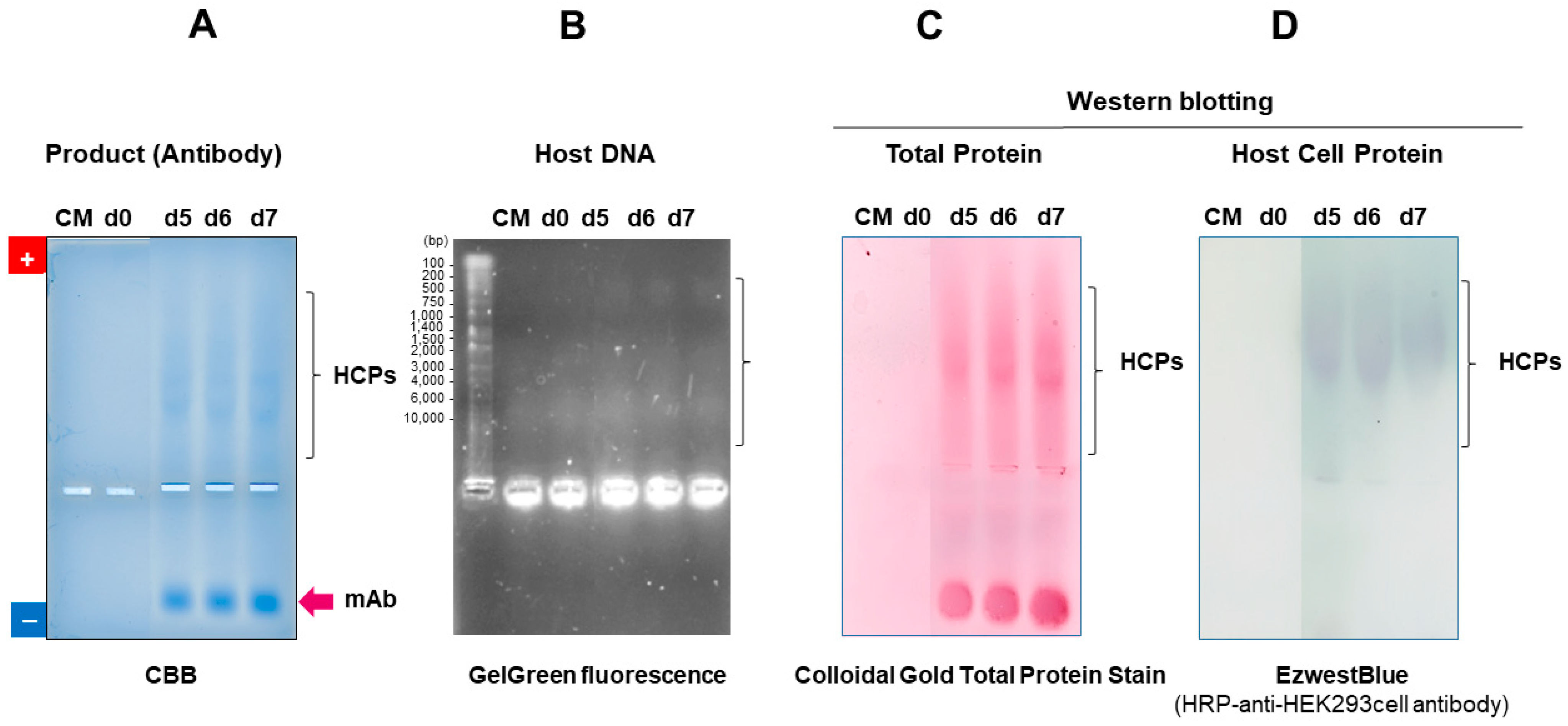
7.2.3. Detection of Contamination
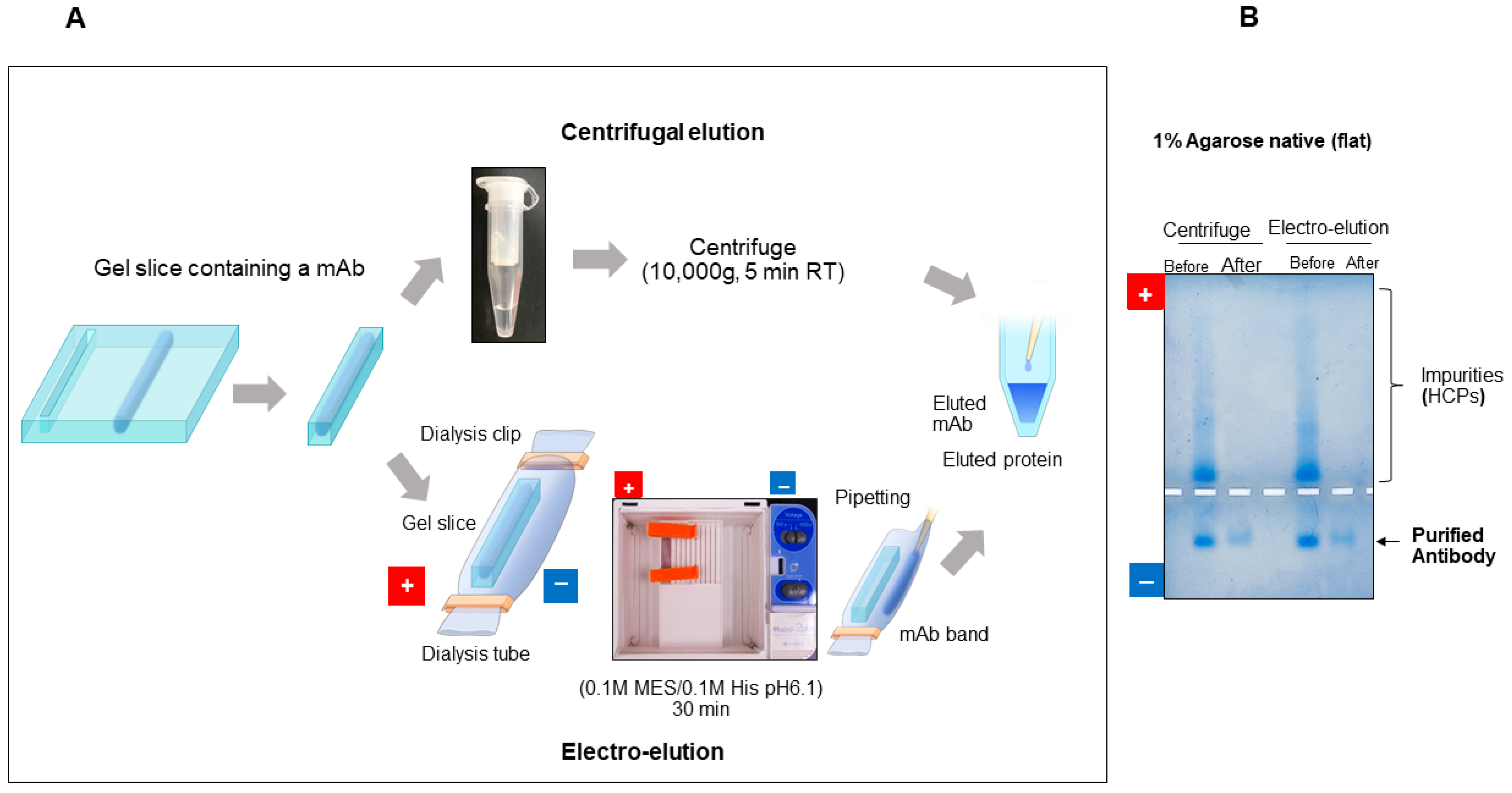
7.2.4. Purification of Protein by Gel Extraction
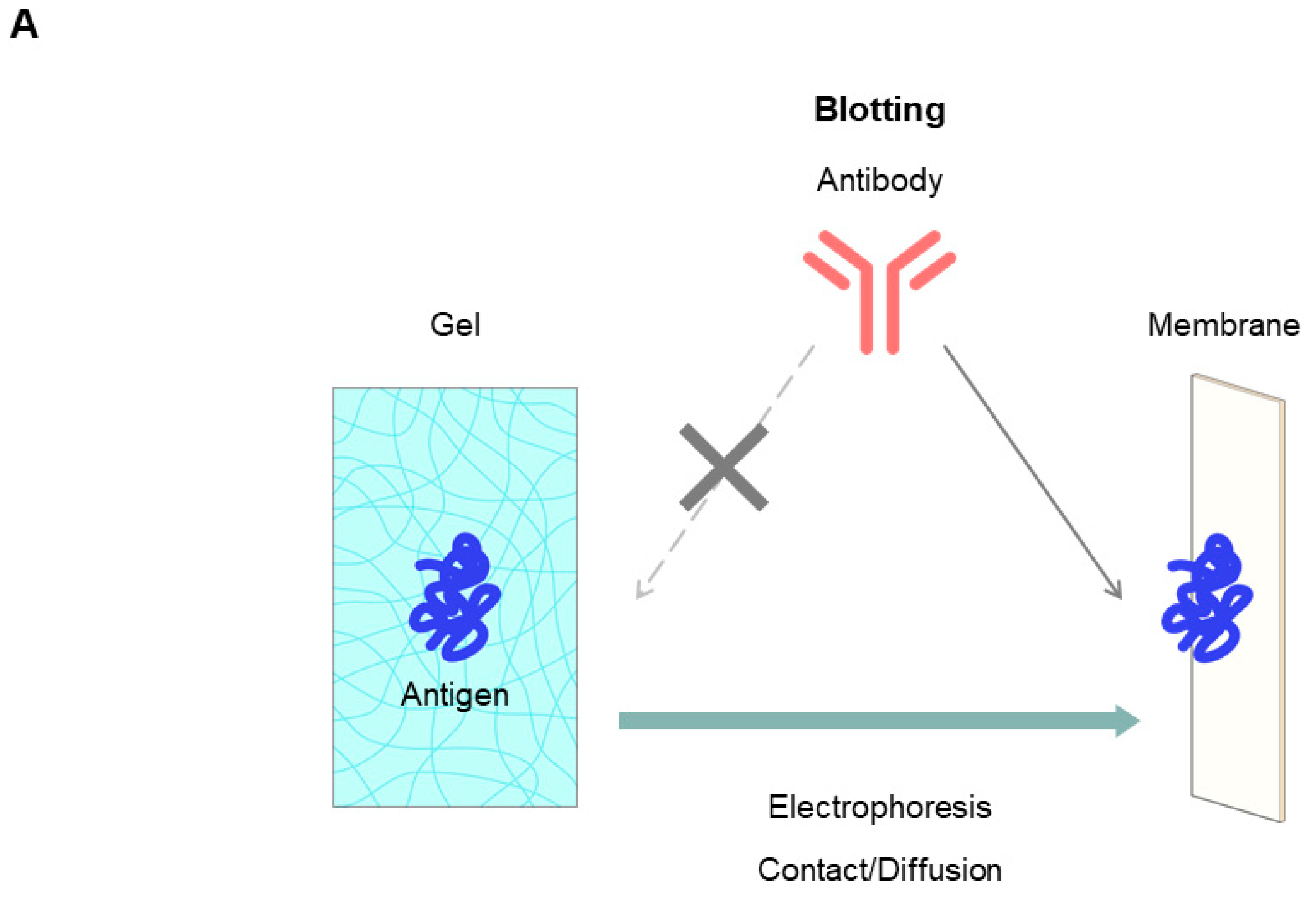
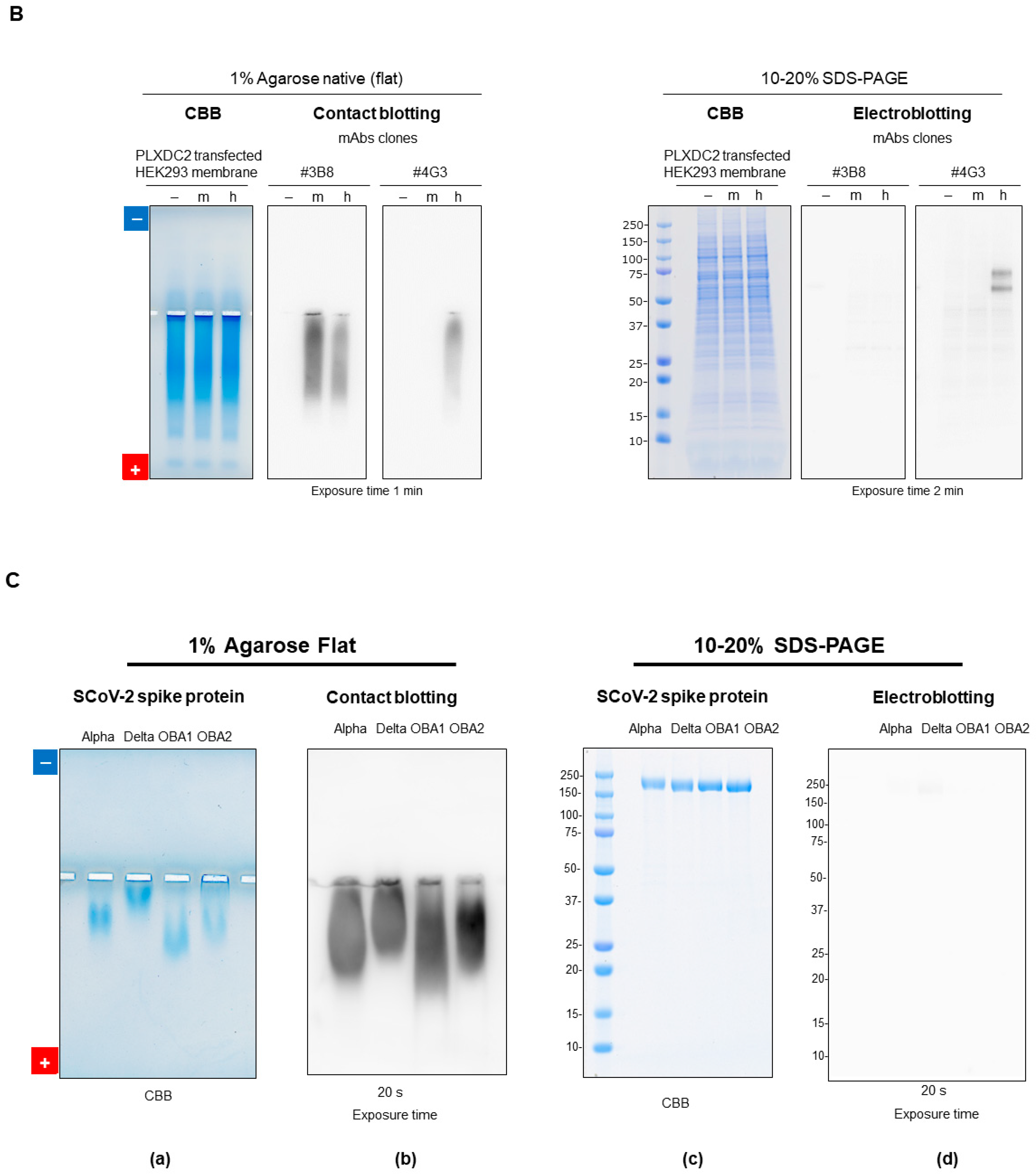
7.2.5. Native Western Blotting by Contact
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-forming algae polysaccha rides: From seaweed to biomedical applications. Macromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Tombs, M.P. Biotechnology of protein and polysaccharide gels. Biotechnol. Genet. Eng. Rev. 1997, 14, 337–363. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, S.U.; Agaoglu, N.B.; Manto, K.; Muftuoglu, M.; Özbek, U. Cosmic Whirl: Navigating the cosmic trail in DNA: H2AX phosphorylation and the enigma of uncertain significance variants. Genes 2024, 15, 724. [Google Scholar] [CrossRef]
- Weickert, P.; Dürauer, S.; Götz, M.J.; Li, H.Y.; Stingele, J. Electro-elution-based purification of covalent DNA-protein cross-links. Nat. Protoc. 2024. [Google Scholar] [CrossRef] [PubMed]
- Lewis, L.A. Electrophoresis of proteins. CRC. Crit. Rev. Clin. Lab. Sci. 1970, 1, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Niikura, T.; Kita, Y.; Akuta, T. Sodium dodecyl sulfate analogs as a potential molecular biology reagent. Curr. Issues Mol. Biol. 2024, 46, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Cramer, D.A.; Franc, V.; Heidenreich, A.K.; Hook, M.; Adibzadeh, M.; Reusch, D.; Heck, A.J.R.; Haberger, M. Characterization of high-molecular weight by-products in the production of a trivalent bispecific 2 + 1 heterodimeric antibody. mAbs 2023, 15, 2175312. [Google Scholar] [CrossRef]
- Chi, M.C.; Lu, B.Y.; Huang, Y.F.; Wang, S.W.; Lin, M.G.; Wang, T.F. Effects of sodium dodecyl sulfate on the enzyme catalysis and conformation of a recombinant γ-glutamyltranspeptidase from Bacillus licheniformes. Protein J. 2023, 42, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.A. Structure, conformation, and mechanism in the formation of polysaccharide gels and networks. Advan. Carbohyd. Chem. Biochem. 1969, 24, 267–332. [Google Scholar] [CrossRef]
- Arnott, S.; Fulmer, A.; Scott, W.E.; Dea, I.; Moorhouse, R.; Rees, D.A. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 1974, 90, 269–284. [Google Scholar] [CrossRef]
- Maaloum, M.; Pernodet, N.; Tinland, B. Agarose gel structure using atomic force microscopy: Gel concentration and ionic strength effects. Electrophoresis 1998, 19, 1606–1610. [Google Scholar] [CrossRef] [PubMed]
- Sasuga, K.; Ishikawa, A.; Ito, M.; Amano, N.; Ishii, Y.; Kitagawa, Y.; Usui, M. Agar and agarose for electrophoresis. Chem. Times 2011, 4, 8–14. Available online: https://www.kanto.co.jp/dcms_media/other/backno7_pdf04.pdf (accessed on 11 October 2011). (In Japanese).
- Jiang, F.; Xu, X.W.; Chen, F.Q.; Weng, H.F.; Chen, J.; Ru, Y.; Xiao, Q.; Xiao, A.F. Extraction, Modification and Biomedical Application of Agarose Hydrogels: A Review. Mar Drugs. 2023, 21, 299. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.L.; Stellwagen, N.C. Estimation of polyacrylamide gel pore size from Ferguson plots of normal and anolamously migrating DNA fragments. I. Gels containing 3% N,N′-methylenebisacrylamide. Electrophoresis 1991, 12, 253–363. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, N.C. Electrophoresis of DNA in agarose gels, polyacrylamide gels, and in free solution. Electrophoresis 2009, 30, S185–S195. [Google Scholar] [CrossRef] [PubMed]
- Karathanos, V.T.; Saravacos, G.D. Porosity and pore size distribution of starch materials. J. Food Eng. 1993, 18, 259–280. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Liu, W. Bioinspired fabrication of high strength hydrogels from non-covalent interactions. Prog. Polymer Sci. 2017, 71, 1–25. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chang, C.C.; Chan, H.P.; Chung, T.W.; Shu, C.W.; Chuang, K.P.; Duh, T.H.; Yang, M.H.; Tyan, Y.C. Hydrogels: Properties and applications in biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef]
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.-G.; Song, W.; Kim, B.J. Rational design of hyper-crosslinked polymers for biomedical applications. J. Polymer Sci. 2024, 62, 1517–1535. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, G.; Shi, M.; Suo, Z. Fracture, fatiue, and friction of polymers in which entanglements greatly out-number cross-links. Science 2021, 374, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Belashova, T.A.; Velina, A.A.; Sysoev, E.I.; Velizhanina, M.E.; Zelinsky, A.A.; Galkin, A.P. Search and identification of amyloid proteins. Methods Protoc. 2023, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Arakawa, T. Agarose native gel electrophoresis. Int. J. Biol. Macromol. 2019, 140, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M.; Watanabe, C.; Fujiwara, K. Single micrometer-sized gels: Unique mechanics and characters for application. Gels 2018, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.; Kobori, Y.; Yamamoto, J.; Kinjo, M.; Yanagisawa, M. Quantitative analysis of membrane surface and small confinement effects on molecular diffusion. J. Phys. Chem. B 2020, 124, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, M. Cell-size space effects on phase separation of binary polymer blends. Biophys. Rev. 2022, 14, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. Simplified equilibrium model for exploring the combined influences of concentrations, aggregate shape, excluded volume, and surface adsorption upon aggregation propensity and distribution of globular macromolecules. J. Phys. Chem. B 2023, 127, 9303–9311. [Google Scholar] [CrossRef]
- Rivas, G.; Minton, A.P. Influence of nonspecific interactions on protein associations: Implications for biochemistry in vivo. Annu. Rev. Biochem. 2022, 91, 321–351. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, T.; Minton, A.P. Non-specific interactions between macromolecular solutes in concentrated solution: Physico-chemical manifestations and biochemical consequences. Front. Mol. Biosci. 2019, 6, 10. [Google Scholar] [CrossRef]
- Tomioka, Y.; Nakagawa, M.; Sakuma, C.; Nagatoishi, S.; Tsumoto, K.; Arakawa, T.; Akuta, T. Ladder observation of bovine serum albumin by high resolution agarose native gel electrophoresis. Int. J. Biol. Macromol. 2022, 215, 512–520. [Google Scholar] [CrossRef]
- Sambrook, J.; Russel, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Hayward, G.S.; Smith, M.G. The chromosome of bacteriophage T5. I. Analysis of the single-stranded DNA fragments by agarose gel electrophoresis. J. Mol. Biol. 1972, 63, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Borst, P. Ethidium DNA agarose gel electrophoresis: How it started. IUBMB Life 2005, 57, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Fried, M.; Crothers, D.M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981, 9, 6505–6525. [Google Scholar] [CrossRef] [PubMed]
- Garner, M.M.; Revzin, A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: Application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981, 9, 3047–3060. [Google Scholar] [CrossRef] [PubMed]
- Vossen, K.M.; Fried, M.G. Stability of lac repressor-operator complexes in a new agarose-based gel matrix. Nucleic Acids Res. 1995, 23, 2346–2347. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Selby, P.B. Comet assay and hormesis. Environ. Pollut. 2024, 341, 122929. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, Y.; Yang, C. Evaluating in vitro DNA damage using comet assay. J. Vis. Exp. 2017, 11, 56450. [Google Scholar] [CrossRef]
- Walsh, K.D.; Kato, T.A. Alkaline comet assay to detect DNA damage. Methods Mol. Biol. 2023, 2519, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Karbaschi, M.; Abdulwahed, A.; Quinete, N.S.; Evans, M.D.; Cooke, M.S. A high-throughput comet assay approach for assessing cellular DNA damage. J. Vis. Exp. 2022, 183, e63559. [Google Scholar] [CrossRef]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Olive, P.L.; Banáth, J.P. The comet assay: A method to measure DNA damage in individual cells. Nat. Protoc. 2006, 1, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, S.; Zhu, Y.; Matsui, T.; Tipirneni, N.; Yasuhara, Y.; Kaneki, M.; Rosenzweig, A.; Martyn, J.A.J. Comparison of comet assay, electron microscopy, and flow cytometry for detection of apoptosis. J. Histochem. Cytochem. 2003, 51, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.L.; Di Bucchianico, S.; Collins, A.R.; Dusinska, M. Can the comet assay used reliably to detect nanoparticle-induced genotoxicity? Environ. Mol. Mutagen. 2015, 56, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Fairbairn, D.W.; Olive, P.L.; O‘Neill, K.L. The Comet Assay: A comprehensive review. Mutat. Res. 1995, 339, 37–59. [Google Scholar] [CrossRef] [PubMed]
- Gajski, G.; Langie, S.; Zhanataev, A. Recent applications of the Comet Assay: A report from the International Comet Assay Workshop 2019. Toxicol. Lett. 2020, 333, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Irvin-Barnwell, E.A.; Benson, K.M.; Lu, M.; Ragin, A.; Wheeler, J.; Hoffman, R. Environmental Toxins Found Historically in the Polycythemia Vera Cluster Area and their Potential for Inducing DNA Damage. Anal. Toxicol. 2021, 8, 551. [Google Scholar] [CrossRef]
- Azqueta, A.; Gutzkow, K.B.; Priestley, C.C.; Meier, S.; Walker, J.S.; Brunborg, G.; Collins, A.R. A comparative performance test of standard, medium- and high-throughput comet assays. Toxicol. In Vitro 2013, 27, 768–773. [Google Scholar] [CrossRef]
- Azqueta, A.; Collins, A.R. The essential comet assay: A comprehensive guide to measuring DNA damage and repair. Arch. Toxicol. 2013, 87, 949–968. [Google Scholar] [CrossRef] [PubMed]
- Brozovic, G.; Orsolic, N.; Knezevic, F.; Horvat, F.; Knezevic, A.; Benkovic, V.; Sakic, K.; Borojevic, N.; Dikic, D. The in vivo genotoxicity of cisplatin, isoflurane and halothane evaluated by alkaline comet assay in Swiss albino mice. J. Appl. Genet. 2011, 52, 355–361. [Google Scholar] [CrossRef]
- Gutzkow, K.B.; Langleite, T.M.; Meier, S.; Graupner, A.; Collins, A.R.; Brunborg, G. High-throughput comet assay using 96 minigels. Mutagenesis 2013, 28, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shaposhnikov, S.; Azqueta, A.; Henriksson, S.; Meier, S.; Gaivão, I.; Huskisson, N.H.; Smart, A.; Brunborg, G.; Nisson, M.; Collins, A.R. Twelve-gel slide format optimised for comet assay and fluorescent in situ hybridisation. Toxicol. Lett. 2010, 195, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Palosaari, T.; Magdolenova, Z.; Mennecozzi, M.; Gineste, J.M.; Saavedra, L.; Milcamps, A.; Huk, A.; Collins, A.R.; Dusinska, M.; et al. Iron oxide nanoparticle toxicity testing using high-throughput analysis and high-content imaging. Nanotoxicology 2015, 9 (Suppl. S1), 87–94. [Google Scholar] [CrossRef] [PubMed]
- Watson, C.; Ge, J.; Cohen, J.; Pyrgiotakis, G.; Engelward, B.P.; Demokritou, P. High-throughput screening platform for engineered nanoparticle-mediated genotoxicity using CometChip technology. ACS Nano 2014, 8, 2118–2133. [Google Scholar] [CrossRef]
- Li, J.; Beiser, A.; Dey, N.B.; Takeda, S.; Saha, L.K.; Hirota, K.; Parker, L.L.; Carter, M.; Arrieta, M.L.; Sobol, R.W. A high-throughput 384-well CometChip platform reveals a role for 3- methyladenine in the cellular response to etoposide-induced DNA damage. NAR Genom. Bioinform. 2022, 4, lqac065. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Akuta, T.; Nakagawa, M.; Sato, T.; Shibata, T.; Maruyama, T.; Okumura, C.J.; Kurosawa, Y.; Arakawa, T. Agarose native gel electrophoresis for characterization of antibodies. Int. J. Biol. Macromol. 2020, 151, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Tomioka, Y.; Sakuma, C.; Kurosawa, Y.; Shibata, T.; Arakawa, T.; Akuta, T. Development of a novel two-dimensional gel electrophoresis protocol with agarose native gel electrophoresis. Electrophoresis 2023, 44, 1446–1460. [Google Scholar] [CrossRef]
- Wilkesman, J.; Kurz, L. Zymography principles. Methods Mol. Biol. 2017, 1626, 3–10. [Google Scholar] [CrossRef]
- Oloketuyi, S.; Annovi, G.; de Marco, A. Peroxidase zymograms obtained by agarose native gel electrophoresis have unmet resolution and completeness. Int. J. Biol. Macromol. 1984, 156, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, P.; Mishra, V.K.; Swarnakar, S. Zymography and reverse zymography for testing proteases and their inhibitors. Methods Mol. Biol. 2022, 2413, 107–120. [Google Scholar] [CrossRef]
- Sakuma, C.; Sato, T.; Shibata, T.; Nakagwa, M.; Kurosawa, Y.; Okumura, C.J.; Maruyama, T.; Arakawa, T.; Akuta, T. Western blotting analysis of proteins by agarose native gel electrophoresis. Int. J. Biol. Macromol. 2021, 166, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Nakagawa, M.; Ouchi, Y.; Sakuma, C.; Nakajima, Y.; Shimizu, T.; Shibata, T.; Kurosawa, Y.; Maruyama, T.; Okumura, C.J.; et al. A rabbit monoclonalantibody-mediated lateral flow immunoassay for rapid detection of CTX-M extended-spectrum β-lactamase-producing Enterobacterales. Int. J. Biol. Macromol. 2021, 185, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Yigit, H.; Anderson, G.J.; Biddle, J.W.; Steward, C.D.; Rasheed, J.K.; Valera, L.L.; McGowan, J.E.; Tenover, F.C. Carbapenem resistance in a clinical isolate of Enterobacter aerogenes is associated with decreased expression of OmpF and OmpC Porin Analogs. Antimicrob. Agents Chemother. 2001, 46, 3817–3822. [Google Scholar] [CrossRef] [PubMed]
- Kurien, B.T.; Scofield, R.H. Extraction of proteins from gels: A brief review. Methods Mol. Biol. 2012, 869, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Sule, R.; Rivera, G.; Gomes, A.V. Western blotting (immunoblotting): History, theory, uses, protocol and problems. Biotechniques 2023, 75, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Begum, H.; Murugesan, P.; Tangutur, A.D. Western blotting: A powerful staple in scientific and biomedical research. Biotechniques 2022, 73, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Yasuoka, Y.; Izumi, Y.; Sands, J.M.; Kawahara, K.; Nonoguchi, H. Progress in the detection of erythropoietin in blood, urine, and tissue. Molecules 2023, 28, 4446. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, C.; Nakagawa, M.; Tomioka, Y.; Maruyama, T.; Entzminger, K.; Fleming, J.K.; Shibata, T.; Kurosawa, Y.; Okumura, C.J.; Arakawa, T.; et al. Western blotting of native proteins from agarose gels. Biotechniques 2022, 72, 207–218. [Google Scholar] [CrossRef]
- Arakawa, T.; Nakagawa, M.; Tomioka, Y.; Sakuma, C.; Li, C.; Sato, T.; Shibata, Y.; Kurosawa, Y.; Akuta, T. Gel-electrophoresis based method for biomolecular interaction. Methods Cell Biol. 2022, 169, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Akuta, T.; Maruyama, T.; Sakuma, C.; Nakagawa, M.; Tomioka, Y.; Entzminger, K.; Fleming, J.K.; Sato, R.; Shibata, T.; Kurosawa, Y.; et al. A new method to characterize conformation-specific antibody by a combination of agarose native gel electrophoresis and contact blotting. Antibodies 2022, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Pramanick, I.; Sengupta, N.; Mishra, S.; Pandey, S.; Girish, N.; Das, A.; Dutta, S. Conformational flexibility and structural varia bility of SARS-CoV2 S protein. Structure 2021, 29, 834–845. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arakawa, T.; Nakagawa, M.; Sakuma, C.; Tomioka, Y.; Kurosawa, Y.; Akuta, T. Polysaccharide as a Separation Medium for Gel Electrophoresis. Polysaccharides 2024, 5, 380-398. https://doi.org/10.3390/polysaccharides5030024
Arakawa T, Nakagawa M, Sakuma C, Tomioka Y, Kurosawa Y, Akuta T. Polysaccharide as a Separation Medium for Gel Electrophoresis. Polysaccharides. 2024; 5(3):380-398. https://doi.org/10.3390/polysaccharides5030024
Chicago/Turabian StyleArakawa, Tsutomu, Masataka Nakagawa, Chiaki Sakuma, Yui Tomioka, Yasunori Kurosawa, and Teruo Akuta. 2024. "Polysaccharide as a Separation Medium for Gel Electrophoresis" Polysaccharides 5, no. 3: 380-398. https://doi.org/10.3390/polysaccharides5030024
APA StyleArakawa, T., Nakagawa, M., Sakuma, C., Tomioka, Y., Kurosawa, Y., & Akuta, T. (2024). Polysaccharide as a Separation Medium for Gel Electrophoresis. Polysaccharides, 5(3), 380-398. https://doi.org/10.3390/polysaccharides5030024





