Chitosan-Based Membranes: A Comprehensive Review of Nanofiltration, Pervaporation, and Ion Exchange Applications
Abstract
1. Introduction
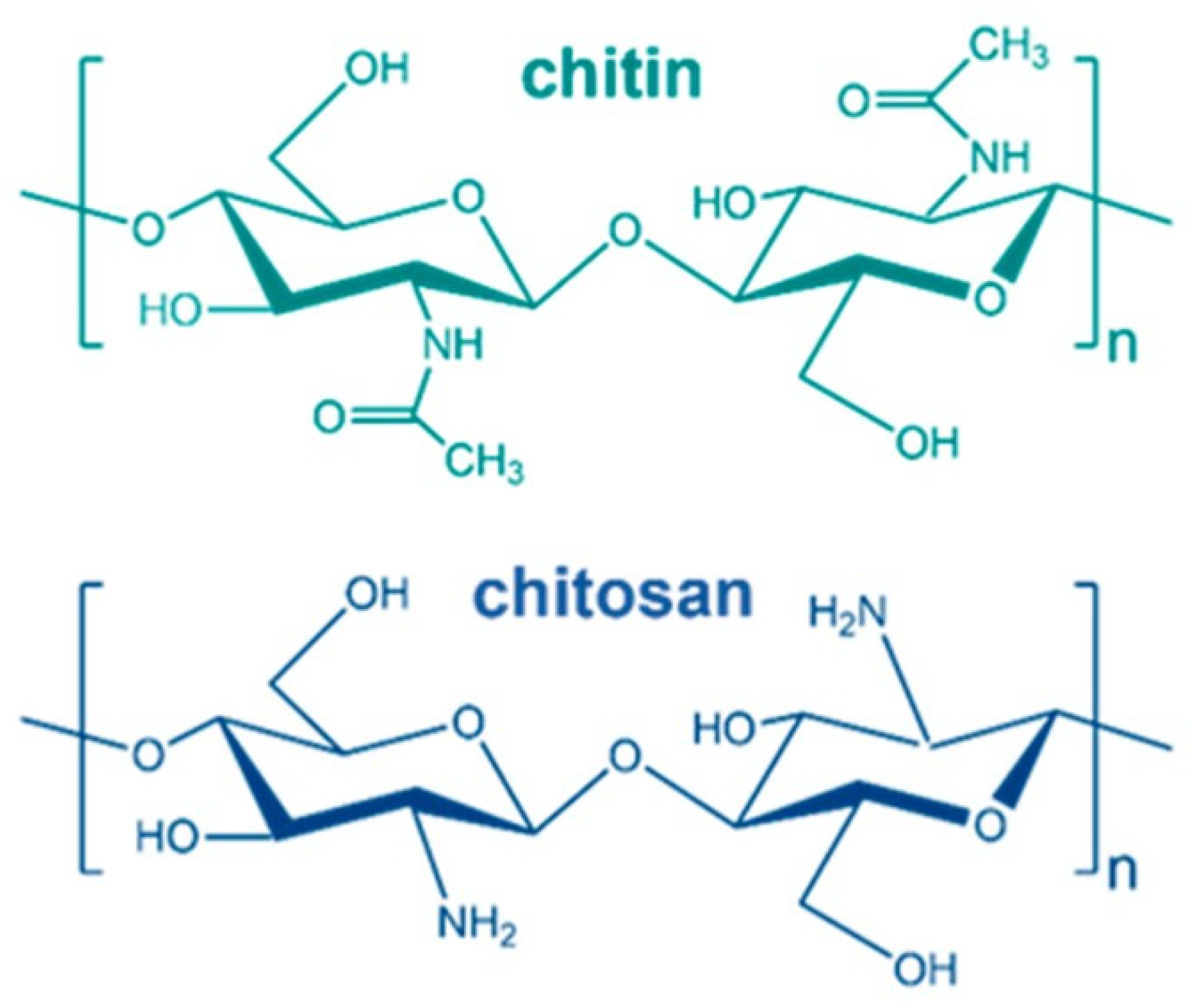
2. Chitosan
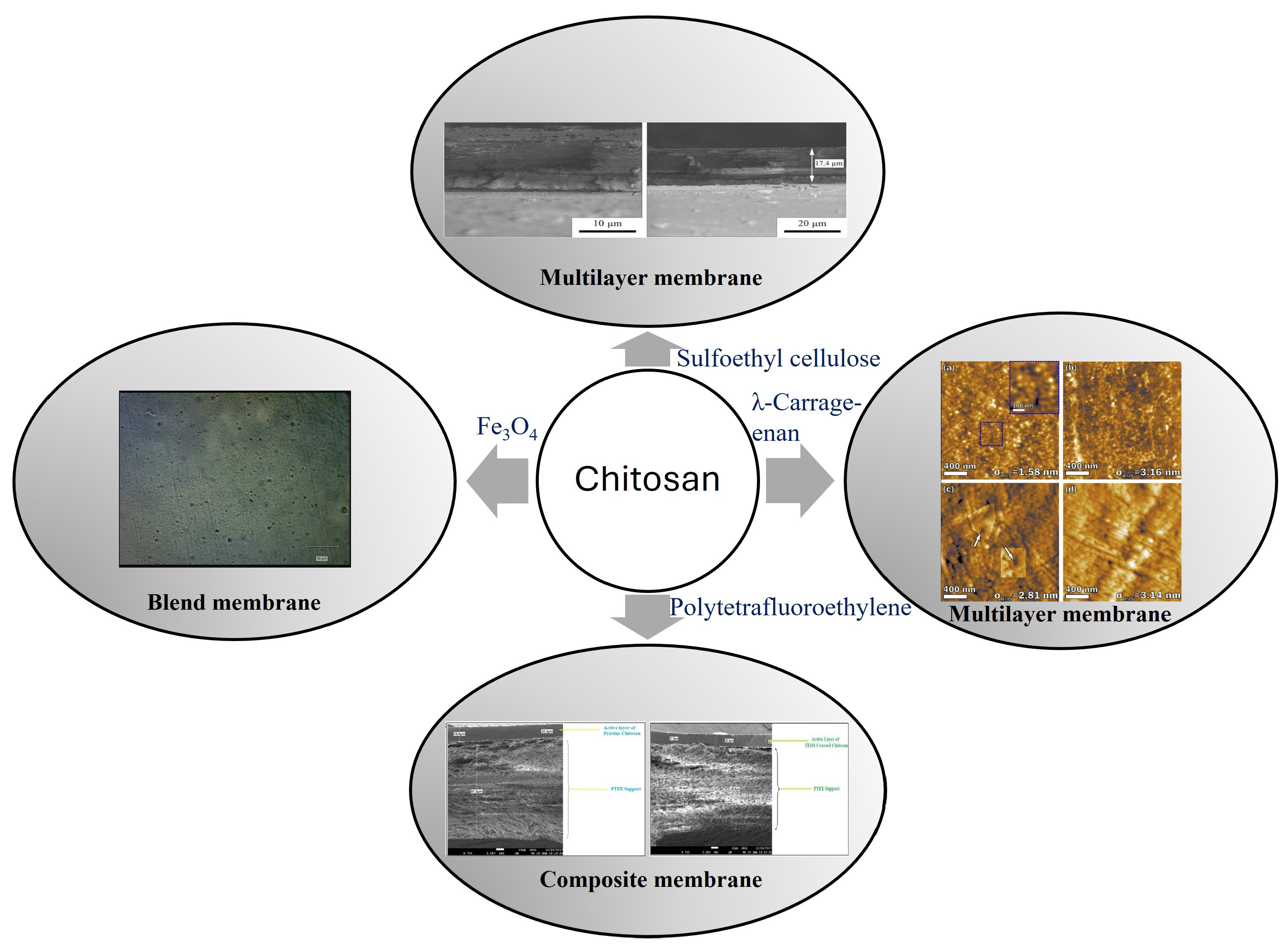
3. Chitosan-Based Membranes
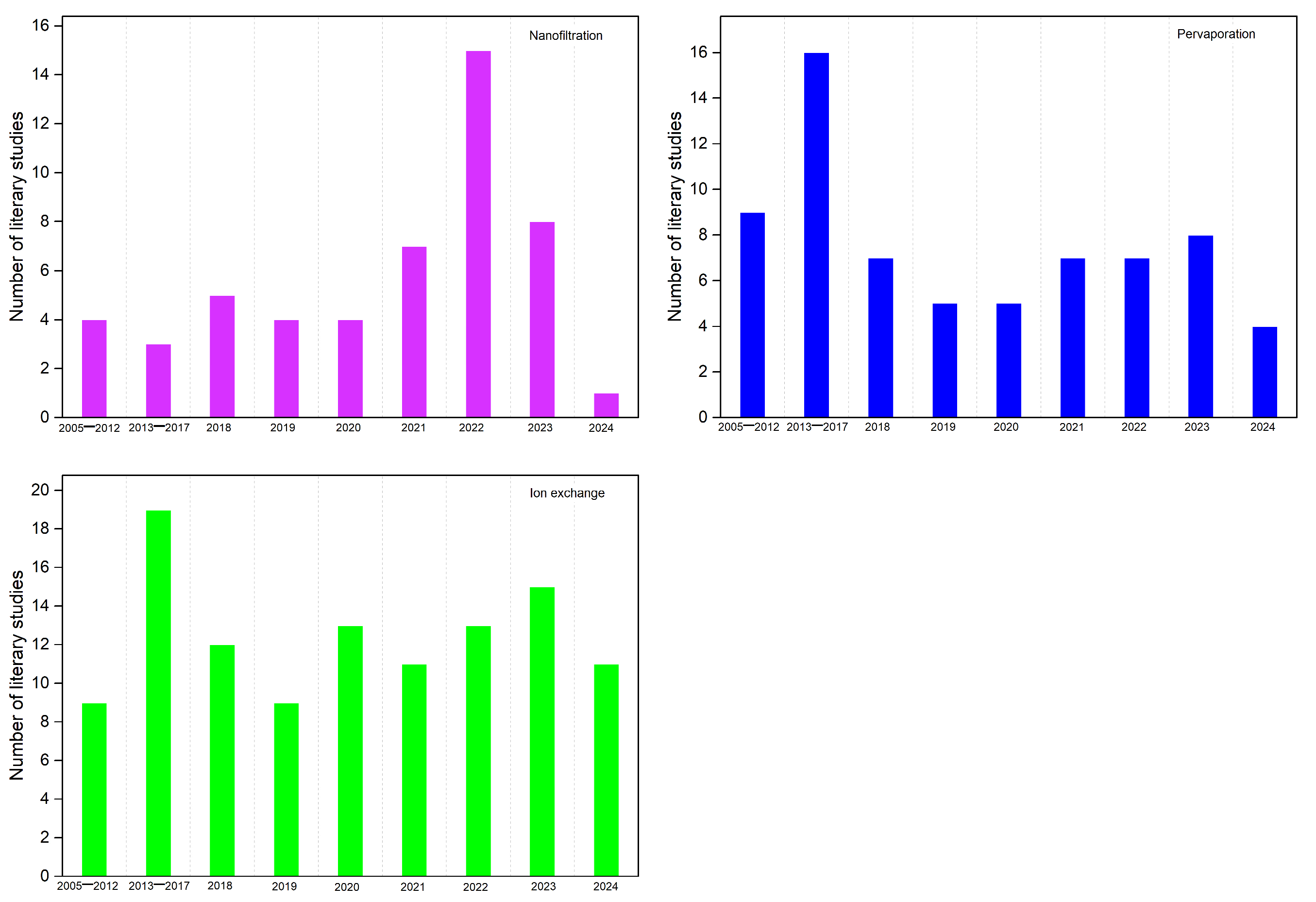
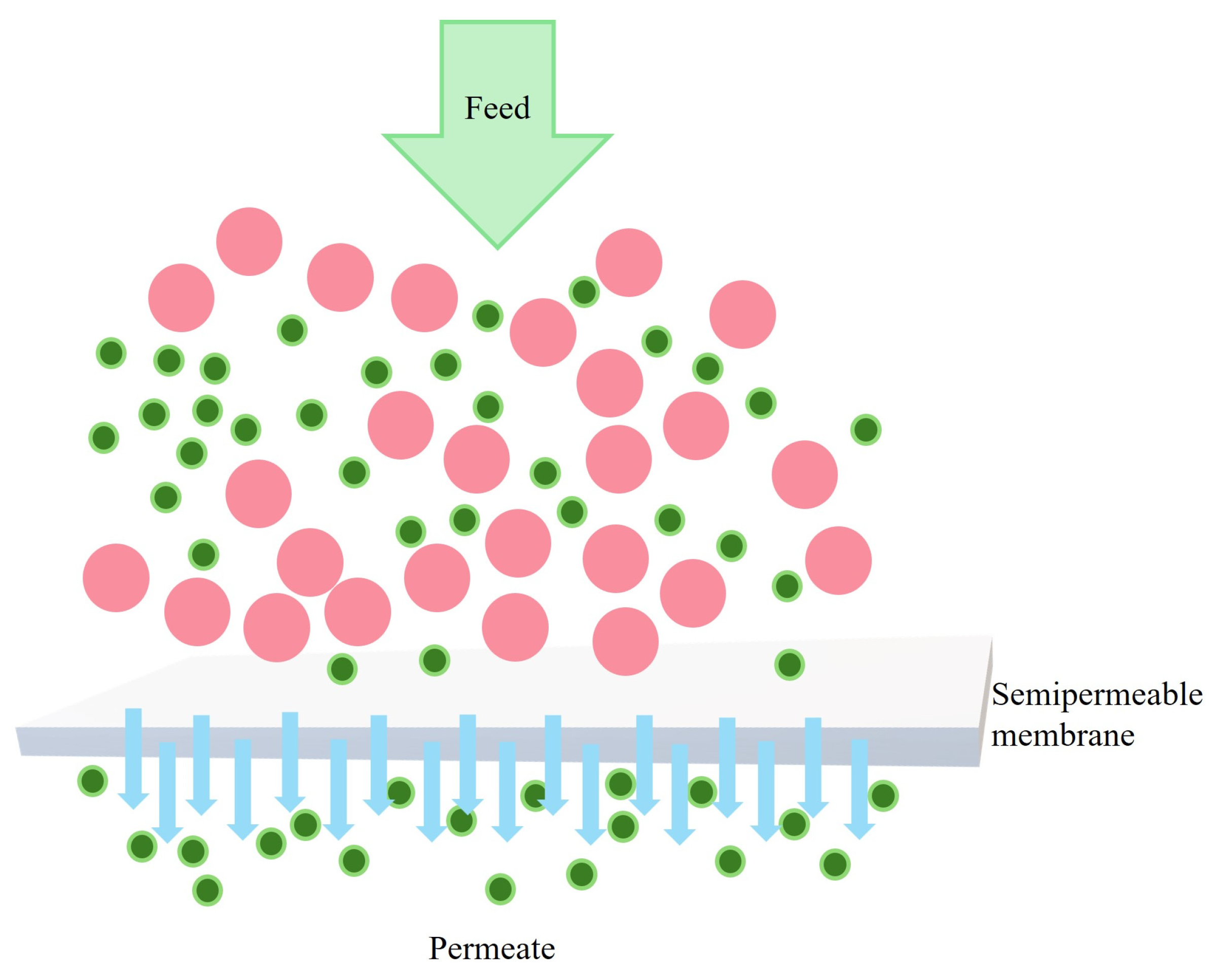
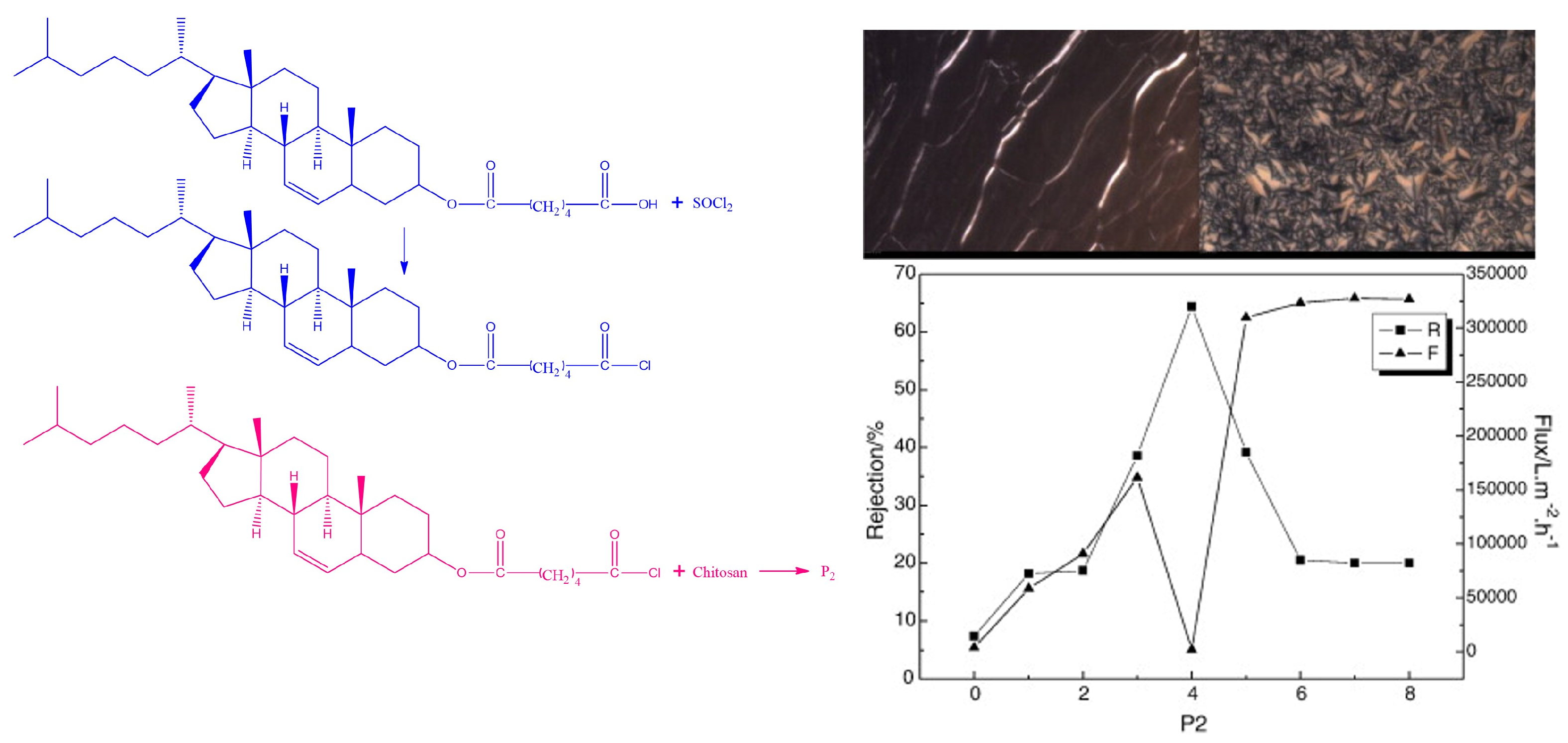
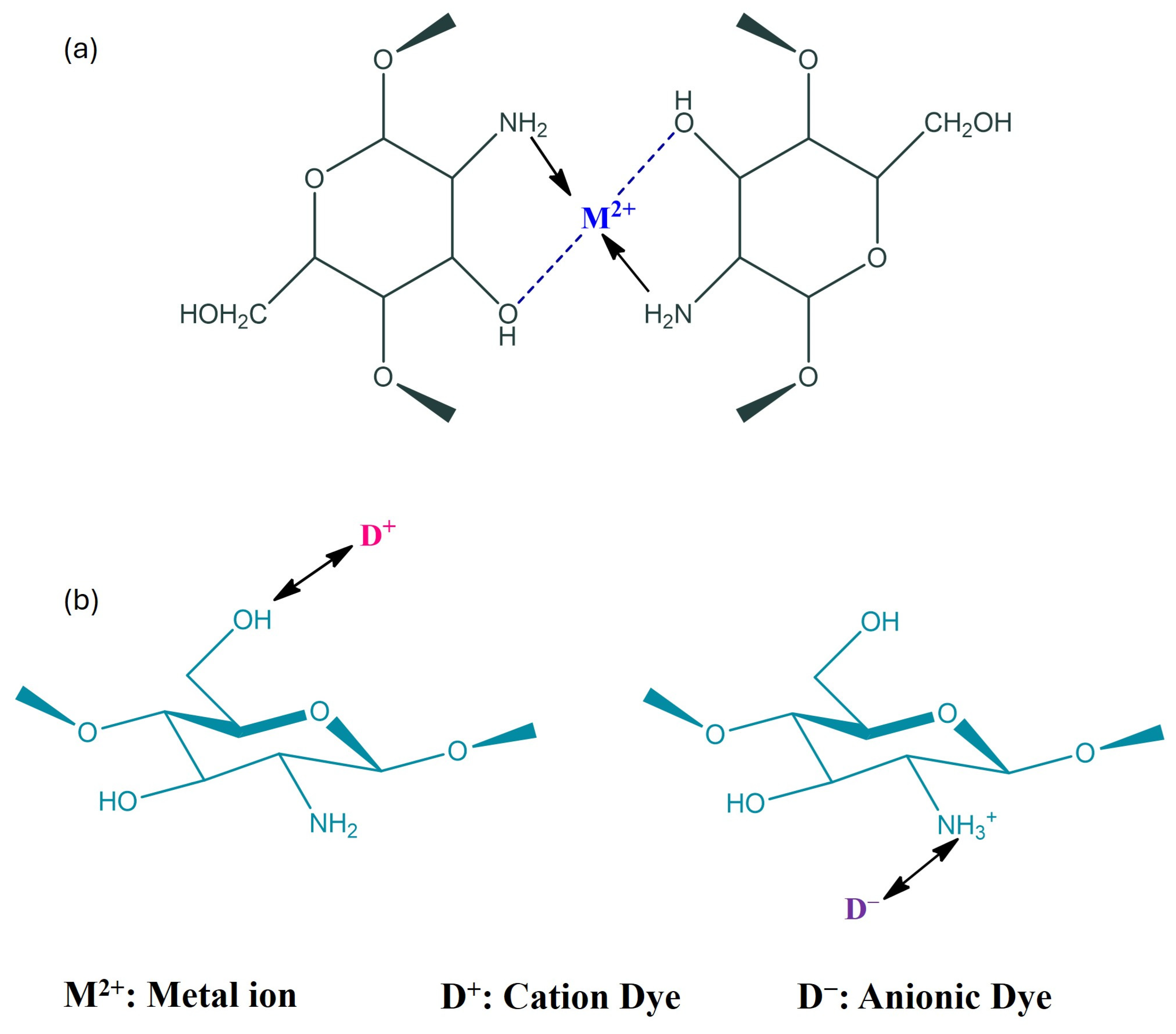
3.1. Application of Chitosan Derived Membranes in Nanofiltration
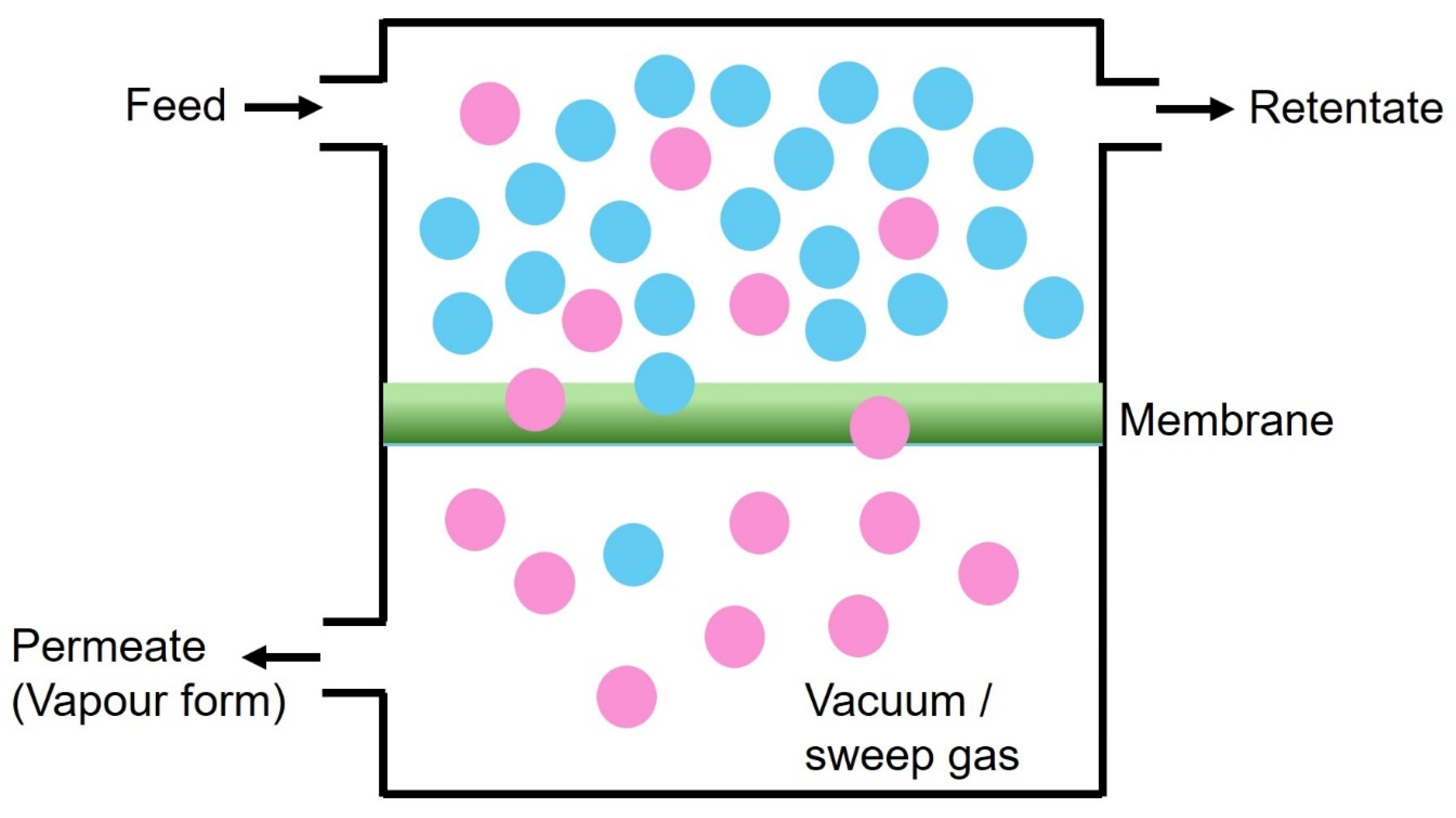
3.2. Use of Chitosan-Based Membranes in Pervaporation
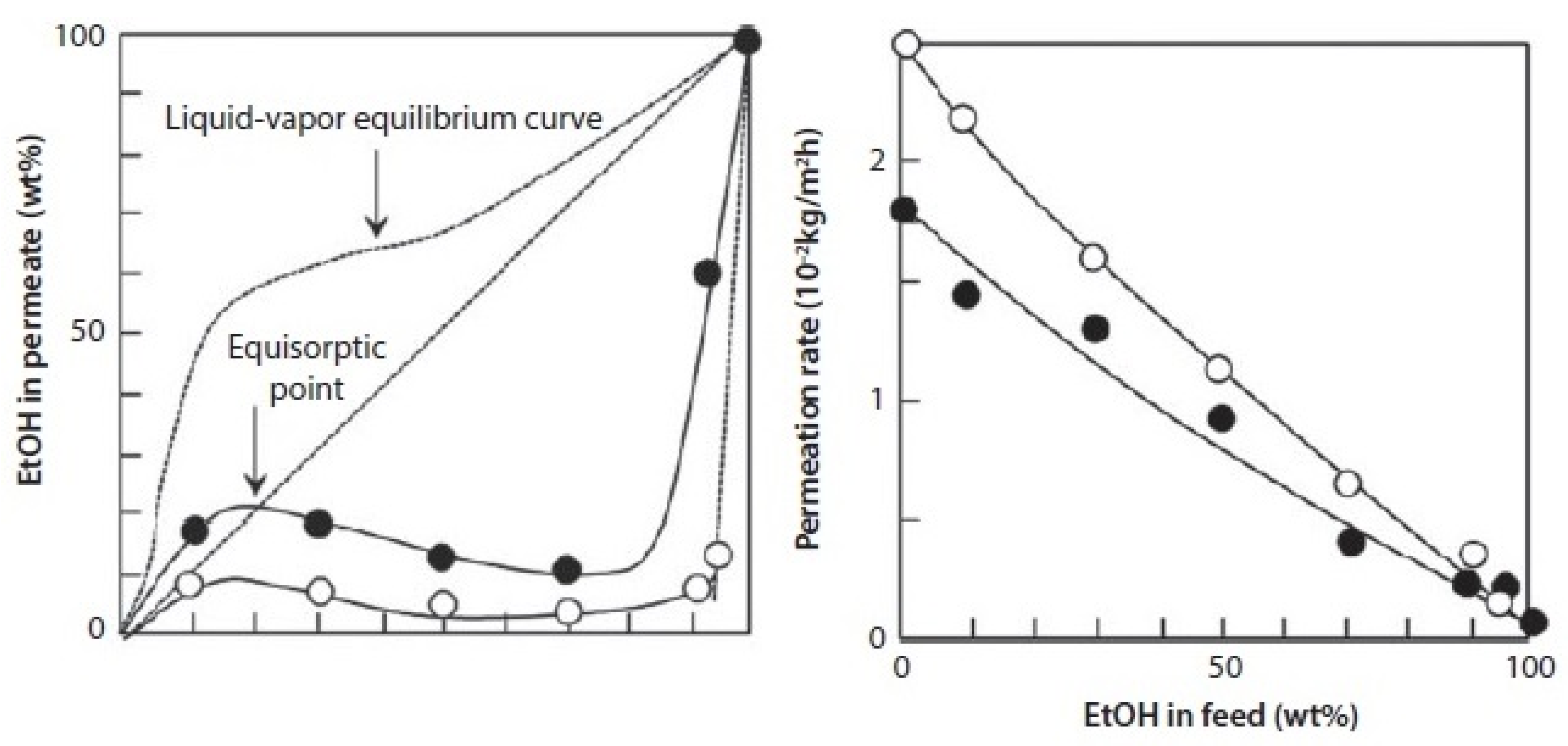
3.2.1. Water Selective Membranes
3.2.2. Organic-Permselective Membranes
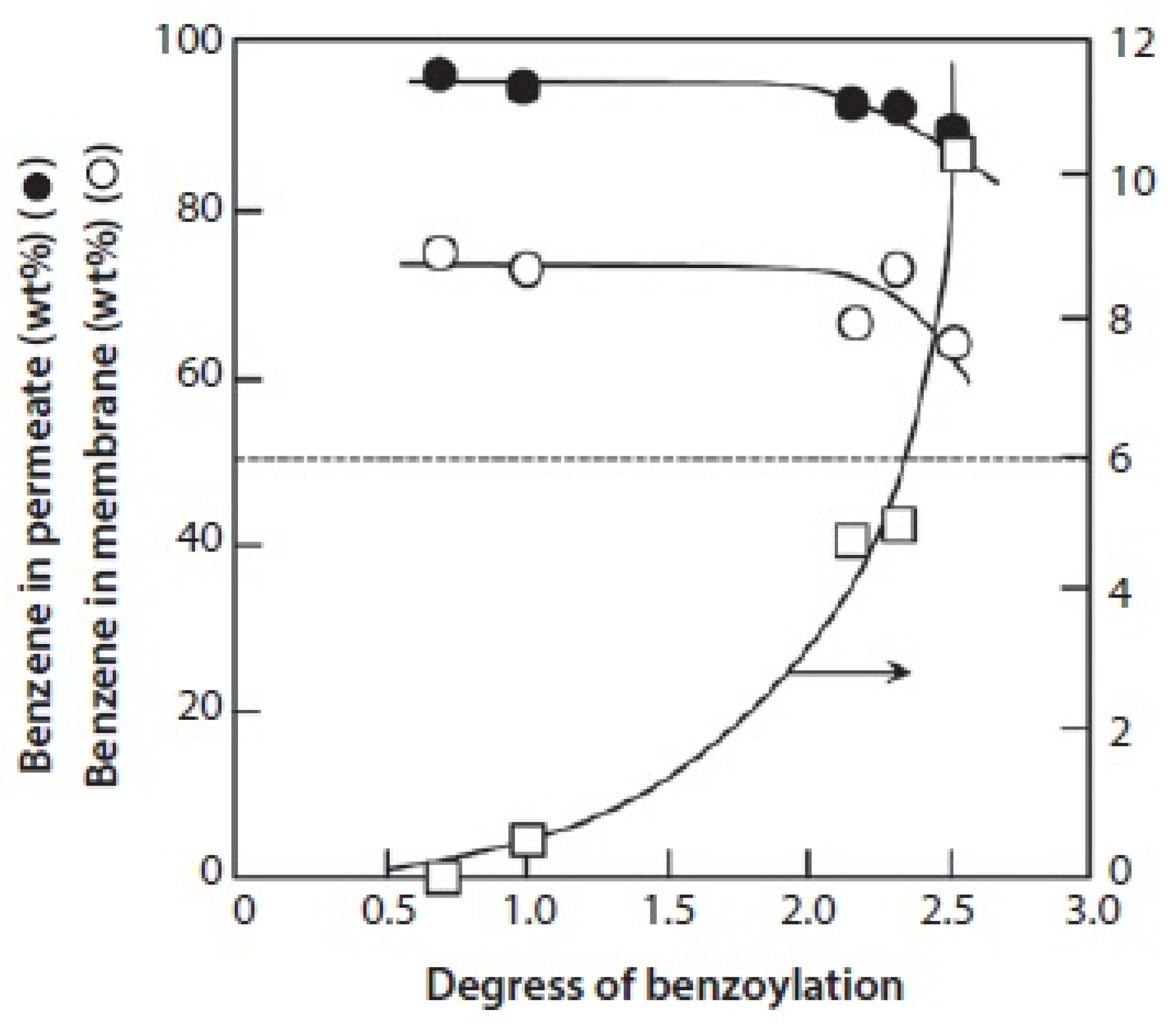
3.2.3. Organic–Organic Separation Membranes
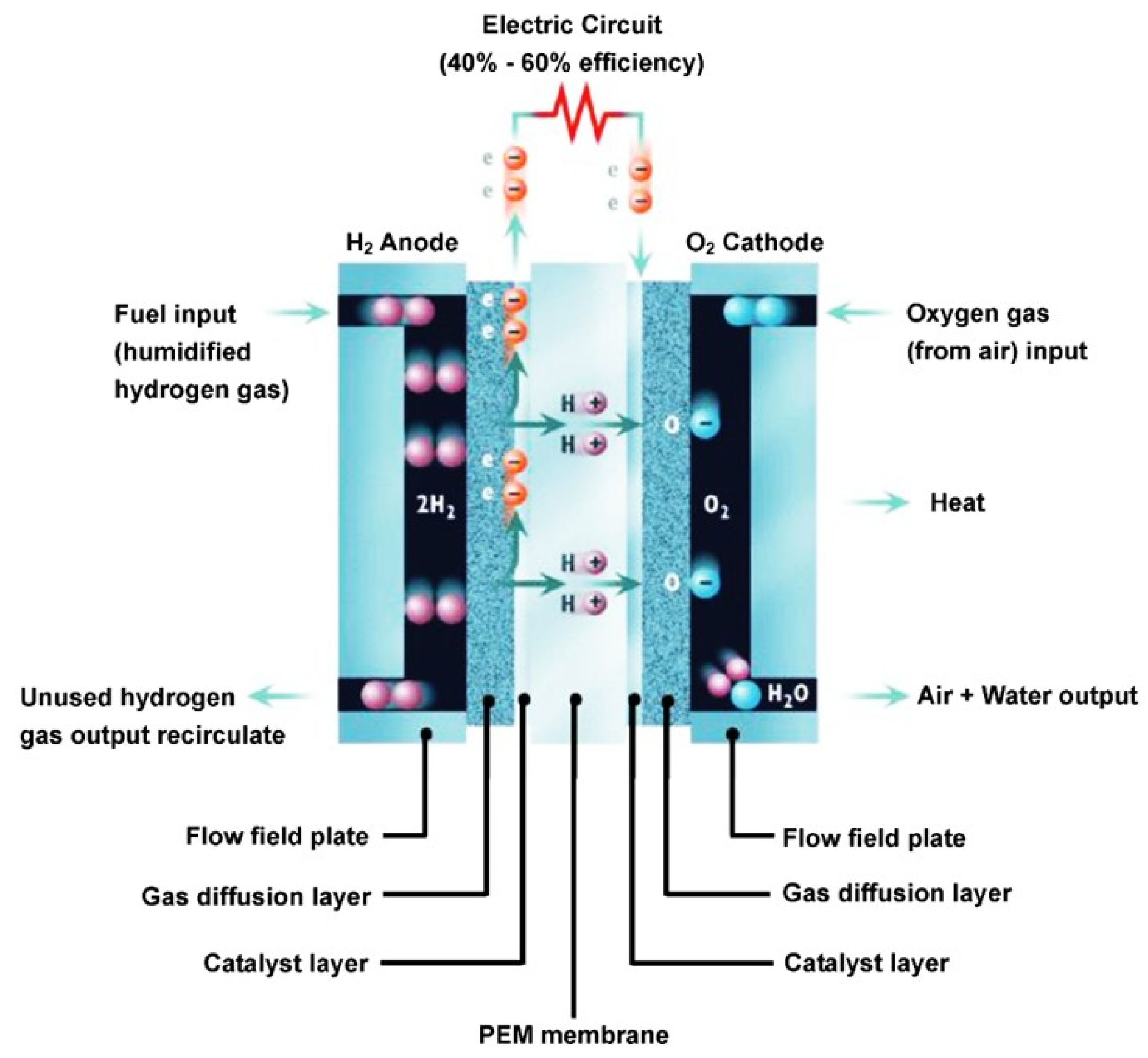
3.3. Chitosan Based Polymer Electrolyte Fuel Cell Membranes (PEMFCs)
4. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baker, R.W. Future Directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar]
- Xu, D.; Hein, S.; Wang, K. Chitosan membrane in separation applications. Mater. Sci. Technol. 2008, 24, 1076–1087. [Google Scholar] [CrossRef]
- Yeo, Z.Y.; Chew, T.L.; Zhu, P.W.; Mohamed, A.R.; Chai, S.P. Conventional processes and membrane technology for carbon dioxide removal from natural gas: A review. J. Nat. Gas Chem. 2012, 21, 282–298. [Google Scholar]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar]
- Poshina, D.N.; Rakshina, A.D.; Skorik, Y.A. Hydrophobic chitosan derivatives for gene and drug delivery in cancer therapies. Polysaccharides 2025, 6, 11. [Google Scholar] [CrossRef]
- Panagopoulos, A.; Michailidis, P. Membrane technologies for sustainable wastewater treatment: Advances, challenges, and applications in zero liquid discharge (zld) and minimal liquid discharge (mld) systems. Membranes 2025, 15, 64. [Google Scholar] [CrossRef]
- Figueiredo, A.S.; Sánchez-Loredo, M.G.; de Pinho, M.N.; Minhalma, M. Surface-charge characterization of nanocomposite cellulose acetate/silver membranes and bsa permeation performance. Membranes 2025, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Li, G.; Liu, Y.; Zhou, S.; Liu, F. Loose polyester nanofiltration membrane designed with hydroxyl-ammonium for efficient dye/salt separation. Membranes 2025, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Hara, N.; Taniguchi, S.; Yamaki, T.; Nguyen, T.T.H.; Kataoka, S. Bi-objective optimization of techno-economic and environmental performance for membrane-based CO2 capture via single-stage membrane separation. Membranes 2025, 15, 57. [Google Scholar] [CrossRef]
- Kang, G.D.; Cao, Y.M. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Membr. Sci. 2014, 463, 145–165. [Google Scholar]
- Krajewska, B. Membrane-based processes performed with use of chitin/chitosan materials. Sep. Purif. Technol. 2005, 41, 305–312. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Kim, S.K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar]
- Khor, E. Chitin: Fulfilling a Biomaterials Promise; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Zhang, S.; Ji, Y.; He, Y.; Dong, J.; Li, H.; Yu, S. Effect of Environmental pH on the Mechanics of Chitin and Chitosan: A Single-Molecule Study. Polymers 2024, 16, 995. [Google Scholar] [CrossRef]
- Clementino, A.; Climani, G.; Bianchera, A.; Buttini, F.; Sonvico, F. Polysaccharides: New frontiers for nasal administration of medicines. Polysaccharides 2025, 6, 6. [Google Scholar] [CrossRef]
- Yang, S.Y.; Im, K.S.; Nikita, K.; Nam, S.Y. Membrane-based direct air capture: A review. App. Chem. Eng. 2024, 35, 85–95. [Google Scholar]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Thomas, S.M.; Gómez-Romero, P.; González-Gil, R.M. Polysaccharides: The sustainable foreground in energy storage systems. Polysaccharides 2025, 6, 5. [Google Scholar] [CrossRef]
- Struszczyk, M.H. Chitin and chitosan: Part III. some aspects of biodegradation and bioactivity. Polimery 2002, 47, 619–629. [Google Scholar] [CrossRef]
- Song, R.; Zhong, Z.; Lin, L. Evaluation of chitosan quaternary ammonium salt modified resin denture base material. Int. J. Biol. Macromol. 2016, 85, 102–110. [Google Scholar] [CrossRef]
- Silvestre, W.P.; Baldasso, C.; Tessaro, I.C. Potential of chitosan-based membranes for the separation of essential oil components by target-organophilic pervaporation. Carbohydr. Polym. 2020, 247, 116676. [Google Scholar]
- Zanotti, A.; Baldino, L.; Cardea, S.; Reverchon, E. Methyl orange adsorption using chitosan-based composite aerogels produced by supercritical gel drying. Polysaccharides 2025, 6, 1. [Google Scholar] [CrossRef]
- Lee, D.J.; Im, K.S.; Ryu, K.Y.; Nam, S.Y. Synthesis and Characterization of Ion Exchange Particles for Application of Anion Exchange Membrane. Membr. J. 2023, 33, 137–147. [Google Scholar] [CrossRef]
- Kwak, G.J.; Kim, D.H.; Nam, S.Y. Development of pore filled anion exchange membrane using UV polymerization method for anion exchange membrane fuel cell application. Membr. J. 2023, 33, 77–86. [Google Scholar] [CrossRef]
- Gonçalves, V.L.; Laranjeira, M.C.M.; Fávere, V.T.; Pedrosa, R.C. Effect of crosslinking agents on chitosan microspheres in controlled release of diclofenac sodium. Polímeros 2006, 15, 6–12. [Google Scholar] [CrossRef]
- Sarode, S.; Upadhyay, P.; Khosa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of wastewater treatment methods with special focus on biopolymer chitinchitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Karakoti, M.; Nam, S.Y. Role of graphene derivatives in anion exchange membrane for fuel cell: Recent trends. Membr. J. 2022, 32, 411–426. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Turczyn, R.; Strzelewicz, A.; Krasowska, M. Pervaporation with chitosan membranes containing iron oxide nanoparticles. Sep. Purif. Technol. 2014, 133, 8–15. [Google Scholar] [CrossRef]
- Kononova, S.V.; Kruchinina, E.V.; Petrova, V.A.; Baklagina, Y.G.; Romashkova, K.A.; Orekhov, A.S.; Klechkovskaya, V.V. Polyelectrolyte complexes of sulfoethyl cellulose–chitosan: Effect of the structure on separation properties of multilayer membranes. Cellulose 2018, 25, 7239–7259. [Google Scholar] [CrossRef]
- Kononova, S.V.; Volod’ko, A.V.; Petrova, V.A.; Kruchinina, E.V.; Baklagina, Y.G.; Chusovitin, E.A.; Skorik, Y.A. Pervaporation multilayer membranes based on a polyelectrolyte complex of λ-carrageenan and chitosan. Carbohydr. Polym. 2018, 181, 86–92. [Google Scholar] [CrossRef]
- Ko, H.; Kim, M.; Nam, S.Y.; Kim, K. Research of cross-linked hydrocarbon based polymer electrolyte membranes for polymer electrolyte membrane fuel cell applications. Membr. J. 2020, 30, 395–408. [Google Scholar] [CrossRef]
- Moulik, S.; Bukke, V.; Sajja, S.C.; Sridhar, S. Chitosan-polytetrafluoroethylene composite membranes for separation of methanol and toluene by pervaporation. Carbohydr. Polym. 2018, 193, 28–38. [Google Scholar] [PubMed]
- Clasen, C.; Wilhelms, T.; Kulicke, W.M. Formation and characterization of chitosan membranes. Biomacromolecules 2006, 7, 3210–3222. [Google Scholar] [PubMed]
- Cui, L.; Gao, S.; Song, X.; Huang, L.; Dong, H.; Liu, J.; Chena, F.; Yua, S. Preparation and characterization of chitosan membranes. RSC Adv. 2018, 8, 28433–28439. [Google Scholar] [PubMed]
- Unlu, D.; Hilmioglu, N.D. Pervaporation catalytic membrane reactor application over functional chitosan membrane. J. Membr. Sci. 2018, 559, 138–147. [Google Scholar]
- Kaewklin, P.; Siripatrawan, U.; Suwanagul, A.; Lee, Y.S. Active packaging from chitosan-titanium dioxide nanocomposite film for prolonging storage life of tomato fruit. Int. J. Biol. Macromol. 2018, 112, 523–529. [Google Scholar] [PubMed]
- Lusiana, R.A.; Protoningtyas, W.P.; Wijaya, A.R.; Siswanta, D.; Mudasir, M.; Santosa, S.J. Chitosan-tripoly phosphate (CS-TPP) synthesis through cross-linking process: The effect of concentration towards membrane mechanical characteristic and urea permeation. Orient. J. Chem. 2017, 33, 2913–2919. [Google Scholar]
- Gayed, H.M.; El Fadl, F.I.A.; Maziad, N.A.; El-Aassar, A.H.M.; Abdel Mottaleb, M.S.A. Surface modification of composite polyamide reverse osmosis membrane by irradiated chitosan and TiO2 nanoparticles. Desalin. Water Treat. 2019, 160, 32–40. [Google Scholar]
- Liao, L.; Fei, P.F.; Cheng, B.W.; Meng, J.Q.; Hu, X.Y.; Song, J. Fabrication and antibacterial properties of cellulose triacetate/chitosan reverse osmosis membrane. Acta Polym. Sin. 2018, 5, 607–616. [Google Scholar]
- Shi, J.; Kang, H.; Li, N.; Teng, K.; Sun, W.; Xu, Z.; Qian, X.; Liu, Q. Chitosan sub-layer binding and bridging for nanofiber-based composite forward osmosis membrane. Appl. Surf. Sci. 2019, 478, 38–48. [Google Scholar]
- Kazemi, M.; Jahanshahi, M.; Peyravi, M. Chitosan-sodium alginate multilayer membrane developed by Fe0@WO3 nanoparticles: Photocatalytic removal of hexavalent chromium. Carbohydr. Polym. 2018, 198, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Figoli, A.; Santoro, S.; Galiano, F.; Basile, A. 2-Pervaporation membranes: Preparation, characterization, and application. In Woodhead Publishing Series in Energy: Pervaporation, Vapour Permeation and Membrane Distillation; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 19–63. [Google Scholar]
- Musale, D.A.; Kumar, A. Solvent and pH resistance of surface crosslinked chitosan/poly(acrylonitrile) composite nanofiltration membranes. J. Appl. Polym. Sci. 2000, 77, 1782–1793. [Google Scholar] [CrossRef]
- Musale, D.A.; Kumar, A. Effects of surface crosslinking on sieving characteristics of chitosan/poly(acrylonitrile) composite nanofiltration membranes. Sep. Purif. Technol. 2000, 21, 27–37. [Google Scholar] [CrossRef]
- Miao, J.; Li, L.; Chen, G.; Gao, C.; Dong, S. Preparation of N, O-carboxymethyl chitosan composite nanofiltration membrane and its rejection performance for the fermentation effluent from a wine factory. Chin. J. Chem. Eng. 2008, 16, 209–213. [Google Scholar] [CrossRef]
- Mu, T.; Cong, Y.; Wang, W.; Zhang, B. Preparation and characterization of novel chitosan composite nanofiltration membrane containing mesogenic units. Desalination 2012, 298, 67–74. [Google Scholar] [CrossRef]
- Ghaee, A.; Shariaty-Niassar, M.; Barzin, J.; Ismail, A.F. Chitosan/polyethersulfone composite nanofiltration membrane for industrial wastewater treatment. Int. J. Nanosci. Nanotechnol. 2013, 9, 213–220. [Google Scholar]
- Mu, T.; Liu, X.; Tan, G.; Wang, K. Novel positively charged chiral chitosan composite nanofiltration membrane. IDA J. Desalin. Water Reuse 2014, 6, 33–38. [Google Scholar]
- Salehi, E.; Khajavian, M.; Sahebjamee, N.; Mahmoudi, M.; Drioli, E.; Matsuura, T. Advances in nanocomposite and nanostructured chitosan membrane adsorbents for environmental remediation: A review. Desalination 2022, 527, 115565. [Google Scholar] [CrossRef]
- Binning, R.; Lee, R.; Jennings, J.; Martin, E. Separation of liquid mixtures by permeation. Ind. Eng. Chem. 1961, 53, 45–50. [Google Scholar]
- Choo, C.Y. Membrane permeation. In Advances in Petrolum Chemistry and Refining, 5th ed.; Kobe, K.A., McKetta, J.J., Eds.; Wiley Interscience: New York, NY, USA, 1958; p. 73. [Google Scholar]
- Uragami, T.; Saito, M.; Takigawa, K. Studies on syntheses and permeabilities of special polymer membranes, 69†. Comparison of permeation and separation characteristics for aqueous alcoholic solutions by pervaporation and new evapomeation methods through chitosan membranes. Makromol. Chem. Rapid Commun. 1988, 9, 361–365. [Google Scholar]
- Uragami, T. Functional separation membranes from chitin and chitosan derivatives. In Handbook of Composites from Renewable Materials; Thakur, V.K., Thakur, M.K., Kessler, M.R., Eds.; Willey: Hoboken, NJ, USA, 2017; Volume 4, pp. 69–120. [Google Scholar]
- Uragami, T.; Saito, M. Analysis of permeation and separation characteristics and new technique for separation of aqueous alcoholic solutions through alginic acid membranes. Sep. Sci. Technol. 1989, 24, 541–554. [Google Scholar]
- Uragami, T.; Takigawa, K. Permeation and separation characteristics of ethanol-water mixtures through chitosan derivative membranes by pervaporation and evapomeation. Polymer 1990, 31, 668–672. [Google Scholar]
- Feng, X.; Huang, R.Y.M. Pervaporation with chitosan membranes. I. separation of water from ethylene glycol by a chitosan/polysulfone composite membrane. J. Membr. Sci. 1996, 116, 67–76. [Google Scholar] [CrossRef]
- Lee, Y.M.; Shin, E.M.; Noh, S.T. Pervaporation separation of water-ethanol through modified chitosan membranes, II. Carboxymethyl, carboxyethyl, cyanoethyl, and amidoxime chitosan membranes. Die Angew. Makromol. Chem. 1991, 192, 169–181. [Google Scholar] [CrossRef]
- Lee, Y.M.; Shin, E.M. Pervaporation separation of water—Ethanol through modified chitosan membranes. IV. Phosphorylated chitosan membranes. J. Membr. Sci. 1991, 64, 145–152. [Google Scholar] [CrossRef]
- Lee, Y.M.; Nam, S.Y.; Kim, J.H. Pervaporation of water- ethanol through poly(vinyl alcohol)/chitosan blend membrane. Polym. Bull. 1992, 29, 423–429. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lee, Y.M. Pervaporation and properties of chitosan-poly(acrylic acid) complex membranes. J. Membr. Sci. 1997, 135, 161–171. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lee, Y.M. Pervaporation of ethylene glycol/water mixtures I. pervaporation performance of surface crosslinked chitosan membranes. J. Membr. Sci. 1999, 153, 155–162. [Google Scholar]
- Lee, Y.M.; Nam, S.Y.; Woo, D.J. Pervaporatton performance of β-chitosan membrane for water/alcohol mixtures. J. Polym. Eng. 1998, 18, 131–146. [Google Scholar] [CrossRef]
- Lee, Y.M.; Park, H.B.; Nam, S.Y. Effect of deacetylation degree in chitosan composite membranes on pervaporation performance. Sep. Sci. Technol. 1998, 33, 1255–1269. [Google Scholar] [CrossRef]
- Chen, X.; Li, W.; Shao, Z.; Zhong, W.; Yu, T. Separation of alcohol—Water mixture by pervaporation through a novel natural polymer blend membrane—Chitosan/silk fibroin blend membrane. J. Appl. Polym. Sci. 1999, 73, 975–980. [Google Scholar] [CrossRef]
- Uragami, T.; Shirakawa, T.; Miyata, T. Relationship between structures of cross-linked chitosan membranes and their permeation and separation characteristics for an aqueous solution containing urine components. Netw. Polym. 2001, 22, 212–217. [Google Scholar]
- Hirabayashi, Y. Pervaporation membrane system for the removal of ammonia from water. Mater. Trans. 2002, 43, 1074–1077. [Google Scholar] [CrossRef]
- Uragami, T.; Katayama, T.; Miyata, T.; Tamura, H.; Shiraiwa, T.; Higuchi, A. Dehydration of an ethanol/water azeotrope by novel organic−inorganic hybrid membranes based on quaternized chitosan and tetraethoxysilane. Biomacromolecules 2004, 5, 1567–1574. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. Functionalized silica–chitosan hybrid membrane for dehydration of ethanol/water azeotrope: Effect of cross-linking on structure and performance. J. Membr. Sci. 2013, 444, 116–126. [Google Scholar] [CrossRef]
- Xia, Q.; Zhu, T.; Chai, Z.; Wang, Y. TFC membrane with in-situ crosslinked ultrathin chitosan layer for efficient water/ethanol separation enabled by multiple supramolecular interactions. Adv. Membr. 2023, 3, 100062. [Google Scholar] [CrossRef]
- Wang, X.P.; Shen, Z.Q.; Zhang, F.Y.; Zhang, Y.F. Preferential separation of ethanol from aqueous solution through hydrophilic polymer membranes. J. Appl. Polym. Sci. 1999, 73, 1145–1151. [Google Scholar] [CrossRef]
- Nam, S.Y.; Lee, Y.M. Pervaporation separation of methanol/methyl t-butyl pervaporation separation of methanol/methyl t-butyl ether through chitosan composite membrane modified with surfactants. J. Membr. Sci. 1999, 157, 63–71. [Google Scholar]
- Kim, S.G.; Lim, G.T.; Jegal, J.; Lee, K.H. Pervaporation separation of MTBE (methyl tert-butyl ether) and methanol mixtures through polyion complex composite membranes consisting of sodium alginate/chitosan. J. Membr. Sci. 2000, 174, 1–15. [Google Scholar] [CrossRef]
- Cao, S.; Shi, Y.; Chen, G. Properties and pervaporation characteristics of chitosan–poly(N-vinyl-2-pyrrolidone) blend membranes for MeOH–MTBE. J. Appl. Polym. Sci. 1999, 74, 1452–1458. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Moon, G.Y.; Pal, R. Chitosan/anionic surfactant complex membranes for the pervaporation separation of methanol/MTBE and characterization of the polymer/surfactant system. J. Membr. Sci. 2001, 184, 1–15. [Google Scholar] [CrossRef]
- Won, W.; Feng, X.; Lawless, D. Pervaporation with chitosan membranes: Separation of dimethyl carbonate/methanol/water mixtures. J. Membr. Sci. 2002, 209, 493–508. [Google Scholar] [CrossRef]
- Huang, R.Y.M.; Moon, G.Y.; Pal, R. N-acetylated chitosan membranes for the pervaporation separation of alcohol/toluene mixtures. J. Membr. Sci. 2000, 176, 223–231. [Google Scholar]
- Uragami, T.; Tsukamoto, K.; Inui, K.; Miyata, T. Pervaporation characteristics of a benzoylchitosan membrane for benzene-cyclohexane mixtures. Macromol. Chem. Phys. 1998, 199, 49–54. [Google Scholar] [CrossRef]
- Inui, K.; Tsukamoto, K.; Miyata, T.; Uragami, T. Permeation and separation of a benzene/cyclohexane mixture through benzoyl chitosan membranes. J. Membr. Sci. 1998, 138, 67–75. [Google Scholar]
- Castro-Muñoz, R.; Msahel, A.; Galiano, F.; Serocki, M.; Ryl, J.; Hamouda, S.B.; Hafiane, A.; Boczkaj, G.; Figoli, A. Towards azeotropic MEOH-MTBE separation using pervaporation chitosan-based deep eutectic solvent membranes. Sep. Purif. Technol. 2022, 281, 119979. [Google Scholar]
- Galiano, F.; Msahel, A.; Russo, F.; Rovella, N.; Policicchio, A.; Hamouda, S.B.; Hafiane, A.; Castro-Muñoz, R.; Figoli, A. Enhancing the separation performance of chitosan membranes through the blending with deep eutectic solvents for the pervaporation of polar/non-polar organic mixtures. Membranes 2024, 14, 237. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Wei, Z. A review of water management in polymer electrolyte membrane fuel cells. Energies 2009, 2, 1057–1106. [Google Scholar] [CrossRef]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of chitosan-based polymers as proton exchange membranes and roles of chitosan-supported ionic liquids. Int. J. Mol. Sci. 2020, 21, 632. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Ochoa, N.A.; Takara, E.A.; Mollá, S.; Compañ, V. Influence of pectin as a green polymer electrolyte on the transport properties of chitosan-pectin membranes. Carbohydr. Polym. 2017, 157, 1759–1768. [Google Scholar] [PubMed]
- Ranjani, M.; Pannipara, M.; Al-Sehemi, A.G.; Vignesh, A.; Kumar, G.G. Chitosan/sulfonated graphene oxide/silica nanocomposite membranes for direct methanol fuel cells. Solid State Ion. 2019, 338, 153–160. [Google Scholar] [CrossRef]
- Shen, S.; Jia, T.; Jia, J.; Wang, N.; Song, D.; Zhao, J.; Jin, J.; Che, Q. Constructing anhydrous proton exchange membranes through alternate depositing graphene oxide and chitosan on sulfonated poly(vinylidenefluoride) or sulfonated poly(vinylidene fluoride-co-hexafluoropropylene) membranes. Eur. Polym. J. 2021, 142, 110160. [Google Scholar] [CrossRef]
- Jia, T.; Shen, S.; Zhao, J.; Jin, J.; Pan, B.; Duan, X.; Meng, C.; Che, Q. Ultrathin membranes formation via the layer by layer self-assembly of carbon nanotubes-based inorganics as high temperature proton exchange membranes. Int. J. Hydrogen Energy 2020, 45, 14517–14527. [Google Scholar] [CrossRef]
- Anu Karthi, A.K.S.; Cindrella, L. Self-humidifying novel chitosan-geopolymer hybrid membrane for fuel cell applications. Carbohydr. Polym. 2019, 223, 115073. [Google Scholar]
- Xiong, Y.; Liu, Q.L.; Zhang, Q.G.; Zhu, A.M. Synthesis and characterization of cross-linked quaternized poly(vinyl alcohol)/chitosan composite anion exchange membranes for fuel cells. J. Power Sources 2008, 183, 447–453. [Google Scholar] [CrossRef]
- Wan, Y.; Peppley, B.; Creber, K.A.M.; Tam Bui, V.; Halliop, E. Quaternized-chitosan membranes for possible applications in alkaline fuel cells. J. Power Sources 2008, 185, 183–187. [Google Scholar] [CrossRef]
- Hari Gopi, K.; Dhavale, V.M.; Bhat, S.D. Development of polyvinyl alcohol/chitosan blend anion exchange membrane with mono and di quaternizing agents for application in alkaline polymer electrolyte fuel cells. Mater. Sci. Energy Technol. 2019, 2, 194–202. [Google Scholar] [CrossRef]
- Ryu, J.; Seo, J.Y.; Choi, B.N.; Kim, W.J.; Chung, C.H. Quaternized chitosan-based anion exchange membrane for alkaline direct methanol fuel cells. J. Ind. Eng. Chem. 2019, 73, 254–259. [Google Scholar] [CrossRef]
- Khaing, M.M.; Chen, N.; Long, C.; Li, Y.; Wang, F.; Zhu, H. Chitosan-modified poly(2,6-dimethyl-1,4-phenylene oxide) for anion-exchange membrane in fuel cell technology. Polym.-Plast. Technol. Eng. 2018, 57, 1121–1130. [Google Scholar] [CrossRef]
- Zhou, T.; Cai, L.; Qiao, J. Application of a novel pub enhanced semi-interpenetrating chitosan-based anion exchange membrane. Int. J. Energy Res. 2020, 44, 1607–1623. [Google Scholar] [CrossRef]
- Han, X.; Zheng, X.Y.; Song, S.; Wang, J.; Wang, L. Schiff base functionalized chitosan anion exchange membranes with 1,4-dichlorobutane as the crosslinker. J. Mol. Struct. 2019, 1195, 807–814. [Google Scholar]
- Zheng, X.Y.; Shang, C.S.; Yang, J.R.; Wang, J.L.; Wang, L. Preparation and characterization of chitosan-crown ether membranes for alkaline fuel cells. Synth. Met. 2019, 247, 109–115. [Google Scholar]
- Shaari, N.; Kamarudin, S.K. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Zhao, S.; Tsen, W.C.; Gong, C. 3D nanoflower-like layered double hydroxide modified quaternized chitosan/polyvinyl alcohol composite anion conductive membranes for fuel cells. Carbohydr. Polym. 2021, 256, 117439. [Google Scholar] [PubMed]
- Jiang, X.; Sun, Y.; Zhang, H.; Hou, L. Preparation and characterization of quaternized poly(vinyl alcohol)/chitosan/MoS2 composite anion exchange membranes with high selectivity. Carbohydr. Polym. 2018, 180, 96–103. [Google Scholar]
- Gong, C.; Zhao, S.; Tsen, W.C.; Hu, F.; Zhong, F.; Zhang, B.; Liu, H.; Zheng, G.; Qin, C.; Wen, S. Hierarchical layered double hydroxide coated carbon nanotube modified quaternized chitosan/polyvinyl alcohol for alkaline direct methanol fuel cells. J. Power Sources 2019, 441, 227176. [Google Scholar] [CrossRef]
- Jang, S.C.; Tsen, W.C.; Chuang, F.S.; Gong, C. Simultaneously enhanced hydroxide conductivity and mechanical properties of quaternized chitosan/functionalized carbon nanotubes composite anion exchange membranes. Int. J. Hydrogen Energy 2019, 44, 18134–18144. [Google Scholar] [CrossRef]
- Kaker, B.; Hribernik, S.; Mohan, T.; Kargl, R.; Stana Kleinschek, K.; Pavlica, E.; Kreta, A.; Bratina, G.; Lue, S.J.; Božič, M. Novel chitosan-mg(oh)2-based nanocomposite membranes for direct alkaline ethanol fuel cells. ACS Sustain. Chem. Eng. 2019, 7, 19356–19368. [Google Scholar]
- Hu, Y.; Tsen, W.C.; Chuang, F.S.; Jang, S.C.; Zhang, B.; Zheng, G.; Wen, S.; Liu, H.; Qin, C.; Gong, C. Glycine betaine intercalated layered double hydroxide modified quaternized chitosan/polyvinyl alcohol composite membranes for alkaline direct methanol fuel cells. Carbohydr. Polym. 2019, 213, 320–328. [Google Scholar] [CrossRef]
- Almenara, N.; Gueret, R.; Huertas-Alonso, A.J.; Veettil, U.T.; Sipponen, M.H.; Lizundia, E. Lignin–chitosan gel polymer electrolytes for stable zn electrodeposition. ACS Sustain. Chem. Eng. 2023, 11, 2283–2294. [Google Scholar]
- Kusumastuti, E.; Siniwi, W.T.; Mahatmanti, F.W.; Jumaeri; Atmaja, L.; Widiastuti, N. Modification of chitosan membranes with nanosilica particles as polymer electrolyte membranes. AIP Conf. Proc. 2016, 1725, 020037. [Google Scholar]
- Sapna; Sharma, R.; Kumar, D. Chitosan-based membranes for wastewater desalination and heavy metal detoxification. In Micro and Nano Technologies; Thomas, S., Pasquini, D., Leu, S.Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 799–814. [Google Scholar]



| Name | Chemical Structure |
|---|---|
| Glyoxal | 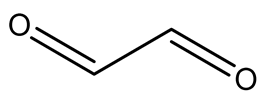 |
| Tetraethyl orthosilicate | 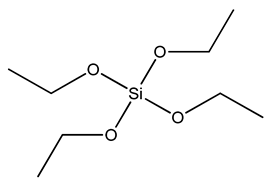 |
| Glutaraldehyde | 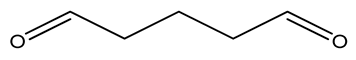 |
| Epichlorohydrin |  |
| N,O-Carboxymethyl chitosan | 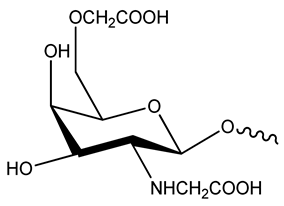 |
| Poly(acrylic acid) | 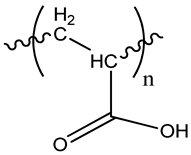 |
| Diethyleneglycol diglycidyl ether |  |
| 2-formyl benzene sulfonate polysiloxane(SBAPTS) | 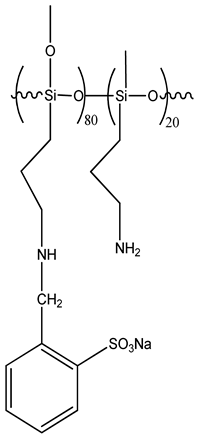 |
| Benzoylchitosan |  |
| Methanol | Feed (wt%) | 0 | 10 | 30 | 50 | 70 | 90 | 100 | |
| Rate of Permeation (103 kg/m2h) | 18.6 | 15.0 | 16.0 | 9.4 | 6.8 | 4.3 | 2.4 | ||
| - | 0.7 | 1 | 1 | 2 | 2 | - | |||
| Ethanol | Feed (wt%) | 0 | 10 | 30 | 50 | 70 | 90 | 96.5 a | 100 |
| Rate of Permeation (103 kg/m2h) | 186.0 | 150.0 | 136.0 | 67.1 | 34.6 | 12.3 | 6.5 a | 2.9 | |
| - | 0.7 | 2 | 13 | 50 | 31 | 17 a | - | ||
| 1-Propanol | Feed (wt%) | 0 | 10 | 30 | 50 | 71.8 a | 90 | 100 | |
| Rate of Permeation (103 kg/m2h) | 337.4 | 111.0 | 141.7 | 106.2 | 50.5 a | 8.2 | 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikita, K.; Vijayakumar, V.; Nam, S.Y. Chitosan-Based Membranes: A Comprehensive Review of Nanofiltration, Pervaporation, and Ion Exchange Applications. Polysaccharides 2025, 6, 31. https://doi.org/10.3390/polysaccharides6020031
Nikita K, Vijayakumar V, Nam SY. Chitosan-Based Membranes: A Comprehensive Review of Nanofiltration, Pervaporation, and Ion Exchange Applications. Polysaccharides. 2025; 6(2):31. https://doi.org/10.3390/polysaccharides6020031
Chicago/Turabian StyleNikita, Km, Vijayalekshmi Vijayakumar, and Sang Yong Nam. 2025. "Chitosan-Based Membranes: A Comprehensive Review of Nanofiltration, Pervaporation, and Ion Exchange Applications" Polysaccharides 6, no. 2: 31. https://doi.org/10.3390/polysaccharides6020031
APA StyleNikita, K., Vijayakumar, V., & Nam, S. Y. (2025). Chitosan-Based Membranes: A Comprehensive Review of Nanofiltration, Pervaporation, and Ion Exchange Applications. Polysaccharides, 6(2), 31. https://doi.org/10.3390/polysaccharides6020031






