Unresectable Hepatocellular Carcinoma: A Review of New Advances with Focus on Targeted Therapy and Immunotherapy
Abstract
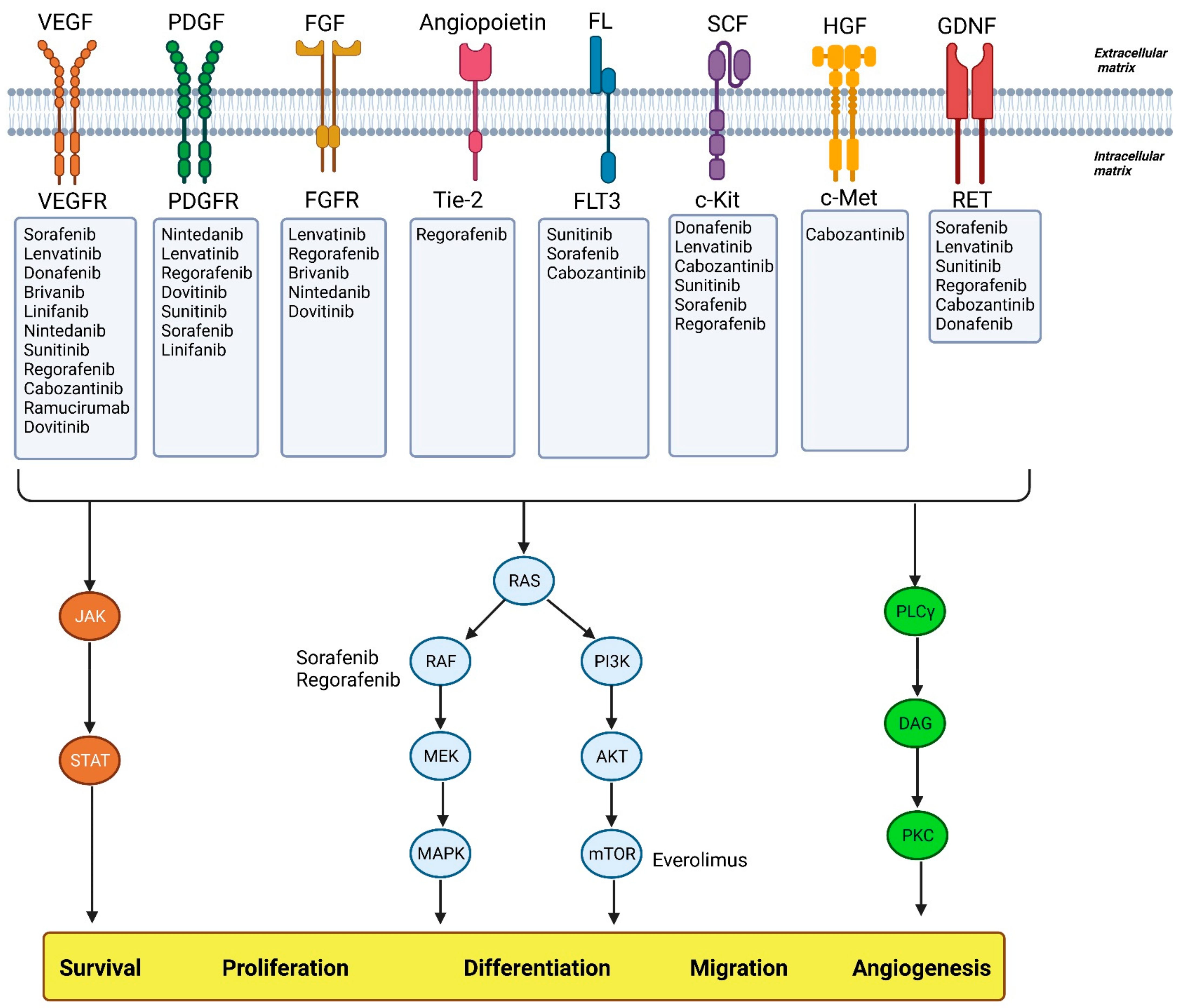
:1. Introduction
2. HCC Stages and Treatment
3. Targeted Therapy and Recent Drug Treatments
3.1. First Line
3.1.1. Sorafenib
3.1.2. Sunitinib
3.1.3. Brivanib
3.1.4. Linifanib
3.1.5. Lenvatinib
3.1.6. Donafenib
3.1.7. Atezolizumab + Bevacizumab
3.1.8. Sintilimab + Bevacizumab
3.1.9. Cediranib
3.1.10. Nintedanib
3.1.11. Dovitinib
3.1.12. Everolimus
3.1.13. Tislelizumab
3.1.14. Regorafenib
3.2. Second Line
3.2.1. Immune Checkpoint Inhibitors
Nivolumab
Nivolumab + Ipilimumab
Pembrolizumab
Avelumab
Tremelimumab + Durvalumab
Durvalumab
Camrelizumab
Spartalizumab
3.2.2. Other Targeting Agents
Ramucirumab
Cabozantinib
Apatinib
3.3. Systemic Chemotherapy
3.4. Herbal Management Role in HCC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Childs, A.; Meyer, T. Hepatocellular Carcinoma: Treatment. In Evidence-Based Gastroenterology and Hepatology 4e; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 703–714. [Google Scholar]
- Kweon, S.-S. Updates on cancer epidemiology in Korea, 2018. Chonnam Med. J. 2018, 54, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Takayama, T.; Mazzaferro, V.; Chau, G.-Y.; Yang, J.; Kudo, M.; Cai, J.; Poon, R.T.; Han, K.-H.; Tak, W.Y.; et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): A phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015, 16, 1344–1354. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Morizane, C.; Ueno, M.; Okusaka, T.; Ishii, H.; Furuse, J. Chemotherapy for hepatocellular carcinoma: Current status and future perspectives. Jpn. J. Clin. Oncol. 2018, 48, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perisetti, A.; Goyal, H.; Yendala, R.; Thandassery, R.B.; Giorgakis, E. Non-cirrhotic hepatocellular carcinoma in chronic viral hepatitis: Current insights and advancements. World J. Gastroenterol. 2021, 27, 3466–3482. [Google Scholar] [CrossRef]
- Geh, D.; Manas, D.M.; Reeves, H.L. Hepatocellular carcinoma in non-alcoholic fatty liver disease—A review of an emerging challenge facing clinicians. Hepatobiliary Surg. Nutr. 2021, 10, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Farrell, A.; Ryan, M.; Howell, J. Epidemiology of non-alcoholic fatty liver disease-related hepatocellular carcinoma: A western perspective. Hepatoma Res. 2020, 6, 18. [Google Scholar] [CrossRef]
- Saitta, C.; Pollicino, T.; Raimondo, G. Obesity and liver cancer. Ann. Hepatol. 2019, 18, 810–815. [Google Scholar] [CrossRef]
- Chew, S.A.; Moscato, S.; George, S.; Azimi, B.; Danti, S. Liver cancer: Current and future trends using biomaterials. Cancers 2019, 11, 2026. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-Y.; Chen, K.-F.; Chen, P.-J. Treatment of liver cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a021535. [Google Scholar] [CrossRef] [Green Version]
- Petrowsky, H.; Fritsch, R.; Guckenberger, M.; De Oliveira, M.L.; Dutkowski, P.; Clavien, P.-A. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 755–772. [Google Scholar] [CrossRef]
- Llovet, J.M.; Burroughs, A.; Bruix, J. Hepatocellular carcinoma. Lancet 2003, 362, 1907–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.-H.; Shen, L.; Li, J.; Zhou, Z.-W.; Liang, H.; Zhang, X.-T.; Tang, L.; Xin, Y.; Jin, J.; Zhang, Y.-J. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun. 2019, 39, 1–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.-M.; Lewandowski, R.-J.; Sato, K.-T.; Gates, V.-L.; Kulik, L.; Mulcahy, M.-F.; Ryu, R.-K.; Omary, R.-A.; Salem, R. Radioembolization for the treatment of unresectable hepatocellular carcinoma: A clinical review. World J. Gastroenterol. 2008, 14, 1664–1669. [Google Scholar] [CrossRef]
- Zhong, Q.; Shuai, Y.; Luo, Q.; Feng, G.; Wu, M.; Fan, E.; Chen, Q.; Yue, G.; Zhang, G. A novel five-gene signature for predicting prognosis in liver cancer. 2020. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H. Precision diagnosis and treatment of liver cancer in China. Cancer Lett. 2018, 412, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Olsen, S.K.; Brown Jr, R.S.; Siegel, A.B. Hepatocellular carcinoma: Review of current treatment with a focus on targeted molecular therapies. Ther. Adv. Gastroenterol. 2010, 3, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lurje, I.; Czigany, Z.; Bednarsch, J.; Roderburg, C.; Isfort, P.; Neumann, U.P.; Lurje, G. Treatment strategies for hepatocellular carcinoma—A multidisciplinary approach. Int. J. Mol. Sci. 2019, 20, 1465. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.M. Local Ablation for Hepatocellular Carcinoma in Taiwan. Liver Cancer 2013, 2, 73–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serper, M.; Parikh, N.D.; Thiele, G.; Ovchinsky, N.; Mehta, S.; Kuo, A.; Ho, C.; Kanwal, F.; Volk, M.; Asrani, S.K. Patient-Reported Outcomes in HCC: A Scoping Review by the Practice Metrics Committee of the American Association for the Study of Liver Diseases. Hepatology 2022, 76, 251–274. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Bruix, J.; Chan, S.L.; Galle, P.R.; Rimassa, L.; Sangro, B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J. Hepatol. 2021, 75, 960–974. [Google Scholar] [CrossRef]
- Lee, W.S.; Yang, H.; Chon, H.J.; Kim, C. Combination of anti-angiogenic therapy and immune checkpoint blockade normalizes vascular-immune crosstalk to potentiate cancer immunity. Exp. Mol. Med. 2020, 52, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Morine, Y.; Yamada, S.; Saito, Y.; Ikemoto, T.; Tokuda, K.; Takasu, C.; Miyazaki, K.; Shimada, M. Nrf2 signaling promotes cancer stemness, migration, and expression of ABC transporter genes in sorafenib-resistant hepatocellular carcinoma cells. PLoS ONE 2021, 16, e0256755. [Google Scholar] [CrossRef] [PubMed]
- Exposito, M.J.; Akce, M.; Alvarez, J.M.; Assenat, E.; Balart, L.; Baron, A.; Decaens, T.; Heurgue-Berlot, A.; Martin, A.; Paik, S. CA209-9DX: Phase III, randomized, double-blind study of adjuvant nivolumab vs placebo for patients with hepatocellular carcinoma (HCC) at high risk of recurrence after curative resection or ablation. Ann. Oncol. 2018, 29, viii267–viii268. [Google Scholar] [CrossRef]
- Rimassa, L.; Santoro, A. Sorafenib therapy in advanced hepatocellular carcinoma: The SHARP trial. Expert Rev. Anticancer Ther. 2009, 9, 739–745. [Google Scholar] [CrossRef]
- Cheng, A.-L.; Guan, Z.; Chen, Z.; Tsao, C.-J.; Qin, S.; Kim, J.S.; Yang, T.-S.; Tak, W.Y.; Pan, H.; Yu, S.J.E.J.o.C. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: Subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur. J. Cancer 2012, 48, 1452–1465. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.-H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.-W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.-Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.-L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.-Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.-W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.-H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracian, A.C.; Acosta-Rivera, M.; Choo, S.P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B.F.; et al. Checkmate-040: Nivolumab (NIVO) in patients (pts) with advanced hepatocellular carcinoma (aHCC) and Child-Pugh B (CPB) status. J. Clin. Oncol. 2019, 37, 327. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.-K.; Yen, C.-J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.-K.; Kim, T.-Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Wilhelm, S.M.; Carter, C.; Tang, L.; Wilkie, D.; McNabola, A.; Rong, H.; Chen, C.; Zhang, X.; Vincent, P.; McHugh, M.; et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004, 64, 7099–7109. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Kudo, M.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.F.; de Guevara, L.L.; Papandreou, C.; et al. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): Second interim analysis. Int. J. Clin. Pract. 2014, 68, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Lencioni, R.; Kudo, M.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.F.; Ladrón de Guevara, L.; Papandreou, C.; et al. First interim analysis of the GIDEON (Global Investigation of therapeutic decisions in hepatocellular carcinoma and of its treatment with sorafeNib) non-interventional study. Int. J. Clin. Pract. 2012, 66, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.H.; de Guevara, L.L.; et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Ganten, T.M.; Stauber, R.; Schott, E.; Malfertheiner, P.; Buder, R.; Galle, P.R.; Goehler, T.; Bernard, I.; Gerken, G. Final Analysis of Overall Survival Per Subgroups of Hcc Patients in the Prospective, Non-Interventional Insight Study Treated with Sorafenib. Ann. Oncol. 2014, 25, iv246. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Sacco, R.; de Stefano, G.; Montesarchio, V.; Cabibbo, G.; Zolfino, T.; Carucci, P.; Pisconti, S.; De Vita, F.; Giovanis, P.; et al. Safety and efficacy of sorafenib in stella study, a Multicenter, Observational, Phase IV Study In Italian Centers. Ann. Oncol. 2015, 26, vi95. [Google Scholar] [CrossRef]
- Iavarone, M.; Cabibbo, G.; Piscaglia, F.; Zavaglia, C.; Grieco, A.; Villa, E.; Cammà, C.; Colombo, M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011, 54, 2055–2063. [Google Scholar] [CrossRef]
- Ford, R.; Schwartz, L.; Dancey, J.; Dodd, L.E.; Eisenhauer, E.A.; Gwyther, S.; Rubinstein, L.; Sargent, D.; Shankar, L.; Therasse, P.; et al. Lessons learned from independent central review. Eur. J. Cancer 2009, 45, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Yang, X.-R.; Chung, W.-Y.; Dennison, A.R.; Zhou, J. Targeted therapy for hepatocellular carcinoma. Signal Transduct. Target. Ther. 2020, 5, 1–13. [Google Scholar] [CrossRef]
- Hironaka, S. Anti-angiogenic therapies for gastric cancer. Asia-Pac. J. Clin. Oncol. 2019, 15, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.L.; Kang, Y.K.; Lin, D.Y.; Park, J.W.; Kudo, M.; Qin, S.; Chung, H.C.; Song, X.; Xu, J.; Poggi, G.; et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J. Clin. Oncol. 2013, 31, 4067–4075. [Google Scholar] [CrossRef]
- Meyer, T.; Fox, R.; Ma, Y.T.; Ross, P.J.; James, M.W.; Sturgess, R.; Stubbs, C.; Stocken, D.D.; Wall, L.; Watkinson, A.; et al. Sorafenib in combination with transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma (TACE 2): A randomised placebo-controlled, double-blind, phase 3 trial. Lancet Gastroenterol. Hepatol. 2017, 2, 565–575. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.J.; Qin, S.; Park, J.-W.; Poon, R.; Raoul, J.-L.; Philip, P.A.; Hsu, C.-H.; Hu, T.-H.; Heo, J.; Xu, J. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef] [Green Version]
- Le Grazie, M.; Biagini, M.R.; Tarocchi, M.; Polvani, S.; Galli, A. Chemotherapy for hepatocellular carcinoma: The present and the future. World J. Hepatol. 2017, 9, 907–920. [Google Scholar] [CrossRef]
- Zhong, Y.; Qiu, R.-Z.; Sun, S.-L.; Zhao, C.; Fan, T.-Y.; Chen, M.; Li, N.-G.; Shi, Z.-H. Small-molecule Fms-like tyrosine kinase 3 inhibitors: An attractive and efficient method for the treatment of acute myeloid leukemia. J. Med. Chem. 2020, 63, 12403–12428. [Google Scholar] [CrossRef] [PubMed]
- Toh, H.C.; Chen, P.-J.; Carr, B.I.; Knox, J.J.; Gill, S.; Ansell, P.; McKeegan, E.M.; Dowell, B.; Pedersen, M.; Qin, Q.; et al. Phase 2 trial of linifanib (ABT-869) in patients with unresectable or metastatic hepatocellular carcinoma. Cancer 2012, 119, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Cainap, C.; Qin, S.; Huang, W.T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.K.; Chen, P.J.; Toh, H.C.; et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Marzin, K.; Kretschmar, G.; Luedtke, D.; Kraemer, S.; Kuelzer, R.; Schlenker-Herceg, R.; Schmid, U.; Schnell, D.; Dallinger, C. Pharmacokinetics of nintedanib in subjects with hepatic impairment. J. Clin. Pharmacol. 2018, 58, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Maucort-Boulch, D.; de Martel, C.; Franceschi, S.; Plummer, M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int. J. Cancer 2018, 142, 2471–2477. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, M.; Mitsunaga, S.; Ohno, I.; Hashimoto, Y.; Takahashi, H.; Watanabe, K.; Umemoto, K.; Okusaka, T. Systemic Chemotherapy for Advanced Hepatocellular Carcinoma: Past, Present, and Future. Diseases 2015, 3, 360–381. [Google Scholar] [CrossRef] [Green Version]
- Al-Salama, Z.T.; Syed, Y.Y.; Scott, L.J. Lenvatinib: A review in hepatocellular carcinoma. Drugs 2019, 79, 665–674. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, T.-H.; Yau, T.; Hsu, C. Novel systemic therapy for hepatocellular carcinoma. Hepatol. Int. 2020, 14, 638–651. [Google Scholar] [CrossRef]
- Li, X.; Qiu, M.; Wang, S.; Zhu, H.; Feng, B.; Zheng, L. A Phase I dose-escalation, pharmacokinetics and food-effect study of oral donafenib in patients with advanced solid tumours. Cancer Chemother Pharm. 2020, 85, 593–604. [Google Scholar] [CrossRef]
- Bi, F.; Qiu, M.; Chai, X.; Niu, J.; Ding, Y.; Bai, Y.; Wu, L.; Shentu, J.-z.; Hao, P.; Chen, J.; et al. A multicenter phase II study of donafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2017, 35, e15682. [Google Scholar] [CrossRef]
- Bi, F.; Qin, S.; Gu, S.; Bai, Y.; Chen, Z.; Wang, Z.; Ying, J.; Lu, Y.; Meng, Z.; Pan, H.; et al. Donafenib versus sorafenib as first-line therapy in advanced hepatocellular carcinoma: An open-label, randomized, multicenter phase II/III trial. J. Clin. Oncol. 2020, 38, 4506. [Google Scholar] [CrossRef]
- Cui, X.; Jia, H.; Xin, H.; Zhang, L.; Chen, S.; Xia, S.; Li, X.; Xu, W.; Chen, X.; Feng, Y.; et al. A Novel Bispecific Antibody Targeting PD-L1 and VEGF With Combined Anti-Tumor Activities. Front. Immunol. 2021, 12, 778978. [Google Scholar] [CrossRef] [PubMed]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P.; Goff, L.; Gupta, S.; Guy, J.; Harris, W.P. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Abou-Alfa, G.K.; Cheng, A.-L.; Duffy, A.G.; El-Khoueiry, A.B.; Finn, R.S.; Galle, P.R.; Goyal, L.; He, A.R.; Kaseb, A.O. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of hepatocellular carcinoma. J. Immunother. Cancer 2021, 9, e002794. [Google Scholar] [CrossRef] [PubMed]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. FDA Approval Summary: Atezolizumab Plus Bevacizumab for the Treatment of Patients with Advanced Unresectable or Metastatic Hepatocellular Carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- Ren, Z.; Xu, J.; Bai, Y.; Xu, A.; Cang, S.; Du, C.; Li, Q.; Lu, Y.; Chen, Y.; Guo, Y.; et al. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): A randomised, open-label, phase 2-3 study. Lancet Oncol. 2021, 22, 977–990. [Google Scholar] [CrossRef]
- Deininger, S.; Törzsök, P.; Oswald, D.; Lusuardi, L. Current Systemic Treatment Options in Metastatic Urothelial Carcinoma after Progression on Checkpoint Inhibition Therapy-A Systemic Review Combined with Single-Group Meta-Analysis of Three Studies Testing Enfortumab Vedotin. Cancers 2021, 13, 3206. [Google Scholar] [CrossRef]
- Alberts, S.R.; Fitch, T.R.; Kim, G.P.; Morlan, B.W.; Dakhil, S.R.; Gross, H.M.; Nair, S. Cediranib (AZD2171) in patients with advanced hepatocellular carcinoma: A phase II north central cancer treatment group (NCCTG) Clinical Trial. Am. J. Clin. Oncol. 2012, 35, 329. [Google Scholar] [CrossRef]
- Zhu, A.X.; Ancukiewicz, M.; Supko, J.G.; Sahani, D.V.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Abrams, T.A.; McCleary, N.J.; Bhargava, P.; Muzikansky, A.; et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: A phase II study. Clin. Cancer Res. 2013, 19, 1557–1566. [Google Scholar] [CrossRef] [Green Version]
- Palmer, D.H.; Ma, Y.T.; Peck-Radosavljevic, M.; Ross, P.; Graham, J.; Fartoux, L.; Deptała, A.; Studeny, M.; Schnell, D.; Hocke, J.; et al. A multicentre, open-label, phase-I/randomised phase-II study to evaluate safety, pharmacokinetics, and efficacy of nintedanib vs. sorafenib in European patients with advanced hepatocellular carcinoma. Br. J. Cancer 2018, 118, 1162–1168. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Xie, H.; Hu, M.; Huang, T.; Hu, Y.; Sang, N.; Zhao, Y. Recent progress in treatment of hepatocellular carcinoma. Am. J. Cancer Res. 2020, 10, 2993–3036. [Google Scholar] [PubMed]
- Gardini, A.C.; Santini, D.; Aprile, G.; Silvestris, N.; Felli, E.; Foschi, F.G.; Ercolani, G.; Marisi, G.; Valgiusti, M.; Passardi, A.J.O. Antiangiogenic agents after first line and sorafenib plus chemoembolization: A systematic review. Oncotarget 2017, 8, 66699–66708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, A.-L.; Thongprasert, S.; Lim, H.Y.; Sukeepaisarnjaroen, W.; Yang, T.-S.; Wu, C.-C.; Chao, Y.; Chan, S.L.; Kudo, M.; Ikeda, M.; et al. Randomized, open-label phase 2 study comparing frontline dovitinib versus sorafenib in patients with advanced hepatocellular carcinoma. Hepatology 2016, 64, 774–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woei-A-Jin, F.S.H.; Weijl, N.I.; Burgmans, M.C.; Sarasqueta, A.F.; van Wezel, J.T.; Wasser, M.N.; Coenraad, M.J.; Burggraaf, J.; Osanto, S. Neoadjuvant Treatment with Angiogenesis-Inhibitor Dovitinib Prior to Local Therapy in Hepatocellular Carcinoma: A Phase II Study. Oncologist 2021, 26, 854–864. [Google Scholar] [CrossRef]
- Houghton, P.J. Everolimus. Clin. Cancer Res. 2010, 16, 1368–1372. [Google Scholar] [CrossRef] [Green Version]
- Efeyan, A.; Sabatini, D.M. mTOR and cancer: Many loops in one pathway. Curr. Opin. Cell Biol. 2010, 22, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.X.; Kudo, M.; Assenat, E.; Cattan, S.; Kang, Y.K.; Lim, H.Y.; Poon, R.T.; Blanc, J.F.; Vogel, A.; Chen, C.L.; et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: The EVOLVE-1 randomized clinical trial. JAMA 2014, 312, 57–67. [Google Scholar] [CrossRef]
- Koeberle, D.; Dufour, J.F.; Demeter, G.; Li, Q.; Ribi, K.; Samaras, P.; Saletti, P.; Roth, A.D.; Horber, D.; Buehlmann, M.; et al. Sorafenib with or without everolimus in patients with advanced hepatocellular carcinoma (HCC): A randomized multicenter, multinational phase II trial (SAKK 77/08 and SASL 29). Ann. Oncol. 2016, 27, 856–861. [Google Scholar] [CrossRef]
- Zhu, A.X.; Abrams, T.A.; Miksad, R.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Zheng, H.; Muzikansky, A.; Clark, J.W.; Kwak, E.L.; Schrag, D.; et al. Phase 1/2 study of everolimus in advanced hepatocellular carcinoma. Cancer 2011, 117, 5094–5102. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Song, X.; Xu, L.; Ma, J.; Zhang, Y.; Gong, W.; Zhang, Y.; Zhou, X.; Wang, Z.; Wang, Y.; et al. The binding of an anti-PD-1 antibody to FcγRΙ has a profound impact on its biological functions. Cancer Immunol. Immunother. 2018, 67, 1079–1090. [Google Scholar] [CrossRef] [Green Version]
- Dahan, R.; Sega, E.; Engelhardt, J.; Selby, M.; Korman, A.J.; Ravetch, J.V. FcγRs Modulate the Anti-tumor Activity of Antibodies Targeting the PD-1/PD-L1 Axis. Cancer Cell 2015, 28, 285–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deva, S.; Lee, J.-S.; Lin, C.-C.; Yen, C.-J.; Millward, M.; Chao, Y.; Keam, B.; Jameson, M.; Hou, M.-M.; Kang, Y.-K. A phase Ia/Ib trial of tislelizumab, an anti-PD-1 antibody (ab), in patients (pts) with advanced solid tumors. Ann. Oncol. 2018, 29, x24–x25. [Google Scholar] [CrossRef]
- Qin, S.; Finn, R.S.; Kudo, M.; Meyer, T.; Vogel, A.; Ducreux, M.; Macarulla, T.M.; Tomasello, G.; Boisserie, F.; Hou, J.; et al. RATIONALE 301 study: Tislelizumab versus sorafenib as first-line treatment for unresectable hepatocellular carcinoma. Future Oncol. 2019, 15, 1811–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fouchardière, C. Regorafenib in the treatment of metastatic colorectal cancer. Future Oncol. 2018, 14, 2239–2246. [Google Scholar] [CrossRef]
- Ferraro, D.; Zalcberg, J. Regorafenib in gastrointestinal stromal tumors: Clinical evidence and place in therapy. Ther. Adv. Med. Oncol. 2014, 6, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Mei, L.; Du, W.; Idowu, M.; von Mehren, M.; Boikos, S.A. Advances and Challenges on Management of Gastrointestinal Stromal Tumors. Front. Oncol. 2018, 8, 135. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Tao, C.-C.; Tao, Z.-G.; Zhang, K.; Wu, A.-K.; Wu, J.-X.; Rong, W.-Q. Research progress regarding programmed cell death 1/programmed cell death ligand 1 inhibitors combined with targeted therapy for treating hepatocellular carcinoma. World J. Gastrointest. Surg. 2021, 13, 1136. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Hirooka, Y.; Fujishiro, M. Clinical characteristics and outcomes of candidates for second-line therapy, including regorafenib and ramucirumab, for advanced hepatocellular carcinoma after sorafenib treatment. Hepatol. Res. 2019, 49, 1054–1065. [Google Scholar] [CrossRef]
- Grothey, A.; George, S.; Van Cutsem, E.; Blay, J.-Y.; Sobrero, A.; Demetri, G.D. Optimizing treatment outcomes with regorafenib: Personalized dosing and other strategies to support patient care. Oncologist 2014, 19, 669–680. [Google Scholar] [CrossRef] [Green Version]
- Pathak, S.; Sonbol, M. Second-Line Treatment Options for Hepatocellular Carcinoma: Current Landscape and Future Direction. J. Hepatocell. Carcinoma 2021, 8, 1147–1158. [Google Scholar] [CrossRef]
- Kumar, V.; Shinagare, A.B.; Rennke, H.G.; Ghai, S.; Lorch, J.H.; Ott, P.A.; Rahma, O.E. The Safety and Efficacy of Checkpoint Inhibitors in Transplant Recipients: A Case Series and Systematic Review of Literature. Oncologist 2020, 25, 505–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gellrich, F.F.; Schmitz, M.; Beissert, S.; Meier, F. Anti-PD-1 and novel combinations in the treatment of melanoma—An update. J. Clin. Med. 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, L.; Chen, S.; Yang, L.; Li, Y. The role of PD-1 and PD-L1 in T-cell immune suppression in patients with hematological malignancies. J. Hematol. Oncol. 2013, 6, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghahremanloo, A.; Soltani, A.; Modaresi, S.M.S.; Hashemy, S.I. Recent advances in the clinical development of immune checkpoint blockade therapy. Cell. Oncol. 2019, 42, 609–626. [Google Scholar] [CrossRef]
- Kitahara, M.; Mizukoshi, E.; Terashima, T.; Nakagawa, H.; Horii, R.; Iida, N.; Arai, K.; Yamashita, T.; Sakai, Y.; Yamashita, T. Safety and long-term outcome of intratumoral injection of ok432-stimulated dendritic cells for hepatocellular carcinomas after radiofrequency ablation. Transl. Oncol. 2020, 13, 100777. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, Y.; Lee, M.; Heo, M.K.; Song, J.-S.; Kim, K.-H.; Lee, H.; Yi, N.-J.; Lee, K.-W.; Suh, K.-S. A phase I/IIa study of adjuvant immunotherapy with tumour antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Br. J. Cancer 2015, 113, 1666–1676. [Google Scholar] [CrossRef] [Green Version]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.-C.; Lee, W.-B.; Lee, H.-S.; Bae, Y.-S. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhang, L.; Chang, R.; Yan, X. Supramolecular Cancer Photoimmunotherapy Based on Precise Peptide Self-Assembly Design. Chem. Commun. 2022, 58, 2247–2258. [Google Scholar] [CrossRef]
- Vafaei, S.; Zekiy, A.O.; Khanamir, R.A.; Zaman, B.A.; Ghayourvahdat, A.; Azimizonuzi, H.; Zamani, M. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022, 22, 2. [Google Scholar] [CrossRef]
- Zhulai, G.; Oleinik, E. Targeting regulatory T cells in anti-PD-1/PD-L1 cancer immunotherapy. Scand. J. Immunol. 2021, 95, e13129. [Google Scholar] [CrossRef]
- Kojima, K.; Sakamoto, T.; Kasai, T.; Kagawa, T.; Yoon, H.; Atagi, S. PD-L1 expression as a predictor of postoperative recurrence and the association between the PD-L1 expression and EGFR mutations in NSCLC. Sci. Rep. 2021, 11, 17522. [Google Scholar] [CrossRef]
- Dietz, H.; Weinmann, S.C.; Salama, A.K. Checkpoint Inhibitors in Melanoma Patients with Underlying Autoimmune Disease. Cancer Manag. Res. 2021, 13, 8199–8208. [Google Scholar] [CrossRef]
- Lisi, L.; Lacal, P.M.; Martire, M.; Navarra, P.; Graziani, G. Clinical experience with CTLA-4 blockade for cancer immunotherapy: From the monospecific monoclonal antibody ipilimumab to probodies and bispecific molecules targeting the tumor microenvironment. Pharmacol. Res. 2022, 175, 105997. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Mahn, R.; Vogt, A.; Kupczyk, P.; Sadeghlar, F.; van Beekum, K.; Hüneburg, R.; Meyer, C.; Toma, M.; Ahmadzadehfar, H.; Essler, M. Programmed cell death protein 1 (PD-1)-inhibition in hepatocellular carcinoma (HCC): A single center experience. Scand. J. Gastroenterol. 2020, 55, 1057–1062. [Google Scholar] [CrossRef]
- Kudo, M.; Matilla, A.; Santoro, A.; Melero, I.; Gracián, A.C.; Acosta-Rivera, M.; Choo, S.-P.; El-Khoueiry, A.B.; Kuromatsu, R.; El-Rayes, B. CheckMate 040 Cohort 5: A phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J. Hepatol. 2021, 5, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Hammers, H.; Lipson, E.J. Nivolumab: Targeting PD-1 to bolster antitumor immunity. Future Oncol. 2015, 11, 1307–1326. [Google Scholar] [CrossRef]
- Tella, S.H.; Mahipal, A.; Kommalapati, A.; Jin, Z. Evaluating the Safety and Efficacy of Nivolumab in Patients with Advanced Hepatocellular Carcinoma: Evidence to Date. OncoTargets Ther. 2019, 12, 10335–10342. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Lee, S.Y. Clinical Characteristics and Treatment of Immune-Related Adverse Events of Immune Checkpoint Inhibitors. Immune Netw. 2020, 20, e9. [Google Scholar] [CrossRef]
- Ji, H.-h.; Tang, X.-w.; Dong, Z.; Song, L.; Jia, Y.-t. Adverse event profiles of anti-CTLA-4 and anti-PD-1 monoclonal antibodies alone or in combination: Analysis of spontaneous reports submitted to FAERS. Clin. Drug Investig. 2019, 39, 319–330. [Google Scholar] [CrossRef]
- Stein, A.; Moehler, M.; Trojan, J.; Goekkurt, E.; Vogel, A. Immuno-oncology in GI tumours: Clinical evidence and emerging trials of PD-1/PD-L1 antagonists. Crit. Rev. Oncol./Hematol. 2018, 130, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; Garcia-Manero, G.; Basu, S.; Boddu, P.C.; Alfayez, M.; Cortes, J.E.; Konopleva, M.; Ravandi-Kashani, F.; Jabbour, E.; Kadia, T. Efficacy, safety, and biomarkers of response to azacitidine and nivolumab in relapsed/refractory acute myeloid leukemia: A nonrandomized, open-label, phase II study. Cancer Discov. 2019, 9, 370–383. [Google Scholar] [CrossRef] [Green Version]
- Azoury, S.C.; Straughan, D.M.; Shukla, V. Immune Checkpoint Inhibitors for Cancer Therapy: Clinical Efficacy and Safety. Curr. Cancer Drug Targets 2015, 15, 452–462. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Yau, T.; Kang, Y.-K.; Kim, T.-Y.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.-M.; Matilla, A.; et al. Nivolumab (NIVO) plus ipilimumab (IPI) combination therapy in patients (Pts) with advanced hepatocellular carcinoma (aHCC): Long-term results from CheckMate 040. J. Clin. Oncol. 2021, 39, 269. [Google Scholar] [CrossRef]
- Feun, L.G.; Li, Y.-Y.; Wu, C.; Wangpaichitr, M.; Jones, P.D.; Richman, S.P.; Madrazo, B.; Kwon, D.; Garcia-Buitrago, M.; Martin, P.; et al. Phase 2 study of pembrolizumab and circulating biomarkers to predict anticancer response in advanced, unresectable hepatocellular carcinoma. Cancer 2019, 125, 3603–3614. [Google Scholar] [CrossRef] [PubMed]
- Bangaru, S.; Marrero, J.A.; Singal, A.G. New therapeutic interventions for advanced hepatocellular carcinoma. Aliment. Pharmacol. Ther. 2020, 51, 78–89. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, phase III trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Pembrolizumab for the treatment of hepatocellular carcinoma. Liver Cancer 2019, 8, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Cho, E.J.; Lee, J.H.; Yu, S.J.; Kim, Y.J.; Yoon, J.H.; Kim, T.Y.; Han, S.W.; Oh, D.Y.; Im, S.A.; et al. Phase II Study of Avelumab in Patients with Advanced Hepatocellular Carcinoma Previously Treated with Sorafenib. Clin. Cancer Res. 2021, 27, 713–718. [Google Scholar] [CrossRef]
- Kudo, M.; Motomura, K.; Wada, Y.; Inaba, Y.; Sakamoto, Y.; Kurosaki, M.; Umeyama, Y.; Kamei, Y.; Yoshimitsu, J.; Fujii, Y.; et al. Avelumab in Combination with Axitinib as First-Line Treatment in Patients with Advanced Hepatocellular Carcinoma: Results from the Phase 1b VEGF Liver 100 Trial. Liver Cancer 2021, 10, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Motomura, K.; Wada, Y.; Inaba, Y.; Sakamoto, Y.; Kurosaki, M.; Umeyama, Y.; Kamei, Y.; Yoshimitsu, J.; Fujii, Y.; et al. First-line avelumab + axitinib in patients with advanced hepatocellular carcinoma: Results from a phase 1b trial (VEGF Liver 100). J. Clin. Oncol. 2019, 37, 4072. [Google Scholar] [CrossRef]
- Kelley, R.K.; Sangro, B.; Harris, W.; Ikeda, M.; Okusaka, T.; Kang, Y.-K.; Qin, S.; Tai, D.W.-M.; Lim, H.Y.; Yau, T.; et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: Randomized expansion of a phase I/II study. J. Clin. Oncol. 2021, 39, 2991–3001. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Abou-Alfa, G.K.; Bendell, J.C.; Kim, T.-Y.; Borad, M.J.; Yong, W.-P.; Morse, M.; Kang, Y.-K.; Rebelatto, M.; Makowsky, M.; et al. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): Phase I safety and efficacy analyses. J. Clin. Oncol. 2017, 35, 4073. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sangro, B.; Morse, M.; Zhu, A.X.; Kim, R.D.; Cheng, A.L.; Kudo, M.; Kang, Y.K.; Chan, S.L.; Antal, J.; et al. Phase 1/2 study of durvalumab and tremelimumab as monotherapy and in combination in patients with unresectable hepatocellular carcinoma (HCC). J. Clin. Oncol. 2016, 34, TPS3103. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Segal, N.H.; Jaeger, D.; Lee, K.-H.; Marshall, J.; Antonia, S.J.; Butler, M.; Sanborn, R.E.; Nemunaitis, J.J.; Carlson, C.A. Safety and clinical activity of durvalumab monotherapy in patients with hepatocellular carcinoma (HCC). J. Clin. Oncol. 2017, 35, 4071. [Google Scholar] [CrossRef]
- Markham, A.; Keam, S.J. Camrelizumab: First Global Approval. Drugs 2019, 79, 1355–1361. [Google Scholar] [CrossRef]
- Qin, S.; Chen, Z.; Liu, Y.; Xiong, J.; Ren, Z.; Meng, Z.; Gu, S.; Wang, L.; Zou, J. A phase II study of anti–PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J. Clin. Oncol. 2019, 37, 4074. [Google Scholar] [CrossRef]
- Naing, A.; Gainor, J.F.; Gelderblom, H.; Forde, P.M.; Butler, M.O.; Lin, C.C.; Sharma, S.; Ochoa de Olza, M.; Varga, A.; Taylor, M.; et al. A first-in-human phase 1 dose escalation study of spartalizumab (PDR001), an anti-PD-1 antibody, in patients with advanced solid tumors. J. Immunother. Cancer 2020, 8, e000530. [Google Scholar] [CrossRef] [Green Version]
- Minami, H.; Doi, T.; Toyoda, M.; Imamura, Y.; Kiyota, N.; Mitsuma, A.; Shimokata, T.; Naito, Y.; Matsubara, N.; Tajima, T.; et al. Phase I study of the antiprogrammed cell death-1 Ab spartalizumab (PDR001) in Japanese patients with advanced malignancies. Cancer Sci. 2021, 112, 725–733. [Google Scholar] [CrossRef]
- Prat, A.; Paz-Ares, L.; Juan, M.; Felip, E.; Garralda, E.; González, B.; Arance, A.; Martín-Liberal, J.; Gavilá, J.; López-González, A.; et al. SOLTI-1904 ACROPOLI TRIAL: Efficacy of spartalizumab monotherapy across tumor-types expressing high levels of PD1 mRNA. Future Oncol. 2022, 18, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Falcon, B.L.; Chintharlapalli, S.; Uhlik, M.T.; Pytowski, B. Antagonist antibodies to vascular endothelial growth factor receptor 2 (VEGFR-2) as anti-angiogenic agents. Pharmacol. Ther. 2016, 164, 204–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.X.; Finn, R.S.; Mulcahy, M.; Gurtler, J.; Sun, W.; Schwartz, J.D.; Dalal, R.P.; Joshi, A.; Hozak, R.R.; Xu, Y.; et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin. Cancer Res. 2013, 19, 6614–6623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, A.X.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Poon, R.; Pastorelli, D.; Blanc, J.F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Baron, A.D.; Malfertheiner, P.; Kudo, M.; Kawazoe, S.; Pezet, D.; Weissinger, F.; Brandi, G.; Barone, C.A.; Okusaka, T.; et al. Ramucirumab as Second-Line Treatment in Patients With Advanced Hepatocellular Carcinoma: Analysis of REACH Trial Results by Child-Pugh Score. JAMA Oncol. 2017, 3, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Finn, R.; Galle, P.; Llovet, J.; Blanc, J.F.; Okusaka, T.; Chau, I.; Abada, P.; Hsu, Y.; Kudo, M. Ramucirumab as second-line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated alpha-fetoprotein (AFP) following first-line sorafenib: Pooled efficacy and safety across two global randomized Phase 3 studies (REACH-2 and REACH). Ann. Oncol. 2018, 29, v122. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Kang, Y.K.; Yen, C.J.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Motomura, K.; Ohno, I.; et al. Serum alpha-fetoprotein and clinical outcomes in patients with advanced hepatocellular carcinoma treated with ramucirumab. Br. J. Cancer 2021, 124, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Choucair, K.; Kamran, S.; Saeed, A. Clinical Evaluation of Ramucirumab for the Treatment of Hepatocellular Carcinoma (HCC): Place in Therapy. Onco Targets Ther. 2021, 14, 5521–5532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, X.; Jiang, Y.; Guo, M.; Zhang, S.; Li, J.; He, J.; Liu, J.; Wang, J.; Ouyang, L. Recent advances in the development of dual VEGFR and c-Met small molecule inhibitors as anticancer drugs. Eur. J. Med. Chem. 2016, 108, 495–504. [Google Scholar] [CrossRef]
- Goyal, L.; Zheng, H.; Abrams, T.A.; Miksad, R.; Bullock, A.J.; Allen, J.N.; Yurgelun, M.B.; Clark, J.W.; Kambadakone, A.; Muzikansky, A. A phase II and biomarker study of sorafenib combined with modified FOLFOX in patients with advanced hepatocellular carcinoma. Clin. Cancer Res. 2019, 25, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Finkelmeier, F.; Scheiner, B.; Leyh, C.; Best, J.; Fründt, T.W.; Czauderna, C.; Beutel, A.; Bettinger, D.; Weiß, J.; Meischl, T.; et al. Cabozantinib in Advanced Hepatocellular Carcinoma: Efficacy and Safety Data from an International Multicenter Real-Life Cohort. Liver Cancer 2021, 10, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Apatinib for molecular targeted therapy in tumor. Drug Des. Dev. Ther. 2015, 9, 6075–6081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, A.J.; Messersmith, W.A.; Jimeno, A. Apatinib: A promising oral antiangiogenic agent in the treatment of multiple solid tumors. Drugs Today 2015, 51, 223–229. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Chen, L.; Guo, H.; Lv, F.; Jia, K.; Yv, K.; Wang, F.; Li, C.; Qian, J.; et al. Safety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignancies. BMC Cancer 2010, 10, 529. [Google Scholar] [CrossRef] [Green Version]
- Tian, S.; Quan, H.; Xie, C.; Guo, H.; Lü, F.; Xu, Y.; Li, J.; Lou, L. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011, 102, 1374–1380. [Google Scholar] [CrossRef] [PubMed]
- Qin, S. Apatinib in Chinese patients with advanced hepatocellular carcinoma: A phase II randomized, open-label trial. J. Clin. Oncol. 2014, 32, 4019. [Google Scholar] [CrossRef]
- Qin, S.; Li, Q.; Gu, S.; Chen, X.; Lin, L.; Wang, Z.; Xu, A.; Chen, X.; Zhou, C.; Ren, Z. Apatinib as second-line or later therapy in patients with advanced hepatocellular carcinoma (AHELP): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol. Hepatol. 2021, 6, 559–568. [Google Scholar] [CrossRef]
- García-Hernández, L.; García-Ortega, M.B.; Ruiz-Alcalá, G.; Carrillo, E.; Marchal, J.A.; García, M.Á. The p38 MAPK Components and Modulators as Biomarkers and Molecular Targets in Cancer. Int. J. Mol. Sci. 2022, 23, 370. [Google Scholar] [CrossRef]
- Thomas, B.J.; Porciani, D.; Burke, D.H. Cancer immunomodulation using bispecific aptamers. Mol. Ther.-Nucleic Acids 2022, 27, 894–915. [Google Scholar] [CrossRef]
- Yang, T.; Huo, J.; Xu, R.; Su, Q.; Tang, W.; Zhang, D.; Zhu, M.; Zhan, Y.; Dai, B.; Zhang, Y. Selenium sulfide disrupts the PLAGL2/C-MET/STAT3-induced resistance against mitochondrial apoptosis in hepatocellular carcinoma. Clin. Transl. Med. 2021, 11, e536. [Google Scholar] [CrossRef]
- Raoof, M.; Malhotra, G.; Kohut, A.; O’Leary, M.; Frankel, P.; Tran, T.; Fakih, M.; Chao, J.; Lim, D.; Woo, Y. PIPAC for the treatment of gynecologic and gastrointestinal peritoneal metastases: Technical and logistic considerations of a Phase 1 trial. Ann. Surg. Oncol. 2022, 29, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.S.; Hanley, K.L.; Liang, Y.; Lin, X. Improving the efficacy of liver cancer immunotherapy: The power of combined preclinical and clinical studies. Hepatology 2021, 73, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Brahma, M.K.; Gilglioni, E.H.; Zhou, L.; Trépo, E.; Chen, P.; Gurzov, E.N. Oxidative stress in obesity-associated hepatocellular carcinoma: Sources, signaling and therapeutic challenges. Oncogene 2021, 40, 5155–5167. [Google Scholar] [CrossRef] [PubMed]
- Mabed, M.; Esmaeel, M.; El-Khodary, T.; Awad, M.; Amer, T. A randomized controlled trial of transcatheter arterial chemoembolization with lipiodol, doxorubicin and cisplatin versus intravenous doxorubicin for patients with unresectable hepatocellular carcinoma. Eur. J. Cancer Care 2009, 18, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Talati, C.; Kim, R. Hepatocellular carcinoma (HCC): Beyond sorafenib—Chemotherapy. J. Gastrointest. Oncol. 2017, 8, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yim, H.J.; Suh, S.J.; Um, S.H. Current management of hepatocellular carcinoma: An Eastern perspective. World J. Gastroenterol. 2015, 21, 3826–3842. [Google Scholar] [CrossRef]
- Lee, J.E.; Bae, S.H.; Choi, J.Y.; Yoon, S.K.; You, Y.K.; Lee, M.A. Epirubicin, cisplatin, 5-FU combination chemotherapy in sorafenib-refractory metastatic hepatocellular carcinoma. World J. Gastroenterol. 2014, 20, 235–241. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, X.-P.; Zhong, B.-Y.; Lau, W.Y.; Madoff, D.C.; Davidson, J.C.; Qi, X.; Cheng, S.-Q.; Teng, G.-J. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: Comparing east and west. Lancet Gastroenterol. Hepatol. 2019, 4, 721–730. [Google Scholar] [CrossRef]
- Louafi, S.; Boige, V.; Ducreux, M.; Bonyhay, L.; Mansourbakht, T.; de Baere, T.; Asnacios, A.; Hannoun, L.; Poynard, T.; Taïeb, J. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC) results of a phase II study. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2007, 109, 1384–1390. [Google Scholar]
- Chia, W.K.; Ong, S.; Toh, H.C.; Hee, S.W.; Choo, S.P.; Poon, D.Y.; Tay, M.H.; Tan, C.K.; Koo, W.H.; Foo, K.F. Phase II trial of gemcitabine in combination with cisplatin in inoperable or advanced hepatocellular carcinoma. Ann. Acad. Med. Singap. 2008, 37, 554–558. [Google Scholar] [CrossRef]
- Lombardi, G.; Zustovich, F.; Farinati, F.; Cillo, U.; Vitale, A.; Zanus, G.; Donach, M.; Farina, M.; Zovato, S.; Pastorelli, D. Pegylated liposomal doxorubicin and gemcitabine in patients with advanced hepatocellular carcinoma: Results of a phase 2 study. Cancer 2011, 117, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Bai, Y.; Lim, H.Y.; Thongprasert, S.; Chao, Y.; Fan, J.; Yang, T.-S.; Bhudhisawasdi, V.; Kang, W.K.; Zhou, Y. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J. Clin. Oncol. 2013, 31, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, J.O.; Kim, W.S.; Park, S.H.; Park, K.W.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2004, 54, 385–390. [Google Scholar] [CrossRef]
- Ikeda, M.; Okusaka, T.; Ueno, H.; Takezako, Y.; Morizane, C. A phase II trial of continuous infusion of 5-fluorouracil, mitoxantrone, and cisplatin for metastatic hepatocellular carcinoma. Cancer 2005, 103, 756–762. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.; Han, S.H.; Kwon, S.Y.; Kwon, O.S.; Kim, S.S.; Kim, J.H.; Park, Y.H.; Lee, J.N.; Bang, S.-M. Systemic chemotherapy with doxorubicin, cisplatin and capecitabine for metastatic hepatocellular carcinoma. BMC Cancer 2006, 6, 3–6. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Tsuji, A.; Morita, S.; Horimi, T.; Shirasaka, T.; Kanematsu, T. A phase II study of LFP therapy (5-FU (5-fluorourasil) continuous infusion (CVI) and Low-dose consecutive (Cisplatin) CDDP) in advanced biliary tract carcinoma. BMC Cancer 2006, 6, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.H.; Park, J.-W.; Nam, B.H.; Lee, W.J.; Kim, C.-M. Efficacy of combination chemotherapy with capecitabine plus cisplatin in patients with unresectable hepatocellular carcinoma. Cancer Chemother. Pharmacol. 2009, 63, 459–467. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.; Oh, D.; Kim, J.; Im, S.; Kim, T.; Bang, Y.-J. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann. Oncol. 2009, 20, 1402–1407. [Google Scholar] [CrossRef]
- Patt, Y.Z.; Hassan, M.M.; Aguayo, A.; Nooka, A.K.; Lozano, R.D.; Curley, S.A.; Vauthey, J.N.; Ellis, L.M.; Schnirer, I.I.; Wolff, R.A. Oral capecitabine for the treatment of hepatocellular carcinoma, cholangiocarcinoma, and gallbladder carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 101, 578–586. [Google Scholar] [CrossRef]
- Porta, C.; Moroni, M.; Nastasi, G.; Arcangeli, G. 5-Fluorouracil and d, l-leucovorin calcium are active to treat unresectable hepatocellular carcinoma patients: Preliminary results of a phase II study. Oncology 1995, 52, 487–491. [Google Scholar] [CrossRef]
- Olweny, C.L.; Toya, T.; Katongole-Mbidde, E.; Mugerwa, J.; Kyalwazi, S.K.; Cohen, H. Treatment of hepatocellular carcinoma with adriamycin. Preliminary communication. Cancer 1975, 36, 1250–1257. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.S.; Lin, Y.C.; Chen, J.S.; Wang, H.M.; Wang, C.H. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2000, 89, 750–756. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Clark, J.W.; Ryan, D.P.; Kulke, M.H.; Kim, H.; Earle, C.C.; Vincitore, M.; Mayer, R.J.; Stuart, K.E. A phase II trial of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer 2002, 94, 3186–3191. [Google Scholar] [CrossRef] [PubMed]
- Kubicka, S.; Rudolph, K.L.; Tietze, M.K.; Lorenz, M.; Manns, M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepato-Gastroenterol. 2001, 48, 783–789. [Google Scholar]
- O’Reilly, E.M.; Stuart, K.E.; Sanz-Altamira, P.M.; Schwartz, G.K.; Steger, C.M.; Raeburn, L.; Kemeny, N.E.; Kelsen, D.P.; Saltz, L.B. A phase II study of irinotecan in patients with advanced hepatocellular carcinoma. Cancer 2001, 91, 101–105. [Google Scholar] [CrossRef]
- Boige, V.; Taïeb, J.; Hebbar, M.; Malka, D.; Debaere, T.; Hannoun, L.; Magherini, E.; Mignard, D.; Poynard, T.; Ducreux, M. Irinotecan as first-line chemotherapy in patients with advanced hepatocellular carcinoma: A multicenter phase II study with dose adjustment according to baseline serum bilirubin level. Eur. J. Cancer 2006, 42, 456–459. [Google Scholar] [CrossRef]
- Leung, T.W.; Patt, Y.Z.; Lau, W.-y.; Ho, S.K.; Simon, C.; Chan, A.T.; Mok, T.S.; Yeo, W.; Liew, C.-t.; Leung, N.W. Complete pathological remission is possible with systemic combination chemotherapy for inoperable hepatocellular carcinoma. Clin. Cancer Res. 1999, 5, 1676–1681. [Google Scholar]
- Abou-Alfa, G.K.; Niedzwieski, D.; Knox, J.J.; Kaubisch, A.; Posey, J.; Tan, B.R.; Kavan, P.; Goel, R.; Murray, J.J.; Bekaii-Saab, T.S.; et al. Phase III randomized study of sorafenib plus doxorubicin versus sorafenib in patients with advanced hepatocellular carcinoma (HCC): CALGB 80802 (Alliance). J. Clin. Oncol. 2016, 34, 192. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Yang, T.; Hsu, C.; Toh, H.; Epstein, R.; Hsiao, L.; Chen, P.; Lin, Z.; Chao, T.-Y.; Cheng, A. Efficacy and tolerability of bevacizumab plus capecitabine as first-line therapy in patients with advanced hepatocellular carcinoma. Br. J. Cancer 2010, 102, 981–986. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Sohal, D.; Haller, D.G.; Mykulowycz, K.; Rosen, M.; Soulen, M.C.; Caparro, M.; Teitelbaum, U.R.; Giantonio, B.; O’Dwyer, P.J. Phase 2 trial of bevacizumab, capecitabine, and oxaliplatin in treatment of advanced hepatocellular carcinoma. Cancer 2011, 117, 3187–3192. [Google Scholar] [CrossRef]
- Asnacios, A.; Fartoux, L.; Romano, O.; Tesmoingt, C.; Louafi, S.S.; Mansoubakht, T.; Artru, P.; Poynard, T.; Rosmorduc, O.; Hebbar, M. Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab in patients with progressive advanced stage hepatocellular carcinoma: Results of a multicenter phase 2 study. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2008, 112, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Blaszkowsky, L.S.; Ryan, D.P.; Clark, J.W.; Muzikansky, A.; Horgan, K.; Sheehan, S.; Hale, K.E.; Enzinger, P.C.; Bhargava, P. Phase II study of gemcitabine and oxaliplatin in combination with bevacizumab in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2006, 24, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Abass, S.A.; Mohamed, A.A.; Muneam Hamid, D. Herbal management of hepatocellular carcinoma through cutting the pathways of the common risk factors. Biomed. Pharmacother. 2018, 107, 1246–1258. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Zaghloul, R.A.; Abdelghany, A.M.; El Gayar, A.M. Selenium nanoparticles and quercetin suppress thioacetamide-induced hepatocellular carcinoma in rats: Attenuation of inflammation involvement. J. Biochem. Mol. Toxicol. 2022, 36, e22989. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhao, L.; Wang, Y.; Wang, Y. Treatment for hepatocellular carcinoma is enhanced when norcantharidin is encapsulated in exosomes derived from bone marrow mesenchymal stem cells. Mol. Pharm. 2021, 18, 1003–1013. [Google Scholar] [CrossRef] [PubMed]




| First Line Treatment | Targets | Study | Patients | Study Type | Dose Evaluated | ORR | PFS | OS | Most Common Adverse Events | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sorafenib | 1 | VEGFR1-3, KIT kinase, PDGFR-β | SHARP | 602 | III | 400 mg twice daily | 2% | 4.1 | 10.7 | Fatigue, weight loss, Diarrhea, palmar-plantar skin reaction, Low phosphor levels | [26] |
| 2 | VEGFR1-3, KIT kinase, PDGFR-β | ORIENTAL | 226 | III | 400 mg twice daily | 3.3% | 2.8 | 6.5 | Fatigue, weight loss, Diarrhea, palmar-plantar skin reaction, Low phosphor levels | [27] | |
| Lenvatinib | RET, c-KIT, VEGFR1-3, FGFR1-4, PDGFRα | REFLECT | 954 | III | ≥60 kg: 12 ng once daily <60 kg: 8 mg once daily | 24% | 7.4 | 13.6 | HTN, decreased weight | [28] | |
| Atezolizumab + Bevacizumab | PD-L1 VEGF-a | IMBRAVE 150 | 501 | III | A: 1200 mg IV e3w + B: 15 mg/kg IV e3w | 27.3% | 6.8 | __ | HTN, Fatigue, Proteinuria, increased AST, Pruritus, Diarrhea, decreased appetite, increased ALT | [29] | |
| Second line treatment | |||||||||||
| Pembrolizumab | PD-1 | KEYNOTE 224 | 104 | II | 200 mg e3w | 17% | 4.9 | 12.9 | Increased AST, increasing ALT, Fatigue, Hyperbilirubinemia | [30] | |
| Cabozantinib | RET, c-MET, AXL, aMET, VEGFR2 | CELESTIAL | 707 | III | 60 mg once daily | 4% | 5.2 | 10.2 | HTN, elevated liver enzymes, Diarrhea, Fatigue, Palmar-planta erythrodysesthesia | [31] | |
| Regorafenib | VEGFR1-3, FGFR, PDGFR, r-KIT, RET, RAF-1, Tie2, BRAF | RESORCE | 573 | III | 160 mg once daily (a cycle of 3 weeks, 1 week off) | 11% | 3.1 | 10.6 | HTN, Hand-foot-skin reactions, increased AST, Fatigue, Diarrhea | [32] | |
| Nivolumab | PD-1 | CheckMate 040 | 212 | I/II | 3 mg/kg IV e2w | 20% | 3.4 | 15 | Pemphigoid, Adrenal insufficiency, Liver disorder | [33,34] | |
| Ramucirumab | VEGFR2 | REACH-2 | 292 | III | 8 mg/kg once daily e2w | 5% | 2.8 | 8.5 | Fatigue, peripheral edema, decreased appetite | [35] | |
| Nivolumab + Ipilimumab | PD-1 CTLA-4 | CheckMate 040 | 148 | I/II | A: (N: 1 mg/kg + I: 3 mg/kg) e3w4c, then N: 240 mg e2w B: (N: 3 mg/kg + I: 1 mg/kg) e3w4c, then N: 240 mg e2w C: (N: 3 mg/kg e2w + I: 1 mg/kg e6w) | A: 32% B: 27% C: 29% | _ | A: 22.8 B: 12.5 C: 12.7 | Hepatic immune-mediated adverse events | [36] | |
| Drugs | Study Type | Dose Evaluated | Patients | Overall Response Rate | Median Survival Rate | Grade 3 or 4 Toxicities | Ref. |
|---|---|---|---|---|---|---|---|
| Gemcitabine plus oxaliplatin (GEMOX) | Phase II | gemcitabine (1000 mg/m2 by fixed-dose rate infusion) on day 1 was followed by oxaliplatin (100 mg/m2) on day 2, with both drugs repeated every two weeks | 32 | 18% | 11.5 months | Thrombocytopenia, neutropenia (with two cases of febrile neutropenia), anemia, and neuropathy | [161] |
| Gemcitabine plus Cisplatin | Phase II | gemcitabine (1250 mg/m2 on days 1 and 8) and cisplatin (70 mg/m2 on day 1 of every 21-day cycle) | 15 | 20% | 18 weeks | Anemia, neutropenia, thrombocytopenia, transaminitis, and mucositis | [162] |
| Pegylated liposomal doxorubicin (PLD) plus gemcitabine | Phase II | gemcitabine (1000 mg/m2 on days 1 and 8) plus PLD (30 mg/m2 on day 1) every 28 days | 41 | 24% | 22.5 months | Neutropenia and thrombocytopenia | [163] |
| Oxaliplatin Plus, Fluorouracil/Leucovorin (FOLFOX4) Versus Doxorubicin | Phase III | Patients received either FOLFOX4 (OXA 85 mg/m2 intravenously (IV) on day 1; LV 200 mg/m2 IV from hour 0 to 2 on days 1 and 2; and FU 400 mg/m2 IV bolus at hour 2, then 600mg/m2 over 22 h on days 1 and 2, once every 2 weeks) or DOX (50 mg/m2 IV, once every 3 weeks) | 371 | 8% with FOLFOX4 and 3% with doxorubicin | 6.40 months with OLFOX4 and 4.97 months with doxorubicin | there were no significant differences in the rate of grade 3 or 4 toxicities | [164] |
| Cisplatin plus doxorubicin | Phase II | doxorubicin 60 mg/m2 delivered as an intravenous infusion over 30 min on day 1, followed by cisplatin 60 mg/m2 infused over 1 h on day 1. The cycle was repeated every 28 days | 42 | 18.9% | 7.3 months | neutropenia, thrombocytopenia, and diarrhea | [165] |
| Cisplatin, mitoxantrone, and continuous-infusion fluorouracil | Phase II | intravenous administration of 80 mg/m2 cisplatin and 6 mg/m2 mitoxantrone on day 1 and continuous intravenous infusion of 450 mg/m2 5-fluorouracil per day on days 1–5. The treatment was repeated every 4 weeks for a maximum of 6 courses | 51 | 27% | 11.6 months | leukocytopenia, neutropenia, thrombocytopenia, and elevated levels of aspartate aminotransferase and alanine aminotransferase | [166] |
| Cisplatin, epirubicin, and infusional fluorouracil | Phase II | epirubicin 60 mg/m2 on day 2, cisplatinum 50 mg/m2 on day 2, and 5-fluorouracil 200 mg/m2 administered as a continuous infusion from day 1 to day 21. Courses were repeated every 21 days. | 21 | 15% | 10 months | mainly hematological, and one patient experienced a grade 4 renal toxicity | [166] |
| Cisplatin, doxorubicin, and capecitabine | Phase II | doxorubicin 60 mg/m2 and cisplatin 60 mg/m2 on day 1, plus capecitabine 2000 mg/m2/day as an intermittent regimen of 2 weeks of treatment followed by a 1-week rest | 29 | 24% | 7.7 months | febrile neutropenia | [167] |
| low-dose infusional cisplatin plus infusional fluorouracil | Phase II | 5-FU (160 mg/m2/day) was continuously infused over 24 h for 7 consecutive days and CDDP (6 mg/m2/day) was infused for 30 min twice a week as one cycle | 42 | 42.9% | N. A. | appetite loss | [168] |
| Cisplatin plus capecitabine | Phase II | Capecitabine was administered orally at a dose of 1000 mg/m2 twice a day (14 days of treatment followed by a 1- or 2-week rest period) Cisplatin was administered intravenously on days 1 and 8 at a dose of 30 mg/m2 for 30 min | 178 | 19.7% | 10.5 months | Nausea/Vomiting, diarrhea, hand–foot syndrome, hyperbilirubinemia and elevated alanine amino transferase (ALT) | [169] |
| Cisplatin plus capecitabine | Phase II | oral capecitabine (2000 mg/m2/day) with a schedule of 2 weeks on and 1 week off and cisplatin (60 mg/m2) on the first day of the 3-week cycle | 32 | 6.3% | 12.2 months | thrombocytopenia, neutropenia, anemia, elevated hepatic aminotransferase, jaundice, mucositis | [170] |
| Capecitabine | Phase II | 1000 mg/m2 twice daily for 14 of every 21 days | 37 | 25% | 10.1 months | thrombocytopenia | [171] |
| 5-Fluorouracil and Leucovorin Calcium | Phase II | 370 mg/m2 5-fluorouracil (5-FU) plus 200 mg/m2 racemic leucovorin both for 5 consecutive days. | 25 | 28% | N. A. | Mucositis, granulocytopenia | [172] |
| Doxorubicin | Phase II | 75 mg/m2 every 3 weeks | 14 | 78.6% | 8 months | myelosuppression, anorexia, nausea, vomiting, and alopecia | [173] |
| gemcitabine | Phase II | 1250 mg/m2 intravenously over 30 min weekly | 28 | 17.8% | 18.7 weeks | leucopenia, anemia, thrombocytopenia, and hepatotoxicity | [174] |
| gemcitabine | Phase II | 1000 mg/m2 intravenously over 30 min weekly | 30 | 0% | 6.9 months | Neutropenia, thrombocytopenia | [175] |
| gemcitabine | Phase II | once weekly over 30 min for 3 consecutive weeks out of every 4 weeks | 20 | 5% | N. A. | thrombocytopenia | [176] |
| irinotecan | Phase II | 125 mg/m2, weekly for 4 weeks followed by a 2-week break | 14 | 7% | 8.2 months | Neutropenia, diarrhea, nausea and vomiting, fatigue, | [177] |
| irinotecan | Phase II | group A: 350 mg/m2 when total bilirubin level was ≤1.5 times upper limit of normal (ULN0 group B: 200 mg/m2 when total bilirubin level was between 1.51 and 3 ULN | 29 (group A, 23; group B, 6) | 0% | 7.4 months | neutropenia, anemia, and diarrhea | [178] |
| cisplatin, doxorubicin, 5-fluorouracil, and α-IFN (PIAF) | Phase II | cisplatin (20 mg/m2 i.v., days 1–4), doxorubicin (40 mg/m2 i.v., day 1), 5-fluorouracil (400 mg/m2 i.v., days 1–4), and α-IFN (5 MU/m2 s.c., days 1–4) | 50 | 26% | 8.9 months | myelosuppression and mucositis | [179] |
| Drugs | Study Type | Dose Evaluated | Patients | Median OS | ORR | Median PFS | Ref. |
|---|---|---|---|---|---|---|---|
| sorafenib plus doxorubicin (S+D) versus sorafenib (S) | Phase III | Doxorubicin (D) 60 mg/m2 every 21 days plus sorafenib (S) 400 mg PO twice daily (D+S) or S alone | 346 (173 on each of D+S and S) | 9.3 months for D+S, and 10.5 months for S | N. A. | 3.6 months for D+S, and 3.2 months for S | [180] |
| bevacizumab plus capecitabine | Phase II | bevacizumab 7.5 mg kg–1 on day 1 and capecitabine 800 mg m–2 twice daily on days 1–14 every 3 weeks | 45 | 5.9 months | 9% | 2.7 months | [181] |
| bevacizumab, capecitabine, and oxaliplatin | Phase II | bevacizumab 5 mg/kg and oxaliplatin 130 mg/m2 on day 1 of each cycle, and capecitabine 825 mg/m2 orally twice a day from days 1 to 14 of a 21-day cycle | 40 | 9.8 month | N. A. | 6.8 months | [182] |
| Gemcitabine plus oxaliplatin (GEMOX) combined with cetuximab | Phase II | cetuximab at a dose of 400 mg/m2 initially then 250 mg/m2 weekly, plus gemcitabine at a dose of 1000 mg/m2 on Day 1 and oxaliplatin at a dose of 100 mg/m2 on Day 2, every 2 weeks | 45 | 9.5 months | N. A. | 4.7 months | [183] |
| gemcitabine, oxaliplatin and bevacizumab | Phase II | bevacizumab 10 mg/kg was administered alone intravenously on day 1. For cycle 2 and beyond (28 days/cycle), bevacizumab 10 mg/kg was administered on days 1 and 15, gemcitabine 1000 mg/m2 was administered as a dose rate infusion at 10 mg/m2/min followed by oxaliplatin at 85 mg/m2 on days 2 and 16 | 33 | 9.6 months | N. A. | 5.3 months | [184] |
| Agents | Name (Line) | Target | n | RR (%) | DCR (%) | TTP | OS | Most Common Adverse Effects (Grade ≥ 3) |
|---|---|---|---|---|---|---|---|---|
| Molecular Targeted Therapy Agents | ||||||||
| Sorafenib Placebo | SHARP (1st) | Multikinases | 299 303 | 2 1 | 43 32 | 4.1 4.9 | 10.7 7.9 | Diarrhea (8% vs. 2%) Weight Loss (2% vs. 0%) Hand–foot syndrome (8% vs. 1%) Hypophosphatemia (11% vs. 2%) |
| ORIENTAL (1st) | Multikinases | 150 76 | 3.3 1.3 | 35.3 15.8 | 2.8 1.4 | 6.5 4.2 | Hand–foot syndrome (10.7% vs. 0%) Diarrhea (6% vs. 0%) Fatigue (3.4% vs. 1.3%) | |
| Sunitinib Sorafenib | SUN1170 (1st) | VEGFR, PDGFR Multikinases | 530 542 | 6.6 6.1 | 50.8 51.5 | 4.1 3.8 | 7.9 10.2 | Thrombocytopenia (29.7% vs. 4.7%) Neutropenia (25.7% vs. 2.2%) Hand-foot syndrome (13.3% vs. 21.3%) |
| Brivanib Sorafenib | BRISK-FL (1st) | VEGFR, PDGFR, FGFR Multikinases | 577 578 | 12 9 | 66 65 | 4.2 4.1 | 9.5 9.9 | Hyponatremia (23% vs. 9%) Elevated AST (15% vs. 17%) Fatigue (15% vs. 7%) Hand-foot syndrome (2% vs. 15%) Hypertension (13% vs. 5%) |
| Brivanib Placebo | BRISK-PS (2nd) | VEGFR, PDGFR, FGFR | 263 132 | 10 2 | 61 40 | 4.2 2.7 | 9.4 8.2 | Hypertension (17% vs. 2%) Fatigue (13% vs. 1%) Hyponatremia (13% vs. 2%) Decreased Appetite (10% vs. 2%) |
| Linifanib Sorafenib | LiGHT (1st) | VEGFR, PDGFR Multikinases | 514 521 | 13 6.9 | N. A. N. A. | 5.4 4 | 9.1 9.8 | Hypertension (20.8% vs. 10.6%) Fatigue (9.6% vs. 4.8%) Hepatic Encephalopathy (7.3% vs. 3.3%) Asthenia (7.1% vs. 2.1%) Ascites (6.1% vs. 3.3%) Thrombocytopenia (5.3% vs. 2.1%) Hypokalemia (4.7% vs. 2.3%) Vomiting (4.3% vs. 0.8%) Hypoglycemia (3.1% vs. 0.8%) |
| Lenvatinib Sorafenib | REFLECT (1st) | Multikinases Multikinases | 478 476 | 24 10 | 75.5 60.5 | 8.9 3.7 | 13.6 12.3 | Hand–foot syndrome (3% vs. 11%) Diarrhea (4% vs. 4%) Hypertension (23% vs. 14%) Decreased Appetite (5% vs. 1%)Decreased Weight (8% vs. 3%) Fatigue (4% vs. 4%) |
| Donafenib Sorafenib | ZGDH3 (1st) | Multikinases Multikinases | 328 331 | 4.6 2.7 | 30.8 28.7 | 3.7 3.7 | 12.1 10.3 | Hand-foot syndrome (6% vs. 12%) Diarrhea (2% vs. 3%) Decreased Platelet Count (4% vs. 2%) Hypertension (9% vs. 9%) Elevated AST (2% vs. 5%) Elevated ALT (2% vs. 3%) Hypophosphatemia (3% vs. 5%) |
| Nintedanib Sorafenib | NCT00987935 (1st) | Multikinases Multikinases | 63 32 | 6.3 3.1 | 68.3 84.4 | 2.8 3.7 | 10.2 10.7 | Anemia (7.9% vs. 9.4%) Thrombocytopenia (7.9% vs. 6.3%) Diarrhea (6.3% vs. 6.3%) Elevated AST (4.8% vs. 21.9%) Elevated ALT (3.2% vs. 9.4%) Decreased Appetite (4.8% vs. 6.3%) |
| NCT01004003 (1st) | Multikinases Multikinases | 62 31 | 1.6 6.5 | 82.3 90.3 | 5.5 4.6 | 11.9 11.4 | Anemia (6.5% vs. 3.2%) Thrombocytopenia (1.6% vs. 9.7%) Diarrhea (12.9% vs. 3.2%) Elevated AST (11.3% vs. 3.2%) Elevated ALT (8.1% vs. 6.5%) Decreased Appetite (1.6% vs. 3.2%) | |
| Dovitinib Sorafenib | N. A. | Multikinases Multikinases | 82 83 | 6.1 10.8 | 57.3 63.9 | 4.1 4.1 | 8 8.4 | Diarrhea (11% vs. 1%) Decreased Appetite (8% vs. 5%) Fatigue (14% vs. 2%) Elevated AST (20% vs. 24%) Elevated ALT (17% vs. 10%) Hypertension (13% vs. 11%) Decreased Platelet Count (8% vs. 5%) |
| Everolimus Placebo | EVOLVE-1 (2nd) | mTOR | 362 184 | 2.2 1.6 | 56.1 45.1 | 3 2.6 | 7.6 7.3 | Decreased Appetite (6.1% vs. 0.5%) Fatigue (4.5% vs. 3.8%) Asthenia (7.8% vs. 5.5%) Anemia (7.7% vs. 3.3%) Elevated AST (4.5% vs. 5.5%) |
| Tislelizumab Sorafenib | RATIONALE-301 (1st) | PD-1 | 342 332 | 14.3 5.4 | N. A. | 2.1 3.4 | 15.9 14.1 | Elevated AST (8% vs. 10%) Elevated ALT (2% vs. 9%) Hypertension (3% vs. 11%) Hand-foot syndrome (0% vs. 17%) |
| Regorafenib Placebo | RESORCE (2nd) | Multikinases | 379 194 | 11 4 | 65 36 | 3.2 1.5 | 10.6 7.8 | Hypertension (15% vs. 5%) Hand-foot syndrome (13% vs. 1%) Fatigue (9% vs. 5%) Diarrhea (3% vs. 0%) |
| Ramucirumab Placebo | REACH-2 (2nd) | VEGFR2 | 197 95 | 5 1 | 59.9 38.9 | 3 1.6 | 8.5 7.3 | Hypertension (13% vs. 5%) Hyponatremia (6% vs. 0%) Elevated AST (3% vs. 5%) |
| REACH (2nd) | VEGFR2 | 283 282 | 8 1 | 56 46 | 3.5 2.6 | 9.2 7.6 | Ascites (5% vs. 4%) Hypertension (12% vs. 4%) Asthenia (5% vs. 2%) Malignancy Progression (6% vs. 4%) Elevated AST (5% vs. 8%) Thrombocytopenia (5% vs. 1%) Hyperbilirubinemia (1% vs. 5%) | |
| Cabozantinib Placebo | CELESTIAL (2nd) | Multikinases | 470 237 | 4 1 | 64 33 | 5.2 1.9 | 10.2 8 | Diarrhea (10% vs. 2%) Decreased Appetite (6% vs. 1%) Fatigue (10% vs. 4%) Hypertension (16% vs. 2%) Elevated AST (12% vs. 7%) Asthenia (8% vs. 2%) |
| 1: Cabozantinib + Atezolizumab 2: Sorafenib 3: Cabozantinib | COSMIC-312 (1st) | Multikinases + PD-L1 | 432 217 188 | 12 4 6 | 78 65 84 | 7 4.6 6.8 | 15.4 15.5 N. A. | Elevated AST (9% vs. 4% vs. 10%) Elevated ALT (9% vs. 3% vs. 6%) Hypertension (9% vs. 8% vs. 12%) Hand-foot syndrome (8% vs. 8% vs. 9%) |
| Apatinib Sorafenib | AHELP (2nd) | VEGF | 261 132 | 11 2 | 61 29 | 4.7 1.9 | 8.7 6.8 | Hypertension (28% vs. 2%) Hand–foot syndrome (18% vs. 0%) Decreased platelet count (13% vs. 1%) |
| Apatinib + Camrelizumab | RESCUE (1st and 2nd) | VEGF + PD-1 | 1st: 70 2nd: 120 | 34.3 22.5 | 77.1 75.8 | 5.7 5.5 | 12mSR | Hypertension (40% vs. 30.8%) Elevated AST (10% vs. 10.8%) Elevated ALT (8.6% vs. 6.7%) Proteinuria (7.1% vs. 6.7%) Hyperbilirubinemia (14.3% vs. 8.3%) Thrombocytopenia (10% vs. 10%) Hand-foot syndrome (8.6% vs. 9.2%) |
| 74.7 68.2 | ||||||||
| Immune Checkpoint Inhibitors | ||||||||
| Atezolizumab + Bevacizumab Sorafenib | IMbrave150 (1st) | PD-L1 + VEGF Multikinases | 336 165 | 27.3 11.9 | 73.6 55.3 | 11.2 3.6 | 19.2 13.4 | Hypertension (15.2% vs. 12.2%) Fatigue (2.4% vs. 3.2%) Diarrhea (1.8% vs. 5.1%) Decreased Appetite (1.2% vs. 3.8%) Elevated AST (7% vs. 5.1%) |
| Sintilimab + IBI305 Sorafenib | ORIENT-32 (1st) | PD 1 + VEGF Multikinases | 380 191 | 21 4 | 72 64 | 4.6 2.8 | NR 10.4 | Hypertension (14% vs. 6%) Decreased platelet count (8% vs. 3%) Proteinuria (5% vs. 2%) |
| Nivolumab | CheckMate-040 (2nd) | PD-1 Multikinases | 1: 24 2: 25 | 13 12 | 50 60 | 2.2 3.4 | 7.4 9.8 | Elevated Amylase (8% vs. 0%) Elevated AST (8% vs. 0%) |
| Nivolumab Sorafenib | CheckMate-459 (1st) | PD-1 Multikinases | 371 372 | 16 7 | 55 58 | 3.8 3.9 | 16.4 14.7 | Hand-foot syndrome (1% vs. 14%) Elevated AST (6% vs. 4%) Hypertension (0% vs. 7%) |
| Nivolumab + Ipilimumab (3 Arms) | CheckMate-040 (2nd) | CTLA-4 + PD-1 | 50 49 49 | 32 27 29 | 54 43 49 | N. A. | 22.8 12.5 12.7 | Diarrhea (4% vs. 2% vs. 2%) Elevated AST (16% vs. 8% vs. 2%) Elevated ALT (8% vs. 6% vs. 0%) Lipase Increase (12% vs. 6% vs. 8%) Hepatitis (20% vs. 10% vs. 6%) |
| Pembrolizumab | KEYNOTE-224 (2nd) | PD-1 | 104 | 17 | 62 | 4.9 | 12.9 | Elevated AST (7%) Elevated ALT (4%) Fatigue (4%) |
| Pembrolizumab Placebo | KEYNOTE-240 (2nd) | PD-1 | 278 135 | 18.3 4.4 | 62.2 53.3 | 3.8 2.8 | 13.9 10.6 | Elevated AST (13.3% vs. 7.5%) Elevated ALT (6.1% vs. 3%) Bilirubin Increase (7.5% vs. 5.2%) Fatigue (2.5% vs. 1.5%) Diarrhea (1.4% vs. 2.2%) Anemia (3.9% vs. 9%) |
| KEYNOTE-394 (2nd) | PD-1 | 300 153 | 12.7 1.3 | 51 47.1 | 2.7 1.7 | 14.6 13 | Elevated AST (2.3% vs. 2%) Elevated ALT (2% vs. 1.3%) Diarrhea (0.7% vs. 0%) Platelet count Decrease (1.3% vs. 0.7%) | |
| Avelumab | NCT03389126 (2nd) | PD-L1 | 30 | 10 | 73.3 | 4.4 | 14.2 | Elevated AST/ALT (13.3%) Thrombocytopenia (3.3%) Bilirubin Increase (3.3%) |
| Durvalumab + Tremelimumab (4 Arms) | NCT02519348 (2nd) | CTLA-4 + PD-1 | 74 101 69 82 | 24 10.6 7.2 9.5 | 45.3 37.5 49.3 36.9 | 1.86 3.65 1.81 2.86 | 18.7 13.6 15.1 11.3 | AST (12.2% vs. 3% vs. 8.7% vs. 8.5%) ALT (4.1% vs. 0% vs. 4.3% vs. 2.4%) Lipase (6.8% vs. 0% vs. 5.8% vs. 4.9%) Amylase (6.8% vs. 1% vs. 0% vs. 1.2%) |
| STRIDE Arm: Durvalumab + Tremelimumab Durvalumab Arm Sorafenib Arm | HIMALAYA (1st) | CTLA-4 + PD-1 PD-1 Multikinases | 388 388 374 | 20.1 16.9 5.1 | 60.1 54.8 60.7 | N. A. | 16.4 16.6 13.8 | Grade ≥ 3 (25.8% vs. 12.9% vs. 36.9%) |
| Durvalumab | NCT01693562 (1st) | PD-L1 | 39 | 10.3 | N. A. | N. A. | 13.2 | Elevated AST (7.5%) Elevated ALT (5%) |
| Camrelizumab | NCT02989922 (2nd) | PD-1 | 217 | 14.7 | 44.2 | 2.1 | 13.8 | Elevated AST (5%) Elevated Bilirubin (3%) Decreased Platelet Count (2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farasati Far, B.; Rabie, D.; Hemati, P.; Fooladpanjeh, P.; Faal Hamedanchi, N.; Broomand Lomer, N.; Karimi Rouzbahani, A.; Naimi-Jamal, M.R. Unresectable Hepatocellular Carcinoma: A Review of New Advances with Focus on Targeted Therapy and Immunotherapy. Livers 2023, 3, 121-160. https://doi.org/10.3390/livers3010011
Farasati Far B, Rabie D, Hemati P, Fooladpanjeh P, Faal Hamedanchi N, Broomand Lomer N, Karimi Rouzbahani A, Naimi-Jamal MR. Unresectable Hepatocellular Carcinoma: A Review of New Advances with Focus on Targeted Therapy and Immunotherapy. Livers. 2023; 3(1):121-160. https://doi.org/10.3390/livers3010011
Chicago/Turabian StyleFarasati Far, Bahareh, Dorsa Rabie, Parisa Hemati, Parastoo Fooladpanjeh, Neda Faal Hamedanchi, Nima Broomand Lomer, Arian Karimi Rouzbahani, and Mohammad Reza Naimi-Jamal. 2023. "Unresectable Hepatocellular Carcinoma: A Review of New Advances with Focus on Targeted Therapy and Immunotherapy" Livers 3, no. 1: 121-160. https://doi.org/10.3390/livers3010011
APA StyleFarasati Far, B., Rabie, D., Hemati, P., Fooladpanjeh, P., Faal Hamedanchi, N., Broomand Lomer, N., Karimi Rouzbahani, A., & Naimi-Jamal, M. R. (2023). Unresectable Hepatocellular Carcinoma: A Review of New Advances with Focus on Targeted Therapy and Immunotherapy. Livers, 3(1), 121-160. https://doi.org/10.3390/livers3010011






