The Effect of Alginate and κ-Carrageenan on the Stability of Pickering Emulsions Stabilized by Shellac-Based Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Nanoparticle Preparation
2.3. Nanoparticle Characterization
2.4. Emulsion Preparation
2.5. Emulsion Characterization
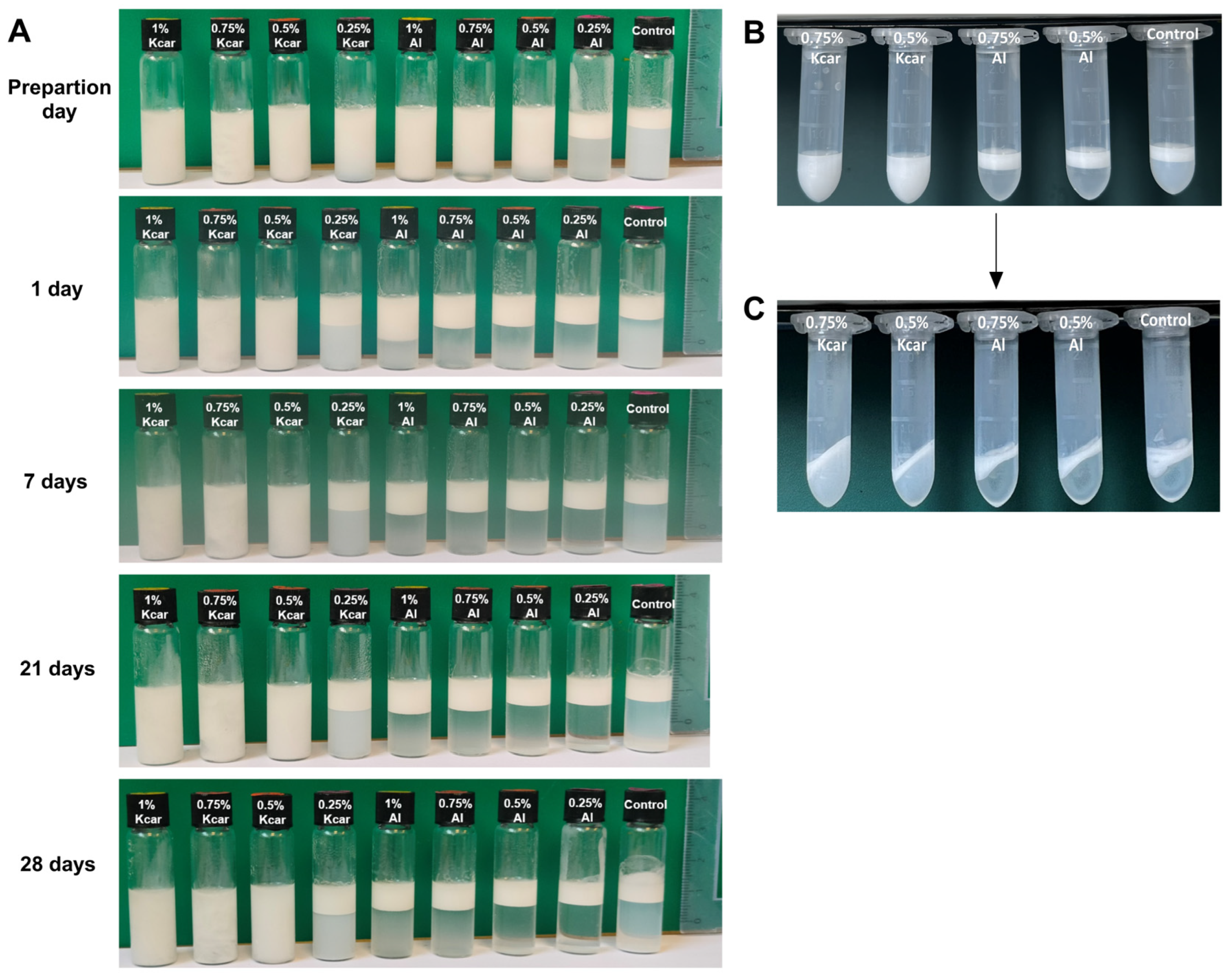
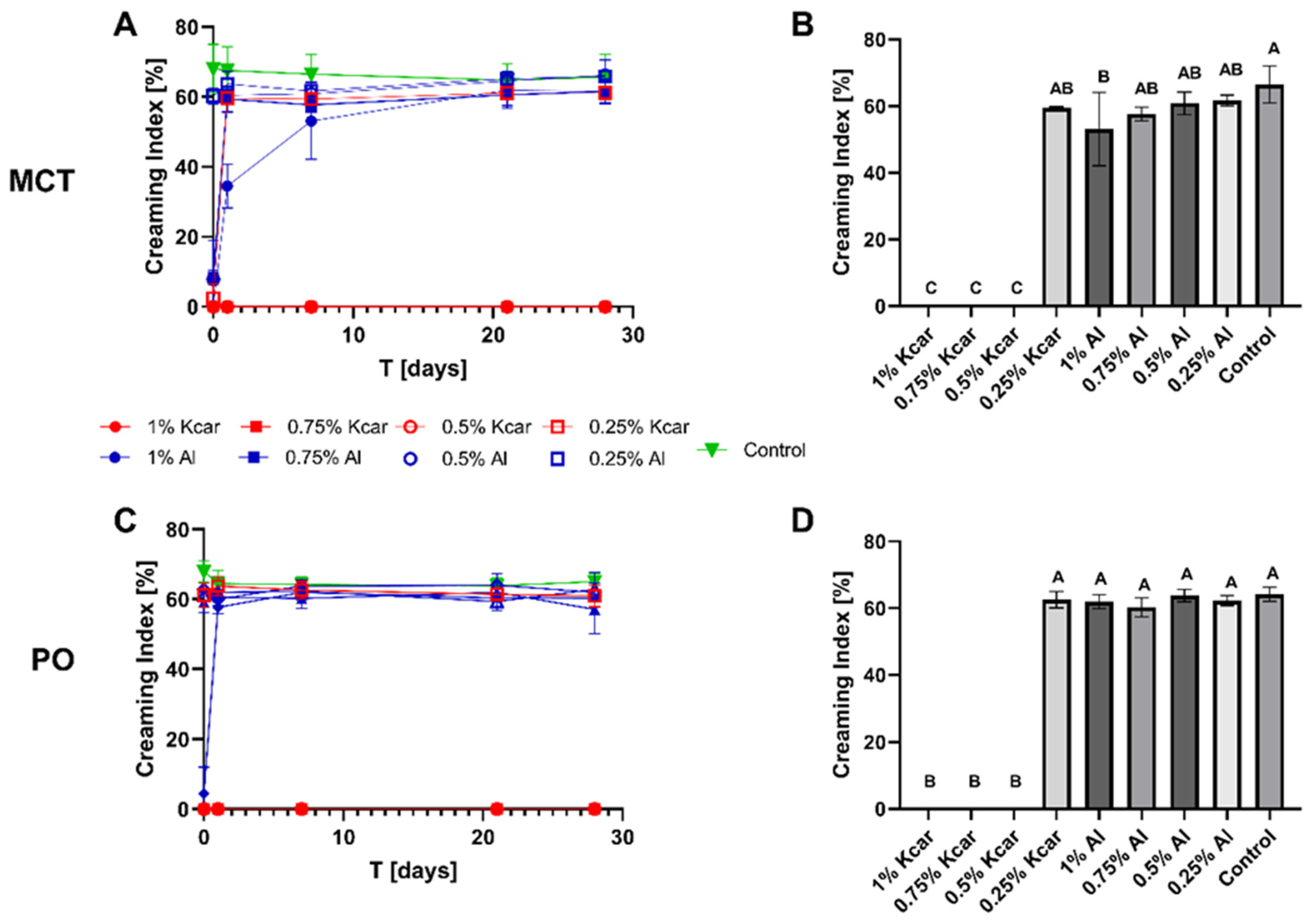
2.5.1. Emulsion Stability
Long-Term Emulsion Appearance
Accelerated Stability Testing
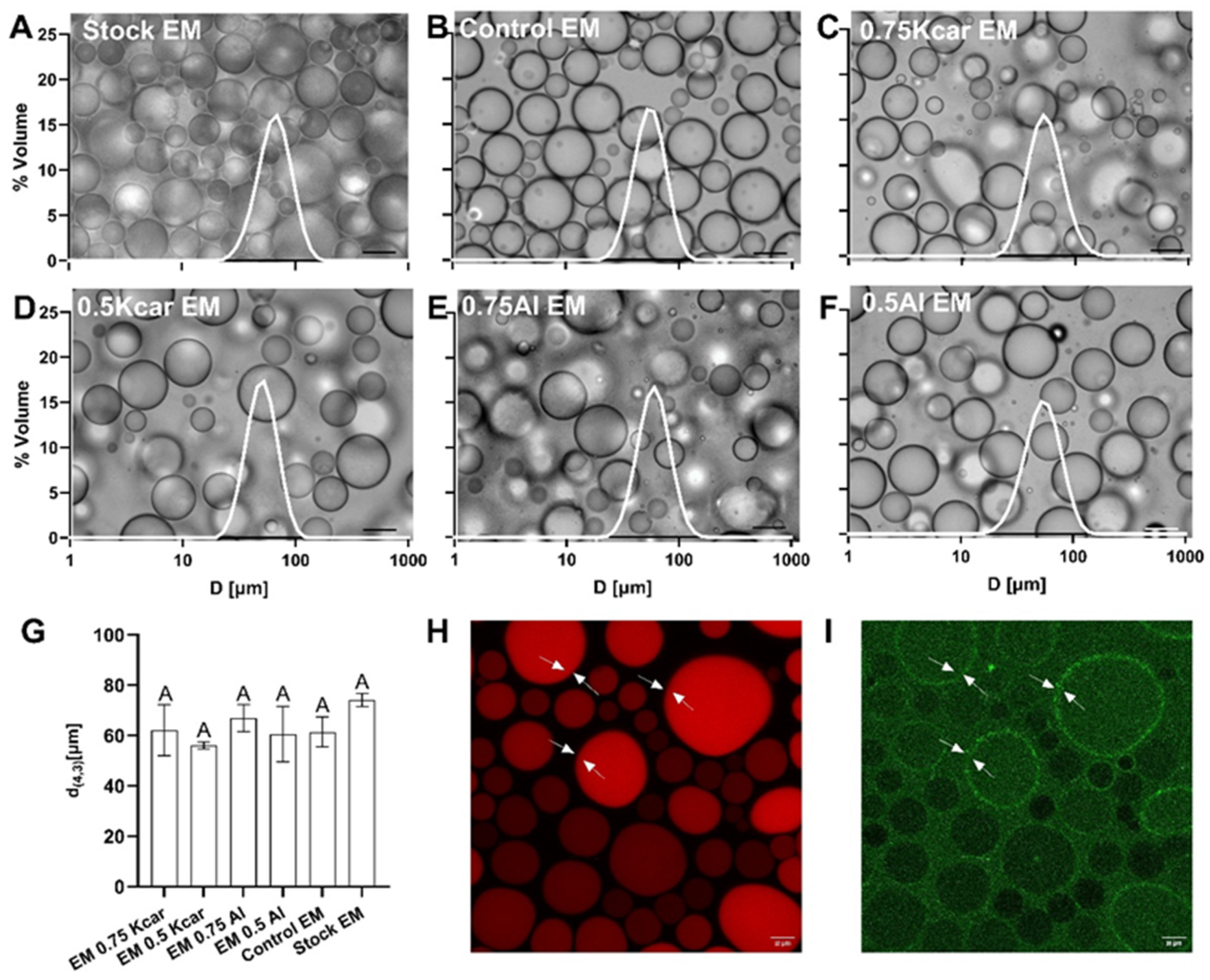
2.5.2. Emulsion Microstructure
Emulsion Droplet Size Analysis
Optical Microscopy
Fluorescent Microscopy
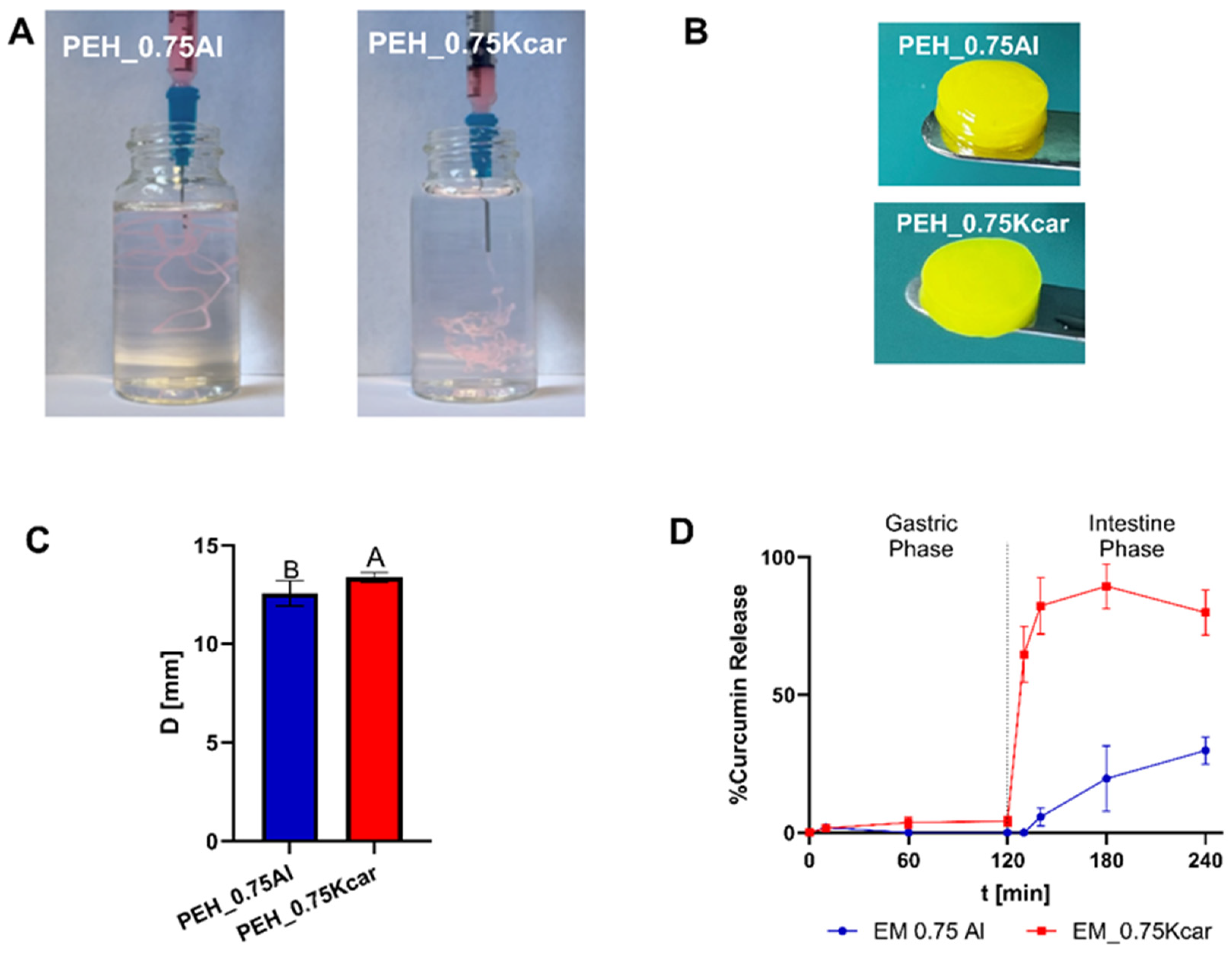
2.6. Pickering Emulsion Hydrogel
2.6.1. Preparation of the Pickering Emulsion Hydrogel
2.6.2. Digestion Experiments
Curcumin Release Measurements
2.7. Polymer–Nanoparticle Interactions
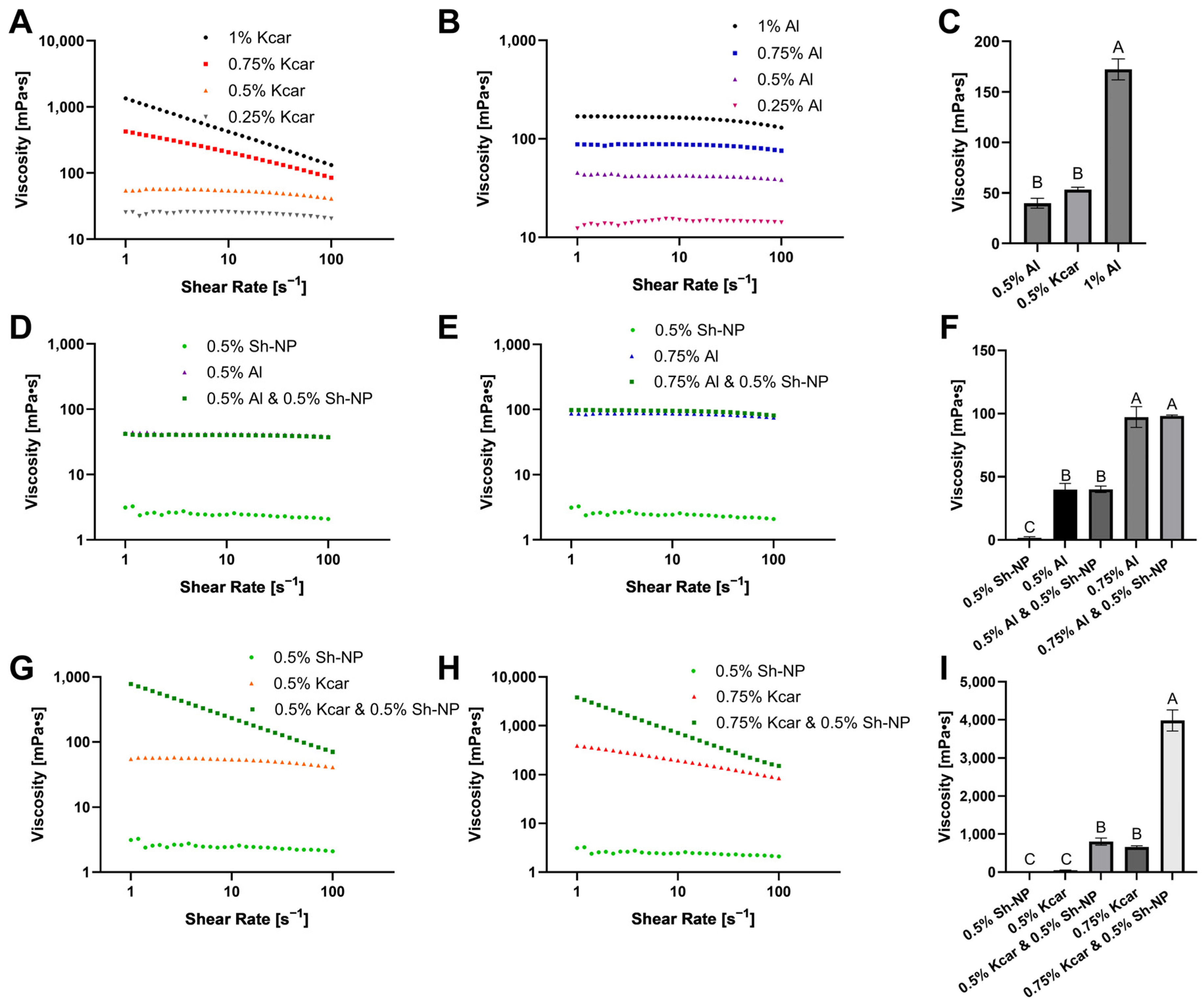
2.7.1. Rheology
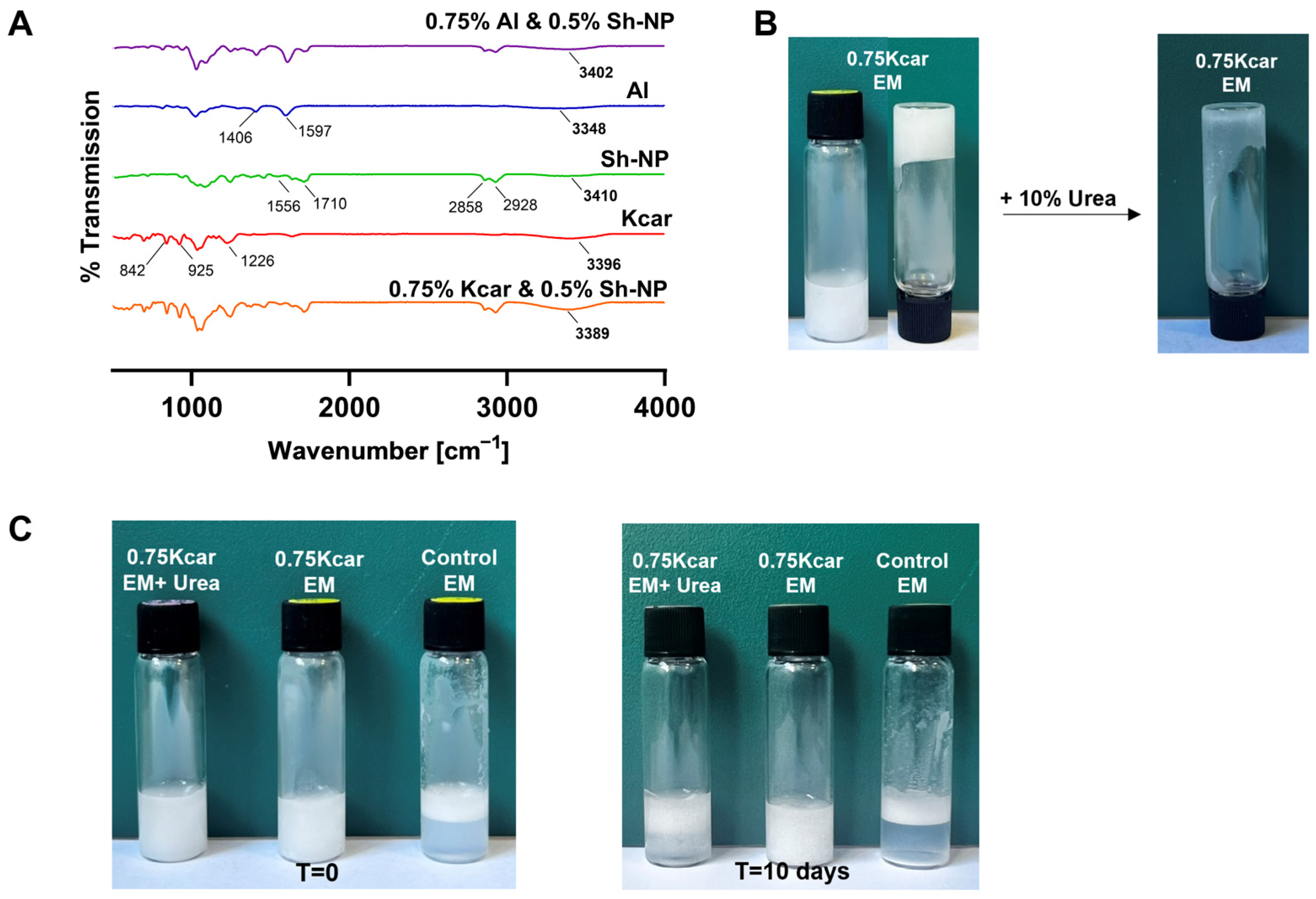
2.7.2. Fourier Transform Infrared Spectroscopy Measurements
2.7.3. Small Angle X-Ray Scattering
2.8. Statistical Analysis
3. Results and Discussion
3.1. Stability of Shellac-Based Pickering Emulsion Following Polysaccharide Addition
3.2. Pickering Emulsion Hydrogels for Curcumin Release
3.3. The Effect of Added Polysaccharide on Shellac-Based Pickering Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Benalaya, I.; Alves, G.; Lopes, J.; Silva, L.R. A Review of Natural Polysaccharides: Sources, Characteristics, Properties, Food, and Pharmaceutical Applications. Int. J. Mol. Sci. 2024, 25, 1322. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef] [PubMed]
- Tzoumaki, M.V.; Moschakis, T.; Scholten, E.; Biliaderis, C.G. In vitro lipid digestion of chitin nanocrystal stabilized o/w emulsions. Food Funct. 2012, 4, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, C.; Zou, S.; Wei, Z.; Tong, Z. Simple, reversible emulsion system switched by pH on the basis of chitosan without any hydrophobic modification. Langmuir 2012, 28, 11017–11024. [Google Scholar] [CrossRef]
- Li, C.; Sun, P.; Yang, C. Emulsion stabilized by starch nanocrystals. Starch/Staerke 2012, 64, 497–502. [Google Scholar] [CrossRef]
- Li, Q.; Xie, B.; Wang, Y.; Wang, Y.; Peng, L.; Li, Y.; Li, B.; Liu, S. Cellulose nanofibrils from Miscanthus floridulus straw as green particle emulsifier for O/W Pickering emulsion. Food Hydrocoll. 2019, 97, 105214. [Google Scholar] [CrossRef]
- Kargar, M.; Fayazmanesh, K.; Alavi, M.; Spyropoulos, F.; Norton, I.T. Investigation into the potential ability of Pickering emulsions (food-grade particles) to enhance the oxidative stability of oil-in-water emulsions. J. Colloid Interface Sci. 2012, 366, 209–215. [Google Scholar] [CrossRef]
- Hernandez-Rodriguez, G.; Tenorio-Garcia, E.; Ettelaie, R.; Lishchuk, S.V.; Harbottle, D.; Murray, B.S.; Sarkar, A. Demulsification of Pickering emulsions: Advances in understanding mechanisms to applications. Soft Matter 2024, 20, 7344–7356. [Google Scholar] [CrossRef]
- Cheon, J.; Haji, F.; Baek, J.; Wang, Q.; Tam, K.C. Pickering emulsions for functional food systems. J. Agric. Food Res. 2023, 11, 100510. [Google Scholar] [CrossRef]
- Chen, L.; Ao, F.; Ge, X.; Shen, W. Food-Grade Pickering Emulsions: Preparation, Stabilization and Applications. Molecules 2020, 25, 3202. [Google Scholar] [CrossRef]
- Mohammadi, A.; Kashi, P.A.; Kashiri, M.; Bagheri, A.; Chen, J.; Ettelaie, R.; Jäger, H.; Shahbazi, M. Self-assembly of plant polyphenols-grafted soy proteins to manufacture a highly stable antioxidative Pickering emulsion gel for direct-ink-write 3D printing. Food Hydrocoll. 2023, 142, 108851. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Farag, Y.; Leopold, C.S. Physicochemical Properties of Various Shellac Types. Dissolution Technol. 2009, 16, 33–39. [Google Scholar] [CrossRef]
- Hagenmaier, R.D.; Shaw, P.E. Permeability of Shellac Coatings to Gases and Water Vapor. J. Agric. Food Chem. 1991, 39, 825–829. [Google Scholar] [CrossRef]
- Delmar, K.; Bianco-Peled, H. Shellac-Based Nanoparticles Provide Highly Stable Pickering Emulsions. Int. J. Biol. Macromol. 2025, 307, 141941. [Google Scholar] [CrossRef]
- McClements, D.J. Critical Review of Techniques and Methodologies for Characterization of Emulsion Stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, S.; Guan, X.; McClements, D.J.; Liu, X.; Liu, F.; Ngai, T. Polysaccharide-based Pickering emulsions: Formation, stabilization and applications. Food Hydrocoll. 2021, 119, 106812. [Google Scholar] [CrossRef]
- Pi, G.; Li, Y.; Bao, M.; Mao, L.; Gong, H.; Wang, Z. Novel and Environmentally Friendly Oil Spill Dispersant Based on the Synergy of Biopolymer Xanthan Gum and Silica Nanoparticles. ACS Sustain. Chem. Eng. 2016, 4, 3095–3102. [Google Scholar] [CrossRef]
- Sriprablom, J.; Suphantharika, M.; Sriprablom Jiratthitikan, J. Influence of xanthan gum on properties and stability of oil-in-water Pickering emulsions stabilized by zein colloidal particles. J. Food Meas. Charact. 2022, 16, 2772–2781. [Google Scholar] [CrossRef]
- Calero, N.; Muñoz, J.; Cox, P.W.; Heuer, A.; Guerrero, A. Influence of chitosan concentration on the stability, microstructure and rheological properties of O/W emulsions formulated with high-oleic sunflower oil and potato protein. Food Hydrocoll. 2013, 30, 152–162. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, D.; Xie, F. The role of alginate in starch nanocrystals-stabilized Pickering emulsions: From physical stability and microstructure to rheology behavior. Food Chem. 2024, 431, 137017. [Google Scholar] [CrossRef] [PubMed]
- Li, X.M.; Meng, R.; Xu, B.C.; Zhang, B. Investigation of the fabrication, characterization, protective effect and digestive mechanism of a novel Pickering emulsion gels. Food Hydrocoll. 2021, 117, 106708. [Google Scholar] [CrossRef]
- Ren, Z.; Huang, X.; Zhao, Y.; Shi, L.; Yang, S.; Jin, R.; Lin, R.; Liu, S.; Liu, Z.; Zhang, Y.; et al. Novel Pickering emulsions using polysaccharide-myosin complexes: Effect of polysaccharide types. Food Hydrocoll. 2024, 157, 110469. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Schmidt, K.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Pectins of different origin and their performance in forming and stabilizing oil-in-water-emulsions. Food Hydrocoll. 2015, 46, 59–66. [Google Scholar] [CrossRef]
- Lim, H.P.; Karandagaspitiya, C.O.; Chan, D.K.H.; Low, L.E.; Tey, B.T.; Chan, E.S. Pickering emulsion ink in additive manufacturing: A state-of-the-art review. Addit. Manuf. 2023, 73, 103677. [Google Scholar] [CrossRef]
- Lim, H.P.; Ho, K.W.; Surjit Singh, C.K.; Ooi, C.W.; Tey, B.T.; Chan, E.S. Pickering emulsion hydrogel as a promising food delivery system: Synergistic effects of chitosan Pickering emulsifier and alginate matrix on hydrogel stability and emulsion delivery. Food Hydrocoll. 2020, 103, 105659. [Google Scholar] [CrossRef]
- Machour, M.; Hen, N.; Goldfracht, I.; Safina, D.; Davidovich-Pinhas, M.; Bianco-Peled, H.; Levenberg, S. Print-and-Grow within a Novel Support Material for 3D Bioprinting and Post-Printing Tissue Growth. Adv. Sci. 2022, 9, 2200882. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, H.; Wang, J.; Wang, J.; Yan, L.; Liu, C.; Zheng, L. Pickering emulsion hydrogel based on alginate-gellan gum with carboxymethyl chitosan as a pH-responsive controlled release delivery system. Int. J. Biol. Macromol. 2022, 216, 850–859. [Google Scholar] [CrossRef]
- Wang, K.; Ni, J.; Tian, X.; Xiang, S.; Li, H.; Shang, W.; Liu, B.; Tan, M.; Su, W. Survivability of probiotics in Pickering emulsion gels stabilized by salmon by-product protein/sodium alginate soluble complexes at neutral pH. Int. J. Biol. Macromol. 2024, 255, 128190. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, C.; Zhang, J.; Li, Y.; Li, B.; Liu, S. Crosslinking Alginate at Water-in-Water Pickering Emulsions Interface to Control the Interface Structure and Enhance the Stress Resistance of the Encapsulated Probiotics. J. Colloid Interface Sci. 2024, 655, 653–663. [Google Scholar] [CrossRef]
- Yan, H.; Chen, X.; Feng, M.; Shi, Z.; Zhang, W.; Wang, Y.; Ke, C.; Lin, Q. Entrapment of bacterial cellulose nanocrystals stabilized Pickering emulsions droplets in alginate beads for hydrophobic drug delivery. Colloids Surf. B Biointerfaces 2019, 177, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.-X.; Lei, Y.-C.; Wang, Y.; Li, D.; Wang, L.-J. Rheological and structural properties of sodium caseinate as influenced by locust bean gum and κ-carrageenan. Food Hydrocoll. 2021, 112, 106251. [Google Scholar] [CrossRef]
- Ganesan, K.; Ratke, L. Facile preparation of monolithic κ-carrageenan aerogels. Soft Matter 2014, 10, 3218–3224. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, J.J.; Kurukji, D.; Norton, I.T.; Spyropoulos, F. Investigation of the fabrication and subsequent emulsifying capacity of potato protein isolate/κ-carrageenan electrostatic complexes. Food Hydrocoll. 2017, 71, 282–289. [Google Scholar] [CrossRef]
- Zhang, X.; Ning, Y.; Chai, L.; Yin, Y.; Luo, D.; Xu, W. Physicochemical properties and in vitro digestive behavior of astaxanthin loaded Pickering emulsion gel regulated by konjac glucomannan and κ-carrageenan. Int. J. Biol. Macromol. 2024, 278, 134710. [Google Scholar] [CrossRef]
- Hegde, M.; Girisa, S.; BharathwajChetty, B.; Vishwa, R.; Kunnumakkara, A.B. Curcumin Formulations for Better Bioavailability: What We Learned from Clinical Trials Thus Far? ACS Omega 2023, 8, 10713–10746. [Google Scholar] [CrossRef]
- Renard, D.; Van De Velde, F.; Visschers, R.W. The gap between food gel structure, texture and perception. Food Hydrocoll. 2006, 20, 423–431. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, R.; Han, L.; Zhang, T.; Ngai, T. Pickering emulsions stabilized by aminated gelatin nanoparticles: Are gelatin nanoparticles acting as genuine Pickering stabilizers or structuring agents? Food Hydrocoll. 2022, 123, 107151. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Shani-Levi, C.; Levi-Tal, S.; Lesmes, U. Comparative performance of milk proteins and their emulsions under dynamic in vitro adult and infant gastric digestion. Food Hydrocoll. 2013, 32, 349–357. [Google Scholar] [CrossRef]
- Shani Levi, C.; Goldstein, N.; Portmann, R.; Lesmes, U. Emulsion and protein degradation in the elderly: Qualitative insights from a study coupling a dynamic in vitro digestion model with proteomic analyses. Food Hydrocoll. 2017, 69, 393–401. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Le Feunteun, S.; Sarkar, A.; Grundy, M.M.L.; Carrière, F.; et al. A standardised semi-dynamic in vitro digestion method suitable for food—An international consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Chen, F.; Gao, C.; Zhang, Y.; Tang, X. Environmental stability and curcumin release properties of Pickering emulsion stabilized by chitosan/gum arabic nanoparticles. Int. J. Biol. Macromol. 2020, 157, 202–211. [Google Scholar] [CrossRef]
- Josef, E.; Bianco-Peled, H. Conformation of a natural polyelectrolyte in semidilute solutions with no added salt. Soft Matter 2012, 8, 9156–9165. [Google Scholar] [CrossRef]
- Kratky, O.G.O. Small Angle X-Ray Scattering; Academic Press: New York, NY, USA, 1982. [Google Scholar]
- Chen, W.R.; Butler, P.D.; Magid, L.J. Incorporating intermicellar interactions in the fitting of SANS data from cationic wormlike micelles. Langmuir 2006, 22, 6539–6548. [Google Scholar] [CrossRef]
- Pedersen, J.S.; Schurtenberger, P. Scattering Functions of Semiflexible Polymers with and without Excluded Volume Effects. Macromolecules 1996, 29, 7602–7612. [Google Scholar] [CrossRef]
- Shimode, M.; Mimura, M.; Urakawa, H.; Yamanaka, S.; Kajiwara, K. Interaction between the dyestuff aggregates in aqueous solution. Sen’i Gakkaishi 1996, 52, 301–309. Available online: https://www.jstage.jst.go.jp/article/fiber1944/52/6/52_6_301/_article/-char/ja/ (accessed on 17 September 2024). [CrossRef]
- Yuguchi, Y.; Mimura, M.; Urakawa, H.; Kitamura, S.; Ohno, S.; Kajiwara, K. Small angle X-ray characterization of gellan gum containing a high content of sodium in aqueous solution. Carbohydr. Polym. 1996, 30, 83–93. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Bizot, H.; Cathala, B.; Capron, I. New pickering emulsions stabilized by bacterial cellulose nanocrystals. Langmuir 2011, 27, 7471–7479. [Google Scholar] [CrossRef]
- Wang, J.; Deng, H.; Sun, Y.; Yang, C. Montmorillonite and alginate co-stabilized biocompatible Pickering emulsions with multiple-stimulus tunable rheology. J. Colloid Interface Sci. 2020, 562, 529–539. [Google Scholar] [CrossRef]
- Zhang, B.; Meng, R.; Li, X.L.; Liu, W.J.; Cheng, J.S.; Wang, W. Preparation of Pickering emulsion gels based on κ-carrageenan and covalent crosslinking with EDC: Gelation mechanism and bioaccessibility of curcumin. Food Chem. 2021, 357, 129726. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Sun, H.; Niu, Z.; Xu, W.; Li, Z.; He, Y.; Luo, D.; Xi, W.; Wei, J.; Zhang, C. Carrageenan-Based Pickering Emulsion Gels Stabilized by Xanthan Gum/Lysozyme Nanoparticle: Microstructure, Rheological, and Texture Perspective. Foods 2022, 11, 3757. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Sun, N.; Kumar, P.; Li, Q.; Liu, B.; Li, A.; Wang, W.; Gao, Z. Real-Time Analysis of the Stability of Oil-In-Water Pickering Emulsion by Electrochemical Impedance Spectroscopy. Molecules 2020, 25, 2904. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiao, B.; Li, S.; Faisal, S.; Shi, A.; Fu, W.; Chen, Y.; Wang, Q. Recent Advances on Pickering Emulsions Stabilized by Diverse Edible Particles: Stability Mechanism and Applications. Front. Nutr. 2022, 9, 864943. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Li, Y.; Li, H.; Li, L.; Xia, Q. Encapsulation of resveratrol-loaded Pickering emulsions in alginate/pectin hydrogel beads: Improved stability and modification of digestive behavior in the gastrointestinal tract. Int. J. Biol. Macromol. 2022, 222, 337–347. [Google Scholar] [CrossRef]
- Soukoulis, C.; Fisk, I.D.; Bohn, T.; Hoffmann, L. Study of intragastric structuring ability of sodium alginate based o/w emulsions under in vitro physiological pre-absorptive digestion conditions. Carbohydr. Polym. 2016, 140, 26–34. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Stokke, B.T. Similarities and differences between alginic acid gels and ionically crosslinked alginate gels. Food Hydrocoll. 2006, 20, 170–175. [Google Scholar] [CrossRef]
- Gurikov, P.; Smirnova, I. Non-Conventional Methods for Gelation of Alginate. Gels 2018, 4, 14. [Google Scholar] [CrossRef]
- Lin, D.; Kelly, A.L.; Miao, S. The impact of pH on mechanical properties, storage stability and digestion of alginate-based and soy protein isolate-stabilized emulsion gel beads with encapsulated lycopene. Food Chem. 2022, 372, 131262. [Google Scholar] [CrossRef]
- Wu, B.; Li, Y.; Li, Y.; Li, H.; Xia, Q. The influence of Ca2+/K+ weight ratio on the physicochemical properties and in vitro digestion behavior of resveratrol-loaded Pickering emulsions encapsulated in alginate/κ-carrageenan hydrogel beads. React. Funct. Polym. 2022, 181, 105414. [Google Scholar] [CrossRef]
- Olímpio, F.M.P.; Mendes, A.A.; Trevisan, M.G.; Garcia, J.S. Preparation and Delayed Release Study on Pancreatin Encapsulated into Alginate, Carrageenan and Pectin Hydrogels. J. Braz. Chem. Soc. 2020, 31, 320–330. [Google Scholar] [CrossRef]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa-Carrageenan Hydrogels for Tissue Engineering Applications. Adv. Healthc. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Ferreira, L.; Gil, A.M.; Trindade, T. Synthesis and swelling behavior of temperature responsive κ-carrageenan nanogels. J. Colloid Interface Sci. 2011, 355, 512–517. [Google Scholar] [CrossRef]
- Tu, X.; Ren, H.; Bu, S. Therapeutic effects of curcumin on constipation-predominant irritable bowel syndrome is associated with modulating gut microbiota and neurotransmitters. Front. Microbiol. 2023, 14, 1274559. [Google Scholar] [CrossRef]
- Deng, W.; Xiong, X.; Lu, M.; Huang, S.; Luo, Y.; Wang, Y.; Ying, Y. Curcumin suppresses colorectal tumorigenesis through restoring the gut microbiota and metabolites. BMC Cancer 2024, 24, 1141. [Google Scholar] [CrossRef]
- Maderuelo, C.; Lanao, J.M.; Zarzuelo, A. Enteric coating of oral solid dosage forms as a tool to improve drug bioavailability. Eur. J. Pharm. Sci. 2019, 138, 105019. [Google Scholar] [CrossRef]
- Hua, S. Advances in Oral Drug Delivery for Regional Targeting in the Gastrointestinal Tract—Influence of Physiological, Pathophysiological and Pharmaceutical Factors. Front. Pharmacol. 2020, 11, 524. [Google Scholar] [CrossRef]
- Yue, M.; Huang, M.; Zhu, Z.; Huang, T.; Huang, M. Effect of ultrasound assisted emulsification in the production of Pickering emulsion formulated with chitosan self-assembled particles: Stability, macro, and micro rheological properties. LWT 2022, 154, 112595. [Google Scholar] [CrossRef]
- Avallone, P.R.; Russo Spena, S.; Acierno, S.; Esposito, M.G.; Sarrica, A.; Delmonte, M.; Pasquino, R.; Grizzuti, N. Thermorheological Behavior of κ-Carrageenan Hydrogels Modified with Xanthan Gum. Fluids 2023, 8, 119. [Google Scholar] [CrossRef]
- Morris, E.R.; Cutler, A.N.; Ross-Murphy, S.B.; Rees, D.A.; Price, J. Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydr. Polym. 1981, 1, 5–21. [Google Scholar] [CrossRef]
- Alexander, M.; Dalgleish, D.G. The interaction of casein micelles with κ-carrageenan studied by diffusing wave spectroscopy. Food Hydrocoll. 2007, 21, 128–136. [Google Scholar] [CrossRef]
- Yuan, C.; Sang, L.; Wang, Y.; Cui, B. Influence of cyclodextrins on the gel properties of kappa-carrageenan. Food Chem. 2018, 266, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.; Cölfen, H.; Antonietti, M. Iron oxyhydroxide colloids stabilized with polysaccharides. Colloid Polym. Sci. 2000, 278, 491–501. [Google Scholar] [CrossRef]
- Yan, J.N.; Du, Y.N.; Jiang, X.Y.; Han, J.R.; Pan, J.F.; Wu, H.T. Intermolecular interaction in the hybrid gel of scallop (Patinopecten yessoensis) male gonad hydrolysates and κ-carrageenan. J. Food Sci. 2021, 86, 792–802. [Google Scholar] [CrossRef]
- Peleg-Evron, O.; Davidovich-Pinhas, M.; Bianco-Peled, H. Crosslinking konjac-glucomannan with kappa-carrageenan nanogels: A step toward the design of sacrificial materials. Int. J. Biol. Macromol. 2023, 227, 654–663. [Google Scholar] [CrossRef]
- Yang, L.; Ge, J.; Wu, H.; Guo, H.; Shan, J.; Zhang, G. Phase behavior of colloidal nanoparticles and their enhancement effect on the rheological properties of polymer solutions and gels. RSC Adv. 2024, 14, 8513–8525. [Google Scholar] [CrossRef]
- Buitrago-Rincon, D.L.; Sadtler, V.; Mercado, R.A.; Roques-Carmes, T.; Benyahia, L.; Durand, A.; Ferji, K.; Marchal, P.; Pedraza-Avella, J.A.; Lemaitre, C. Effect of Silica Nanoparticles in Xanthan Gum Solutions: Evolution of Viscosity over Time. Nanomaterials 2022, 12, 1906. [Google Scholar] [CrossRef]
- Wang, J.; Chen, L.; He, Y. Preparation of environmental friendly coatings based on natural shellac modified by diamine and its applications for copper protection. Prog. Org. Coat. 2008, 62, 307–312. [Google Scholar] [CrossRef]
- Luangtana-anan, M.; Soradech, S.; Saengsod, S.; Nunthanid, J.; Limmatvapirat, S. Enhancement of Moisture Protective Properties and Stability of Pectin through Formation of a Composite Film: Effects of Shellac and Plasticizer. J. Food Sci. 2017, 82, 2915–2925. [Google Scholar] [CrossRef]
- Smith, B.C. The C=O bond, part III: Carboxylic acids. Spectroscopy 2018, 33, 14–20. [Google Scholar]
- Licchelli, M.; Malagodi, M.; Somaini, M.; Weththimuni, M.; Zanchi, C. Surface treatments of wood by chemically modified shellac. Surf. Eng. 2013, 29, 121–127. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between gelatin and sodium alginate: UV and FTIR studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Zhang, Y.; Man, J.; Li, J.; Xing, Z.; Zhao, B.; Ji, M.; Xia, H.; Li, J. Preparation of the alginate/carrageenan/shellac films reinforced with cellulose nanocrystals obtained from enteromorpha for food packaging. Int. J. Biol. Macromol. 2022, 218, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Huang, C.; Lai, H.; Li, K.; Zeng, X.; Wen, C.; Wu, C.; Pang, J. Effect of carboxylated cellulose nanocrystals on konjac glucomannan/κ-carrageenan composite hydrogels. Food Hydrocoll. 2024, 154, 110060. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Liu, B.; Zheng, M.; Chen, H.; Pang, J. Rheological behavior and molecular dynamics simulation of κ-carrageenan/casein under simulated gastrointestinal electrolyte conditions. Food Hydrocoll. 2023, 136, 108240. [Google Scholar] [CrossRef]
- Zou, Q.; Habermann-Rottinghaus, S.M.; Murphy, K.P. Urea effects on protein stability: Hydrogen bonding and the hydrophobic effect. Proteins Struct. Funct. Bioinform. 1998, 31, 107–115. [Google Scholar] [CrossRef]
- Nishinari, K.; Watase, M.; Williams, P.A.; Phillips, G.O. K-Carrageenan Gels: Effect of Sucrose, Glucose, Urea, and Guanidine Hydrochloride on the Rheological and Thermal Properties. J. Agric. Food Chem. 1990, 38, 1188–1193. [Google Scholar] [CrossRef]
- Yu, A.C.; Smith, A.A.A.; Appel, E.A. Structural considerations for physical hydrogels based on polymer–nanoparticle interactions. Mol. Syst. Des. Eng. 2020, 5, 401–407. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Greer, J.M.; Fenton, O.S.; Kreuels, K.; Anderson, D.G.; Langer, R. Exploiting Electrostatic Interactions in Polymer-Nanoparticle Hydrogels. ACS Macro Lett. 2015, 4, 848–852. [Google Scholar] [CrossRef]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-assembled hydrogels utilizing polymer–nanoparticle interactions. Nat. Commun. 2015, 6, 6295. [Google Scholar] [CrossRef]
- Wu, C.J.; Gaharwar, A.K.; Schexnailder, P.J.; Schmidt, G. Development of Biomedical Polymer-Silicate Nanocomposites: A Materials Science Perspective. Materials 2010, 3, 2986–3005. [Google Scholar] [CrossRef]
- Hilliou, L. Structure–Elastic Properties Relationships in Gelling Carrageenans. Polymers 2021, 13, 4120. [Google Scholar] [CrossRef] [PubMed]
- Yuguchi, Y.; Urakawa, H.; Kajiwara, K. Structural characteristics of carrageenan gels: Various types of counter ions. Food Hydrocoll. 2003, 17, 481–485. [Google Scholar] [CrossRef]
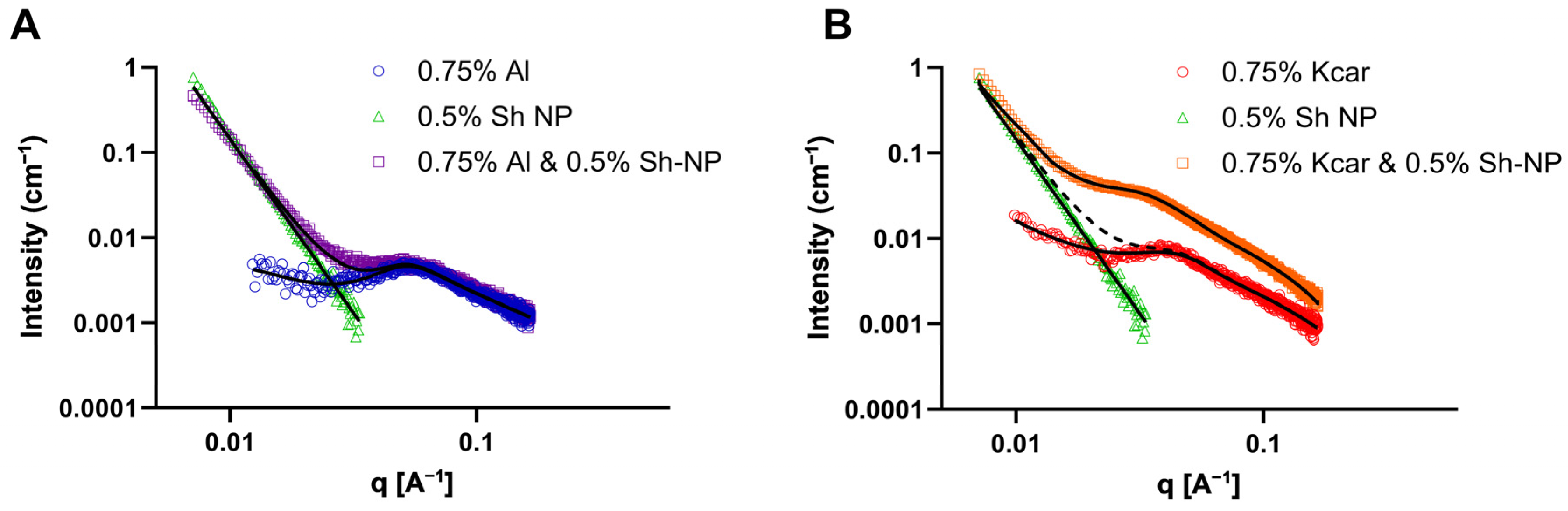
| Al | Kcar | Kcar and NP | |
|---|---|---|---|
| L (Å) | 1638 | 20073 | 20073 |
| b (Å) | 42.4 | 48.2 | 50.3 |
| R (Å) | 4.1 | 8.6 | 11.2 |
| Ce | 15.0 | 6.4 | 5.1 |
| ξe (Å) | 38.7 | 42.4 | 56.4 |
| R2 | 0.908 | 0.994 | 0.997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmar, K.; Kablan, R.; Amiram, G.; Shani Levi, C.; Lesmes, U.; Bianco-Peled, H. The Effect of Alginate and κ-Carrageenan on the Stability of Pickering Emulsions Stabilized by Shellac-Based Nanoparticles. Polysaccharides 2025, 6, 35. https://doi.org/10.3390/polysaccharides6020035
Delmar K, Kablan R, Amiram G, Shani Levi C, Lesmes U, Bianco-Peled H. The Effect of Alginate and κ-Carrageenan on the Stability of Pickering Emulsions Stabilized by Shellac-Based Nanoparticles. Polysaccharides. 2025; 6(2):35. https://doi.org/10.3390/polysaccharides6020035
Chicago/Turabian StyleDelmar, Keren, Reaam Kablan, Gabriela Amiram, Carmit Shani Levi, Uri Lesmes, and Havazelet Bianco-Peled. 2025. "The Effect of Alginate and κ-Carrageenan on the Stability of Pickering Emulsions Stabilized by Shellac-Based Nanoparticles" Polysaccharides 6, no. 2: 35. https://doi.org/10.3390/polysaccharides6020035
APA StyleDelmar, K., Kablan, R., Amiram, G., Shani Levi, C., Lesmes, U., & Bianco-Peled, H. (2025). The Effect of Alginate and κ-Carrageenan on the Stability of Pickering Emulsions Stabilized by Shellac-Based Nanoparticles. Polysaccharides, 6(2), 35. https://doi.org/10.3390/polysaccharides6020035






