Protein Catabolism and the Dysregulation of Energy Intake-Related Hormones May Play a Major Role in the Worsening of Malnutrition in Hospitalized Cirrhotic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anthropometric Measurements and Definition of Malnutrition
2.3. Muscle Function Evaluation
2.4. Nitrogen Balance Evaluation
2.5. Ghrelin and Leptin Assays
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Population
3.2. Nitrogen Balance
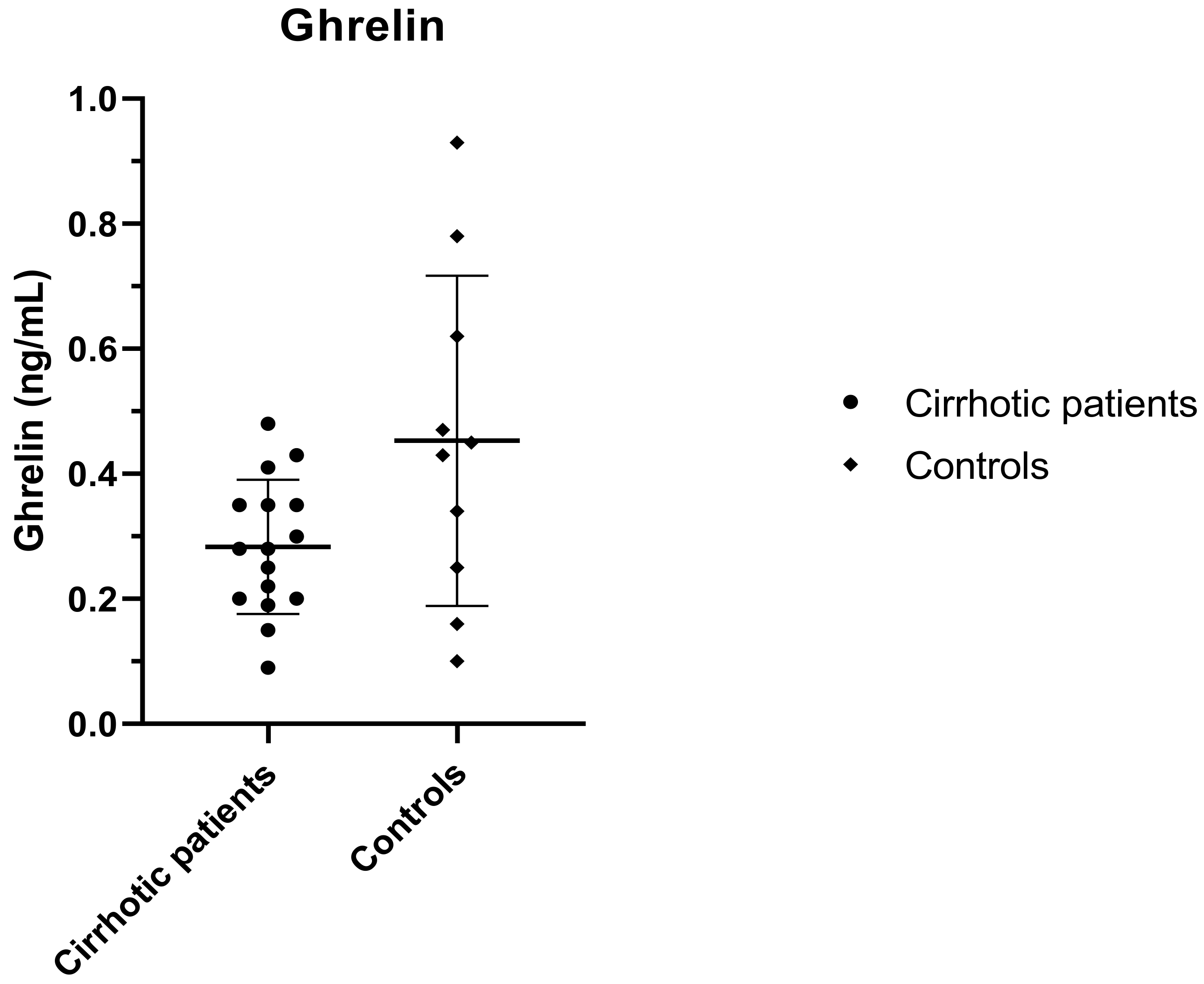
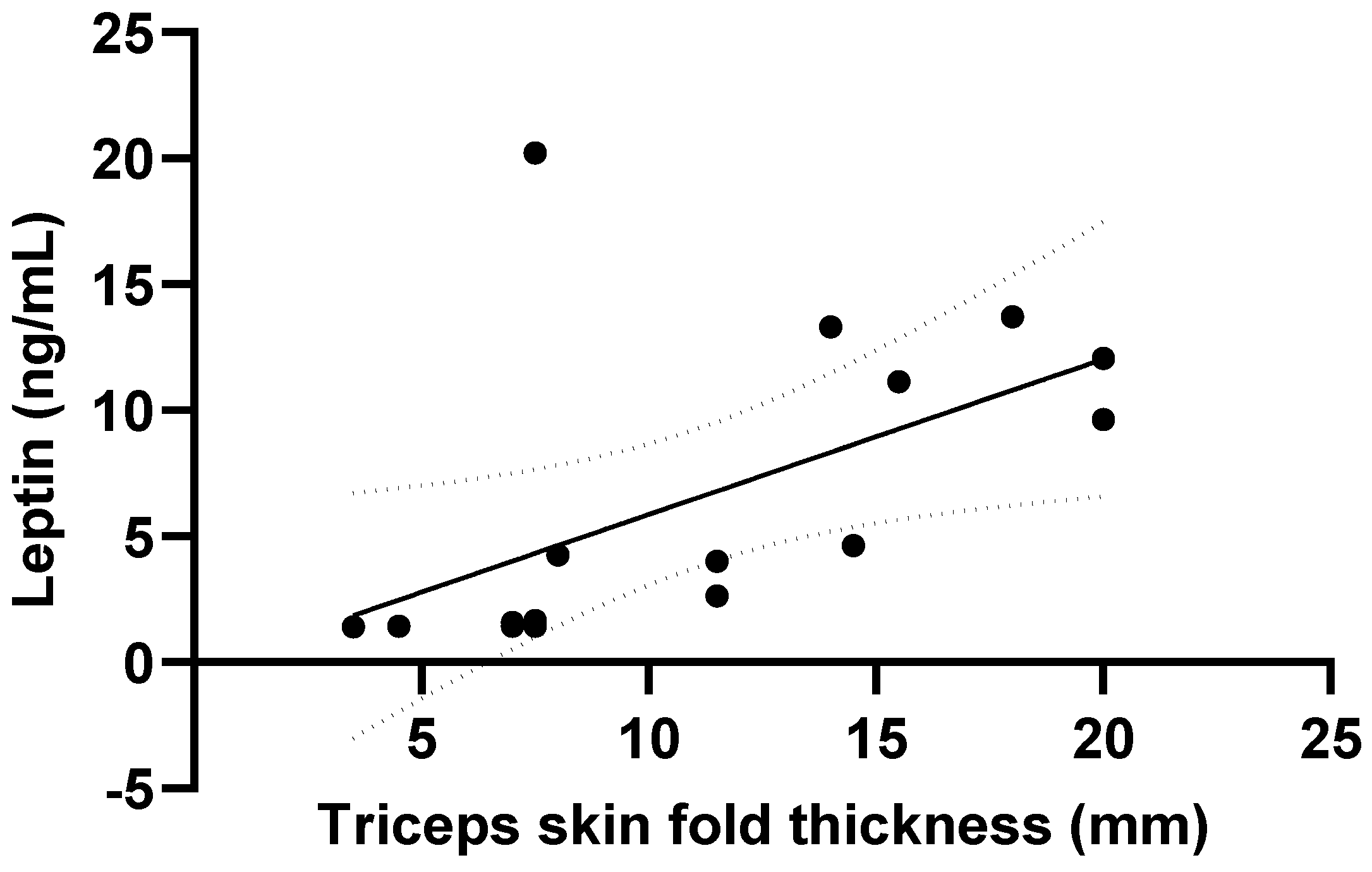
3.3. Ghrelin and Leptin
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Cheung, K.; Lee, S.S.; Raman, M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin. Gastroenterol. Hepatol. 2012, 10, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Daphnee, D.K.; John, S.; Vaidya, A.; Khakhar, A.; Bhuvaneshwari, S.; Ramamurthy, A. Hand grip strength: A reliable, reproducible, cost-effective tool to assess the nutritional status and outcomes of cirrhotics awaiting liver transplant. Clin. Nutr. ESPEN 2017, 19, 49–53. [Google Scholar] [CrossRef]
- McFarlane, M.; Hammond, C.; Roper, T.; Mukarati, J.; Ford, R.; Burrell, J.; Gordon, V.; Burch, N. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis. Clin. Nutr. ESPEN 2018, 23, 156–161. [Google Scholar] [CrossRef]
- Carvalho, L.; Parise, E.R. Evaluation of nutritional status of nonhospitalized patients with liver cirrhosis. Arq. Gastroenterol. 2006, 43, 269. [Google Scholar] [CrossRef] [PubMed]
- Dasarathy, S. Consilience in sarcopenia of cirrhosis. J. Cachexia Sarcopenia Muscle 2012, 3, 225–237. [Google Scholar] [CrossRef]
- Hanai, T.; Shiraki, M.; Nishimura, K.; Ohnishi, S.; Imai, K.; Suetsugu, A.; Takai, K.; Shimizu, M.; Moriwaki, H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition 2015, 31, 193–199. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Aging 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; O’Brien, A. Nutrition in end-stage liver disease: Principles and practice. Gastroenterology 2008, 134, 1729–1740. [Google Scholar]
- Kalaitzakis, E.; Simrén, M.; Olsson, R.; Henfridsson, P.; Hugosson, I.; Bengtsson, M.; Björnsson, E. Gastrointestinal symptoms in patients with liver cirrhosis: Associations with nutritional status and health-related quality of life. Scand. J. Gastroenterol. 2006, 41, 1464–1472. [Google Scholar] [CrossRef]
- Owen, O.E.; Reichle, F.A.; Mozzoli, M.A.; Kreulen, T.; Patel, M.S.; Elfenbein, I.B.; Golsorkhi, M.; Chang, K.H.; Rao, N.S.; Sue, H.S.; et al. Hepatic, gut, and renal substrate flux rates in patients with hepatic cirrhosis. J. Clin. Investig. 1981, 68, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Trapp, V.E.; Reichard, G.A.; Mozzoli, M.A.; Moctezuma, J.; Paul, P.; Skutches, C.L.; Boden, G. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J. Clin. Investig. 1983, 72, 1821–1832. [Google Scholar] [CrossRef] [PubMed]
- Caregaro, L.; Alberino, F.; Amodio, P. Malnutrition in alcoholic and virus-related cirrhosis. Am. J. Clin. Nutr. 1996, 63, 602–609. [Google Scholar] [CrossRef]
- Lautz, H.U.; Selberg, O.; Körber, J.; Bürger, M.; Müller, M.J. Protein-caloric malnutrition in liver cirrhosis. Clin. Investig. 1992, 70, 478–486. [Google Scholar] [CrossRef]
- Sarin, S.K.; Dhingra, N.; Bansal, A. Dietary and nutrition abnormalities in alcoholic liver disease: A comparison with chronic alcoholics without liver disease. Am. J. Gastroenterol. 1997, 92, 777–783. [Google Scholar]
- Sinclair, M.; Gow, P.J.; Grossmann, M.; Angus, P.W. Sarcopenia in cirrhosis—Etiology, implications and potential therapeutic interventions. Aliment. Pharmacol. Ther. 2016, 43, 765–777. [Google Scholar] [CrossRef]
- Dunn, M.A.; Josbeno, D.A.; Tevar, A.D.; Rachakonda, V.; Ganesh, S.R.; Schmotzer, A.R.; Kallenborn, E.A.; Behari, J.; Landsittel, D.P.; DiMartini, A.F.; et al. Frailty as Tested by Gait Speed is an Independent Risk Factor for Cirrhosis Complications that Require Hospitalization. Am. J. Gastroenterol. 2016, 111, 1768–1775. [Google Scholar] [CrossRef]
- Merli, M.; Lucidi, C.; Giannelli, V.; Giusto, M.; Riggio, O.; Falcone, M.; Ridola, L.; Attili, A.F.; Venditti, M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin. Gastroenterol. Hepatol. 2010, 8, 979–985.e1. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, B.; D’Ambrosio, D.; Merli, M. Hepatic Encephalopathy and Sarcopenia: Two Faces of the Same Metabolic Alteration. J. Clin. Exp. Hepatol. 2019, 9, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, S.; Lattanzi, B.; Torrisi, S.; Greco, F.; Farcomeni, A.; Gioia, S.; Merli, M.; Riggio, O. Sarcopenia Is Risk Factor for Development of Hepatic Encephalopathy After Transjugular Intrahepatic Portosystemic Shunt Placement. Clin. Gastroenterol. Hepatol. 2017, 15, 934–936. [Google Scholar] [CrossRef]
- Merli, M.; Giusto, M.; Gentili, F.; Novelli, G.; Ferretti, G.; Riggio, O.; Corradini, S.G.; Siciliano, M.; Farcomeni, A.; Attili, A.F.; et al. Nutritional status: Its influence on the outcome of patients undergoing liver transplantation. Liver Int. 2010, 30, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Maharshi, S.; Sharma, B.C. Malnutrition in cirrhosis increases morbidity and mortality. J. Gastroenterol. Hepatol. 2015, 30, 1507–1513. [Google Scholar]
- Ishizu, Y.; Ishigami, M.; Kuzuya, T.; Honda, T.; Hayashi, K.; Ishikawa, T.; Hirooka, Y.; Goto, H. Low skeletal muscle mass predicts early mortality in cirrhotic patients with acute variceal bleeding. Nutrition 2017, 42, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Montano-Loza, A.J.; Meza-Junco, J.; Baracos, V.E.; Prado, C.M.; Ma, M.; Meeberg, G.; Beaumont, C.; Tandon, P.; Esfandiari, N.; Sawyer, M.B.; et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transplant. 2014, 20, 640–648. [Google Scholar] [CrossRef]
- Kaido, T.; Tamai, Y.; Hamaguchi, Y.; Okumura, S.; Kobayashi, A.; Shirai, H.; Yagi, S.; Kamo, N.; Hammad, A.; Inagaki, N.; et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition 2017, 33, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; de Chen, J.; Wu, W.T.; Huang, K.C.; Lin, H.Y.; Han, D.S. Is sarcopenia associated with hepatic encephalopathy in liver cirrhosis? A systematic review and meta-analysis. J. Formos. Med. Assoc. 2018, 118, 833–842. [Google Scholar] [CrossRef]
- van Vugt, J.L.A.; Alferink, L.J.M.; Buettner, S.; van den Berg, A.P.; Metselaar, H.J.; Jzermans, J.N.M.I. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: A competing risk analysis in a national cohort. J. Hepatol. 2018, 68, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Giusto, M.; Lattanzi, B.; Albanese, C.; Galtieri, A.; Farcomeni, A.; Giannelli, V.; Lucidi, C.; Di Martino, M.; Catalano, C.; Merli, M. Sarcopenia in liver cirrhosis: The role of computed tomography scan for the assessment of muscle mass compared with dual-energy X-ray absorptiometry and anthropometry. Eur. J. Gastroenterol. Hepatol. 2015, 27, 328–334. [Google Scholar] [CrossRef]
- Anand, A.C. Nutrition and Muscle in Cirrhosis. J. Clin. Exp. Hepatol. 2017, 7, 340–357. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef]
- Marr, K.J.; Shaheen, A.A.; Lam, L.; Stapleton, M.; Burak, K.; Raman, M. Nutritional status and the performance of multiple bedside tools for nutrition assessment among patients waiting for liver transplantation: A Canadian experience. Clin. Nutr. ESPEN 2017, 17, 68–74. [Google Scholar] [CrossRef]
- Álvares-Da-Silva, M.R.; da Silveira, T.R. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition 2005, 21, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Rauf, A.; Matin, A.; Agarwal, R.; Tyagi, P.; Arora, A. Handgrip Strength as an Important Bed Side Tool to Assess Malnutrition in Patient with Liver Disease. J. Clin. Exp. Hepatol. 2017, 7, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Feng, S.; Covinsky, K.E.; Hayssen, H.; Zhou, L.Q.; Yeh, B.M.; Lai, J.C. A comparison of muscle function, mass, and quality in liver transplant candidates: Results from the functional assessment in liver transplantation study. Transplantation 2016, 100, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Tanaka-Shintani, M.; Watanabe, M. Distribution of ghrelin-immunoreactive cells in human gastric mucosa: Comparison with that of parietal cells. J. Gastroenterol. 2005, 40, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Ariyasu, H.; Takaya, K.; Tagami, T.; Ogawa, Y.; Hosoda, K.; Akamizu, T.; Suda, M.; Koh, T.; Natsui, K.; Toyooka, S.; et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J. Clin. Endocrinol. Metab. 2001, 86, 4753–4758. [Google Scholar] [CrossRef]
- Date, Y.; Kojima, M.; Hosoda, H.; Sawaguchi, A.; Mondal, M.S.; Suganuma, T.; Matsukura, S.; Kangawa, K.; Nakazato, M. DGhrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–4261. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A Preprandial Rise in Plasma Ghrelin Levels Suggests a Role in Meal Initiation in Humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef]
- Tschöp, M.; Wawarta, R.; Riepl, R.L.; Friedrich, S.; Bidlingmaier, M.; Landgraf, R.; Folwaczny, C. Post-prandial decrease of circulating human ghrelin levels. J. Endocrinol. Investig. 2001, 24, RC19–RC21. [Google Scholar] [CrossRef] [PubMed]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.A.; Ghatei, M.A.; et al. The Novel Hypothalamic Peptide Ghrelin Stimulates Food Intake and Growth Hormone Secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Zhu, X.X.; Levasseur, P.; Meguid, M.M.; Suzuki, S.; Inui, A.; Taylor, J.E.; Halem, H.A.; Dong, J.Z.; Datta, R.; et al. Ghrelin treatment causes increased food intake and retention of lean body mass in a rat model cancer cachexia. Endocrinology 2000, 148, 3004–3012. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Ahima, R.S.; Osei, S.Y. Leptin signaling. Physiol. Behav. 2004, 81, 223–241. [Google Scholar] [CrossRef]
- Takahashi, H.; Kato, A.; Onodera, K.; Suzuki, K. Fasting plasma ghrelin levels reflects malnutrition state in patients with liver cirrhosis. Hepatol. Res. 2006, 34, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Ockenga, J.; Bischoff, S.C.; Tillmann, H.L.; Rifai, K.; Widjaja, A.; Böker, K.H.; Manns, M.P.; Brabant, G. Elevated Bound Leptin Correlates with Energy Expenditure in Cirrhotics. Gastroenterology 2000, 119, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- El-Shehaby, A.M.; Obaia, E.M.; Alwakil, S.S.; Hiekal, A.A. Total and acylated ghrelin in liver cirrhosis: Correlation with clinical and nutritional status. Scand. J. Clin. Lab. Investig. 2010, 70, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Kalaitzakis, E.; Bosaeus, I.; Öhman, L.; Björnsson, E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: Correlations with energy intake and resting energy expenditure. Am. J. Clin. Nutr. 2007, 85, 808–815. [Google Scholar] [CrossRef]
- Marchesini, G.; Bianchi, G.; Lucidi, P.; Villanova, N.; Zoli, M.; de Feo, P. Plasma ghrelin concentrations, food intake, and anorexia in liver failure. J. Clin. Endocrinol. Metab. 2004, 89, 2136–2141. [Google Scholar] [CrossRef]
- Kamath, P.S.; Kim, W.R. The model for end-stage liver disease (MELD). Hepatology 2007, 45, 797–805. [Google Scholar] [CrossRef]
- Frisancho, R. New norms of upper limb fat and muscle areas for assessment of nutritional status. Am. J. Clin. Nutr. 1981, 34, 2540–2545. [Google Scholar] [CrossRef]
- FAO/WHO. Ad Hoc Expert Committee on Energy and Protein Requirements; WHO Technical Report Series No. 522. World Health Organization: Geneva, Switzerland, 1973. [Google Scholar]
- Cecile, D.T.B. Criteria and significance of dietary protein sources in humans. Summary of the workshop with recommendations. J. Nutr. 2000, 130, 1868S–1873S. [Google Scholar]
- Tessari, P. Nitrogen Balance and Protein Requirements: Definition and Measurements. In Cachexia and Wasting a Modern Approach; Springer: Milano, Italy, 2007; pp. 73–79. [Google Scholar]
- Bodwell, C.E.; Schuster, E.M.; Kyle, E.; Brooks, B.; Womack, M.; Steele, P.; Ahrens, R. Obligatory urinary and fecal nitrogen losses in young women, older men, and young men and the factorial estimation of adult human protein requirements. Am. J. Clin. Nutr. 1979, 32, 2450–2459. [Google Scholar] [CrossRef] [PubMed]
- Rand, W.M.; Pellett, P.L.; Young, V.R. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am. J. Clin. Nutr. 2003, 77, 109–127. [Google Scholar] [CrossRef]
- Gaillard, C.; Alix, E.; Boirie, Y.; Berrut, G.; Ritz, P. Are elderly Hospitalized patients getting enough protein? JAGS 2008, 56, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN Guideline ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef] [PubMed]
- Merli, M. Utritional status in cirrhosis. Italian Multicentre Cooperative Project on Nutrition in Liver Cirrhosis. J. Hepatol. 1994, 21, 317–325. [Google Scholar]
- Luna-Heredia, E.; Martín-Peña, G.; Ruiz-Galiana, J. Handgrip dynamometry in healthy adults. Clin. Nutr. 2005, 24, 250–258. [Google Scholar] [CrossRef]
- Campillo, B.; Richardet, J.P.; Scherman, E.; Bories, P.N. Evaluation of Nutritional Practice in Hospitalized Cirrhotic Patients: Result of a prospective study. Nutrition 2003, 19, 515–521. [Google Scholar] [CrossRef]
- Huynh, D.K.; Selvanderan, S.P.; Harley, H.A.J.; Holloway, R.H.; Nguyen, N.Q. Nutritional care in hospitalized patients with chronic liver disease. World J. Gastroenterol. 2015, 21, 12835–12842. [Google Scholar] [CrossRef]
- Leistra, E.; Willeboordse, F.; Visser, M.; Weijs, P.J.; Haans-van den Oord, A.; Oostenbrink, J.; Evers, A.M.; Kruizenga, H.M. Predictors for achieving protein and energy requirements in undernourished hospital patients. Clin. Nutr. 2011, 30, 484–489. [Google Scholar] [CrossRef]
- Millward, D.J.; Roberts, S.B. Protein Requirements of Older Individuals. Nutr. Res. Rev. 2005, 9, 67. [Google Scholar] [CrossRef]
- Horst, D.; Grace, N.D.; Conn, H.O.; Schiff, E.; Schenker, S.; Viteri, A.; Law, D.; Atterbury, C.E. Comparison of dietary protein with an oral, branched chain-enriched amino acid supplement in chronic portal-systemic encephalopathy: A randomized controlled trial. Hepatology 1984, 4, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.; Kondrup, J.; Martinsen, L.; Stilling, B.; Wikman, B. Nutritional assessment and adequacy of dietary intake in hospitalized patients with alcoholic liver cirrhosis. Br. J. Nutr. 1993, 69, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Swart, G.R.; van den Berg, J.W.O.; van Vuure, J.K.; Rietveld, T.; Wattimena, D.L.; Frenkel, M. Minimum protein requirements in liver cirrhosis determined by nitrogen balance measurements at three levels of protein intake. Clin. Nutr. 1989, 8, 329–336. [Google Scholar] [CrossRef]
- Nielsen, K.; Kondrup, J.; Martinsen, L.; Døssing, H.; Larsson, B.; Stilling, B.; Jensen, M.G. Long-term oral refeeding of patients with cirrhosis of the liver. Br. J. Nutr. 2005, 74, 557. [Google Scholar] [CrossRef] [PubMed]
- Pupim, L.B.; Martin, C.J.; Ikizler, T.A. Assessment of protein and energy nutritional status, Nutritional Management of Renal Disease, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Elbadri, A.; Esmat, S.; Abosaif, N.; Morsi, A.; Shaker, O. Study of serum ghrelin changes and its correlation with malnutrition in liver cirrhosis in Egypt. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 638–643. [Google Scholar] [CrossRef]
- Rachakonda, V.; Borhani, A.A.; Dunn, M.A.; Andrzejewski, M.; Martin, K.; Behari, J. Serum leptin is α biomarker of malnutrition in decompensated cirrhosis. PLoS ONE 2016, 11, e0159142. [Google Scholar] [CrossRef]
- Diz-Lois, M.T.; Garcia-Buela, J.; Suarez, F.; Sangiao-Alvarellos, S.; Vidal, O.; Cordido, F. Fasting and postprandial plasma ghrelin levels are decreased in patients with liver failure previous to liver transplantation. Endocrine 2009, 35, 467–476. [Google Scholar] [CrossRef]
- Ataseven, H.; Bahcecioglu, I.H.; Kuzu, N.; Yalniz, M.; Celebi, S.; Erensoy, A.; Ustundag, B. The Levels of Ghrelin, Leptin, TNF-α, and IL-6 in Liver Cirrhosis and Hepatocellular Carcinoma due to HBV and HDV Infection. Mediat. Inflamm. 2006, 2006, 078380. [Google Scholar] [CrossRef]
- Ataseven, H.; Bahcecioglu, I.H.; Kuzu, N.; Yalniz, M.; Celebi, S.; Erensoy, A.; Ustundag, B. Dysregulation of plasma ghrelin in alcoholic cirrhosis. Clin. Endocrinol. 2010, 73, 323–329. [Google Scholar]
- Celinski, K.; Konturek, P.C.; Slomka, M.; Cichoz-Lach, H.; Gonciarz, M.; Bielanski, W.; Reiter, R.J.; Konturek, S.J. Altered basal and postprandial plasma melatonin, gastrin, ghrelin, leptin and insulin in patients with liver cirrhosis and portal hypertension without and with oral administration of melatonin or tryptophan. J. Pineal Res. 2009, 46, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Tacke, F.; Brabant, G.; Kruck, E.; Horn, R.; Schöffski, P.; Hecker, H.; Manns, M.P.; Trautwein, C. Ghrelin in chronic liver disease. J. Hepatol. 2003, 38, 447–454. [Google Scholar] [CrossRef]
- Rieger, R.; Oertelt, S.; Selmi, C.; Invernizzi, P.; Podda, M.; Gershwin, M.E. Decreased serum leptin levels in Primary biliary cirrhosis: A link between metabolism and autoimmunity? Ann. N. Y. Acad. Sci. 2005, 1051, 211–217. [Google Scholar] [CrossRef]
- Greco, A.V.; Mingrone, G.; Favuzzi, A.; Capristo, E.; Gniuli, D.; Addolorato, G.; Brunani, A.; Cavagnin, F.; Gasbarrini, G. Serum leptin levels in post-hepatitis liver cirrhosis. J. Hepatol. 2000, 33, 38–42. [Google Scholar] [CrossRef]
- Onodera, K.; Kato, A.; Suzuki, K. Serum leptin concentrations in liver cirrhosis: Relationship to the severity of liver dysfunction and their characteristic diurnal profiles. Hepatol. Res. 2001, 21, 205–212. [Google Scholar] [CrossRef]
- McCullough, A.J.; Bugianesi, E.; Marchesini, G.; Kalhan, S.C. Gender-dependent alterations in serum leptin in alcoholic cirrhosis. Gastroenterology 1998, 115, 947–953. [Google Scholar] [CrossRef]
- Testa, R.; Franceschini, R.; Giannini, E.; Cataldi, A.; Botta, F.; Fasoli, A.; Tenerelli, P.; Rolandi, E.; Barreca, T. Serum leptin levels in patients with viral chronic hepatitis or liver cirrhosis. J. Hepatol. 2000, 33, 33–37. [Google Scholar] [CrossRef]
- Bolukbas, F.F.; Bolukbas, C.; Horoz, M.; Gumus, M.; Erdogan, M.; Zeyrek, F.; Yayla, A.; Ovunc, O. Child-Pugh classification dependent alterations in serum leptin levels among cirrhotic patients: A case controlled study. BMC Gastroenterol. 2004, 4, 23. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, Y.Y.; Sheu, W.H.-H. Increased serum leptin concentrations correlate with soluble tumour necrosis factor receptor levels in patients with cirrhosis. Clin. Endocrinol. 2002, 57, 805–811. [Google Scholar] [CrossRef]
- Buechler, C.; Haberl, E.M.; Rein-Fischboeck, L.; Aslanidis, C. Adipokines in liver cirrhosis. Int. J. Mol. Sci. 2017, 18, 1392. [Google Scholar]

| Mean ± SD | |
|---|---|
| Age (years) | 58.9 ± 10.8 |
| Female/Male (%) | 30/70 |
| Etiology of liver disease (%): | |
| Alcohol/HCV/HCV + Alcohol/Crypto/Other | 31/29/18/10/12 |
| Reason for the hospitalization (%): | |
| Ascites/Bleeding/HE/Interventional procedure/Other | 36/8/12/12/32 |
| Child Class (%): A/B/C | 21/50/29 |
| MELD | 16.1 ± 6.9 |
| MELD > 15 (%) | 52 |
| HG (kg) | 23.1 ± 9.1 |
| HG < 10° percentile (%) | 37.5 |
| BMI (kg/m2) | 23.9 ± 3.7 |
| MAMC (mm) | 21.6 ± 3.6 |
| MAMC < 5° percentile (%) | 60 |
| Nourished (n = 20) | Malnourished (n = 30) | p | |
|---|---|---|---|
| Weight (kg) | 74.3 ± 14.0 | 64.8 ± 10.8 | <0.05 |
| BMI (kg/m2) | 25.7 ± 3.8 | 22.1 ± 2.8 | <0.001 |
| Triceps skin fold thickness (mm) | 13.4 ± 7.1 | 7.0 ± 3.3 | <0.001 |
| Biceps skin fold thickness (mm) | 7.4 ± 6.0 | 4.2 ± 2.0 | <0.05 |
| HG (kg) | 23.5 ± 10.1 | 22.7 ± 8.3 | n.s. |
| HG < 10° percentile (%) | 20.0 | 55.0 | <0.05 |
| Nourished (n = 20) | Malnourished (n = 30) | p | |
|---|---|---|---|
| Dietary Protein intake (g/kg) | 0.7 ± 0.4 | 0.8 ± 0.6 | n.s. |
| Urinary nitrogen (g/24 h) | 11.1 ± 6.0 | 14.1 ± 8.5 | n.s. |
| Nitrogen balance (g) | −6.1 ± 5.2 | −7.5 ± 10.7 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangitano, E.; Gnessi, L.; Merli, M. Protein Catabolism and the Dysregulation of Energy Intake-Related Hormones May Play a Major Role in the Worsening of Malnutrition in Hospitalized Cirrhotic Patients. Livers 2022, 2, 158-170. https://doi.org/10.3390/livers2030014
Gangitano E, Gnessi L, Merli M. Protein Catabolism and the Dysregulation of Energy Intake-Related Hormones May Play a Major Role in the Worsening of Malnutrition in Hospitalized Cirrhotic Patients. Livers. 2022; 2(3):158-170. https://doi.org/10.3390/livers2030014
Chicago/Turabian StyleGangitano, Elena, Lucio Gnessi, and Manuela Merli. 2022. "Protein Catabolism and the Dysregulation of Energy Intake-Related Hormones May Play a Major Role in the Worsening of Malnutrition in Hospitalized Cirrhotic Patients" Livers 2, no. 3: 158-170. https://doi.org/10.3390/livers2030014
APA StyleGangitano, E., Gnessi, L., & Merli, M. (2022). Protein Catabolism and the Dysregulation of Energy Intake-Related Hormones May Play a Major Role in the Worsening of Malnutrition in Hospitalized Cirrhotic Patients. Livers, 2(3), 158-170. https://doi.org/10.3390/livers2030014








