Role of Mitochondrial Iron Uptake in Acetaminophen Hepatotoxicity †
Abstract
:1. Introduction
1.1. Epidemiology of Acetaminophen Hepatotoxicity
1.2. Metabolism of APAP
1.3. Risk Factors of APAP Hepatotoxicity
1.4. Treatment for APAP Hepatotoxicity
2. Role of Mitochondria in Pathogenesis of APAP Hepatotoxicity
2.1. Mitochondrial Permeability Transition in APAP Hepatotoxicity
2.2. Apoptosis and Necrosis in APAP Hepatotoxicity
2.3. c-Jun N-Terminal Protein Kinase Activation in APAP Hepatotoxicity
3. Role of Oxidative Stress in APAP Hepatotoxicity
4. Iron Metabolism
4.1. Cellular Iron Metabolism
4.2. Mitochondrial Iron Metabolism
4.3. Role of Iron in Common Models of Acute Liver Injury
5. Iron and Acetaminophen Hepatotoxicity
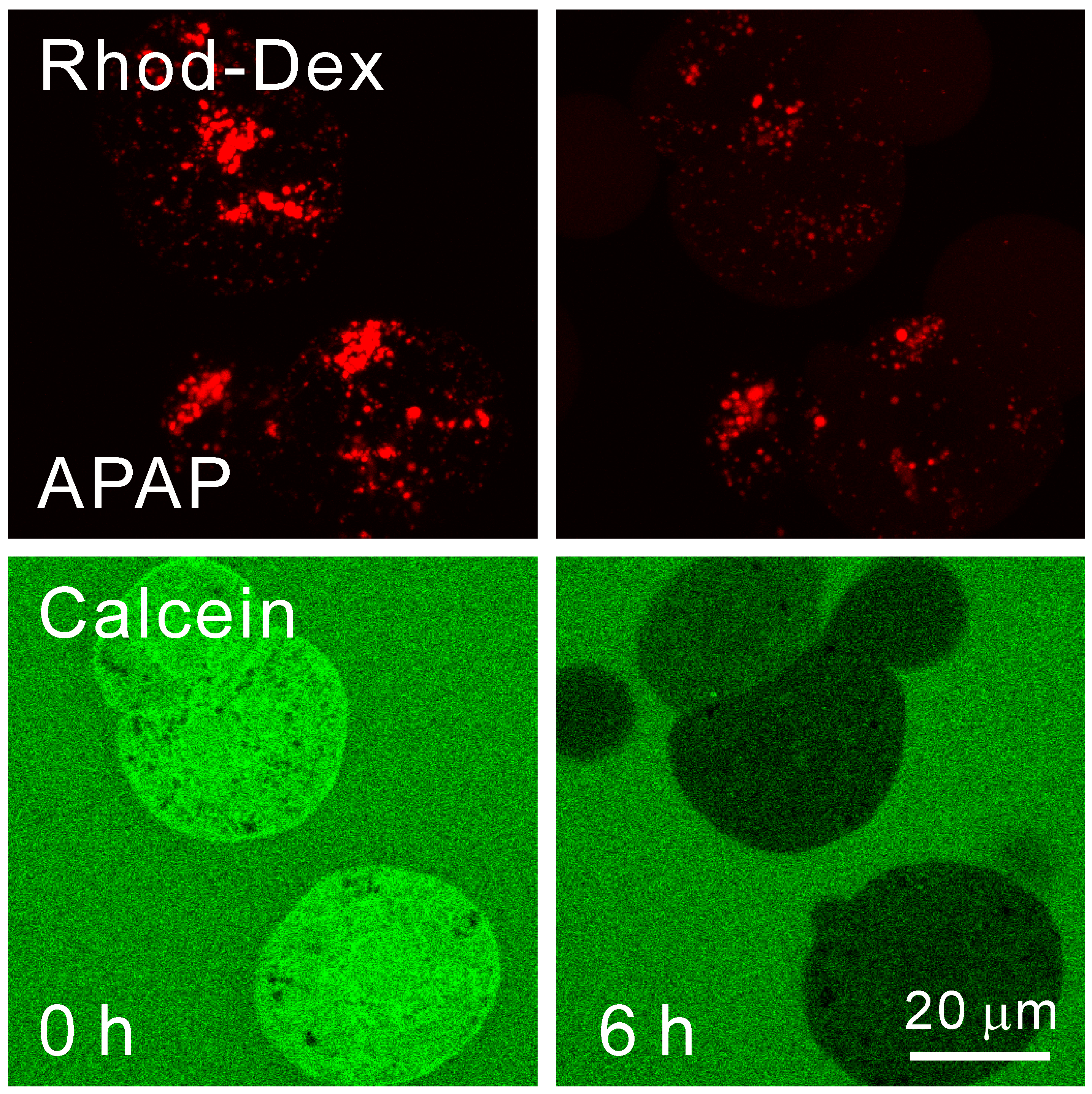
5.1. Evidence for Mitochondrial Iron Uptake in Acetaminophen Hepatotoxicity
5.2. Role of the Mitochondrial Calcium Uniporter in Mitochondrial Iron Uptake during Acetaminophen Hepatotoxicity
5.3. Possible Roles of Kupffer Cells and JNK in Iron-Dependency of Acetaminophen Hepatotoxicity
5.4. ”Two Hit” Hypothesis
5.5. Ferroptosis during Acetaminophen Hepatotoxicity
5.6. Role of Peroxynitrite and Protein Nitration in Acetaminophen Hepatotoxicity
5.7. Aldehydes as Drivers of Acetaminophen Hepatotoxicity
6. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Fisher, E.S.; Curry, S.C. Evaluation and treatment of acetaminophen toxicity. Adv. Pharmacol. 2019, 85, 263–272. [Google Scholar] [CrossRef]
- Michna, E.; Duh, M.S.; Korves, C.; Dahl, J.L. Removal of opioid/acetaminophen combination prescription pain medications: Assessing the evidence for hepatotoxicity and consequences of removal of these medications. Pain Med. 2010, 11, 369–378. [Google Scholar] [CrossRef]
- Blieden, M.; Paramore, L.C.; Shah, D.; Ben-Joseph, R. A perspective on the epidemiology of acetaminophen exposure and toxicity in the United States. Expert Rev. Clin. Pharmacol. 2014, 7, 341–348. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Jollow, D.J.; Potter, W.Z.; Gillette, J.R.; Brodie, B.B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973, 187, 211–217. [Google Scholar]
- McGill, M.R.; Jaeschke, H. Metabolism and disposition of acetaminophen: Recent advances in relation to hepatotoxicity and diagnosis. Pharm. Res. 2013, 30, 2174–2187. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, A.; Jaeschke, H. Mitochondria in Acetaminophen-Induced Liver Injury and Recovery: A Concise Review. Livers 2023, 3, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Rajan, S.S.; Essien, E.J.; Sansgiry, S.S. Effectiveness of FDA’s new over-the-counter acetaminophen warning label in improving consumer risk perception of liver damage. J. Clin. Pharm. Ther. 2012, 37, 681–685. [Google Scholar] [CrossRef]
- Schilling, A.; Corey, R.; Leonard, M.; Eghtesad, B. Acetaminophen: Old drug, new warnings. Cleve Clin. J. Med. 2010, 77, 19–27. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R.; James, L.P.; McCullough, S.S.; Moran, J.H.; Mathews, S.E.; Peterson, E.C.; Fleming, D.P.; Tripod, M.E.; Vazquez, J.H.; Kennon-McGill, S.; et al. Short-Term Safety of Repeated Acetaminophen Use in Patients with Compensated Cirrhosis. Hepatol. Commun. 2022, 6, 361–373. [Google Scholar] [CrossRef]
- Myers, R.P.; Shaheen, A.A.; Li, B.; Dean, S.; Quan, H. Impact of liver disease, alcohol abuse, and unintentional ingestions on the outcomes of acetaminophen overdose. Clin. Gastroenterol. Hepatol. 2008, 6, 918–925, quiz 837. [Google Scholar] [CrossRef]
- Bacle, A.; Pronier, C.; Gilardi, H.; Polard, E.; Potin, S.; Scailteux, L.M. Hepatotoxicity risk factors and acetaminophen dose adjustment, do prescribers give this issue adequate consideration? A French university hospital study. Eur. J. Clin. Pharmacol. 2019, 75, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Vogt, B.L.; Richie, J.P., Jr. Fasting-induced depletion of glutathione in the aging mouse. Biochem. Pharmacol. 1993, 46, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, J.; Riordan, S.M. Paracetamol-induced hepatotoxicity at recommended dosage. J. Intern. Med. 2003, 253, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, H.J.; Maddrey, W.C. Acetaminophen (paracetamol) hepatotoxicity with regular intake of alcohol: Analysis of instances of therapeutic misadventure. Hepatology 1995, 22, 767–773. [Google Scholar] [CrossRef] [PubMed]
- James, L.P.; Alonso, E.M.; Hynan, L.S.; Hinson, J.A.; Davern, T.J.; Lee, W.M.; Squires, R.H.; Pediatric Acute Liver Failure Study, G. Detection of acetaminophen protein adducts in children with acute liver failure of indeterminate cause. Pediatrics 2006, 118, e676–e681. [Google Scholar] [CrossRef] [PubMed]
- Chidiac, A.S.; Buckley, N.A.; Noghrehchi, F.; Cairns, R. Paracetamol (acetaminophen) overdose and hepatotoxicity: Mechanism, treatment, prevention measures, and estimates of burden of disease. Expert Opin. Drug Metab. Toxicol. 2023, 19, 297–317. [Google Scholar] [CrossRef]
- Suzuki, A.; Yuen, N.; Walsh, J.; Papay, J.; Hunt, C.M.; Diehl, A.M. Co-medications that modulate liver injury and repair influence clinical outcome of acetaminophen-associated liver injury. Clin. Gastroenterol. Hepatol. 2009, 7, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef] [PubMed]
- Michaut, A.; Moreau, C.; Robin, M.A.; Fromenty, B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014, 34, e171–e179. [Google Scholar] [CrossRef] [PubMed]
- Larson, A.M. Acetaminophen hepatotoxicity. Clin. Liver Dis. 2007, 11, 525–548. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen-related hepatotoxicity. Clin. Liver Dis. 2013, 17, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Rumack, B.H.; Peterson, R.C.; Koch, G.G.; Amara, I.A. Acetaminophen overdose. 662 cases with evaluation of oral acetylcysteine treatment. Arch. Intern. Med. 1981, 141, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Perry, H.E.; Shannon, M.W. Efficacy of oral versus intravenous N-acetylcysteine in acetaminophen overdose: Results of an open-label, clinical trial. J. Pediatr. 1998, 132, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Yeates, P.J.; Thomas, S.H. Effectiveness of delayed activated charcoal administration in simulated paracetamol (acetaminophen) overdose. Br. J. Clin. Pharmacol. 2000, 49, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.W.; Galanko, J.A.; Shrestha, R.; Fried, M.W.; Watkins, P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004, 10, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Athersuch, T.J.; Antoine, D.J.; Boobis, A.R.; Coen, M.; Daly, A.K.; Possamai, L.; Nicholson, J.K.; Wilson, I.D. Paracetamol metabolism, hepatotoxicity, biomarkers and therapeutic interventions: A perspective. Toxicol. Res. 2018, 7, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Adelusi, O.B.; Ramachandran, A.; Lemasters, J.J.; Jaeschke, H. The role of Iron in lipid peroxidation and protein nitration during acetaminophen-induced liver injury in mice. Toxicol. Appl. Pharmacol. 2022, 445, 116043. [Google Scholar] [CrossRef] [PubMed]
- Anandatheerthavarada, H.K.; Addya, S.; Dwivedi, R.S.; Biswas, G.; Mullick, J.; Avadhani, N.G. Localization of multiple forms of inducible cytochromes P450 in rat liver mitochondria: Immunological characteristics and patterns of xenobiotic substrate metabolism. Arch. Biochem. Biophys. 1997, 339, 136–150. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.A.; Anandatheerthavarada, H.K.; Fang, J.K.; Cudic, M.; Otvos, L.; Avadhani, N.G. Mitochondrial targeted cytochrome P450 2E1 (P450 MT5) contains an intact N terminus and requires mitochondrial specific electron transfer proteins for activity. J. Biol. Chem. 2001, 276, 24680–24689. [Google Scholar] [CrossRef]
- Massart, J.; Begriche, K.; Hartman, J.H.; Fromenty, B. Role of Mitochondrial Cytochrome P450 2E1 in Healthy and Diseased Liver. Cells 2022, 11, 288. [Google Scholar] [CrossRef]
- Kon, K.; Kim, J.S.; Jaeschke, H.; Lemasters, J.J. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology 2004, 40, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, N.; Shinohara, M.; Saberi, B.; Gaarde, W.A.; Han, D.; Kaplowitz, N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008, 283, 13565–13577. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ramshesh, V.K.; McGill, M.R.; Jaeschke, H.; Lemasters, J.J. Low Dose Acetaminophen Induces Reversible Mitochondrial Dysfunction Associated with Transient c-Jun N-Terminal Kinase Activation in Mouse Liver. Toxicol. Sci. 2016, 150, 204–215. [Google Scholar] [CrossRef]
- Dunn, K.W.; Martinez, M.M.; Wang, Z.; Mang, H.E.; Clendenon, S.G.; Sluka, J.P.; Glazier, J.A.; Klaunig, J.E. Mitochondrial depolarization and repolarization in the early stages of acetaminophen hepatotoxicity in mice. Toxicology 2020, 439, 152464. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, M.; Szabo, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Antoniel, M.; Giorgio, V.; Fogolari, F.; Glick, G.D.; Bernardi, P.; Lippe, G. The oligomycin-sensitivity conferring protein of mitochondrial ATP synthase: Emerging new roles in mitochondrial pathophysiology. Int. J. Mol. Sci. 2014, 15, 7513–7536. [Google Scholar] [CrossRef] [PubMed]
- Crompton, M.; Ellinger, H.; Costi, A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988, 255, 357–360. [Google Scholar] [PubMed]
- Waldmeier, P.C.; Feldtrauer, J.J.; Qian, T.; Lemasters, J.J. Inhibition of the mitochondrial permeability transition by the nonimmunosuppressive cyclosporin derivative NIM811. Mol. Pharmacol. 2002, 62, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Krauskopf, A.; Eriksson, O.; Craigen, W.J.; Forte, M.A.; Bernardi, P. Properties of the permeability transition in VDAC1(-/-) mitochondria. Biochim. Biophys. Acta 2006, 1757, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Baines, C.P.; Kaiser, R.A.; Sheiko, T.; Craigen, W.J.; Molkentin, J.D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 2007, 9, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Kokoszka, J.E.; Waymire, K.G.; Levy, S.E.; Sligh, J.E.; Cai, J.; Jones, D.P.; MacGregor, G.R.; Wallace, D.C. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature 2004, 427, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Karch, J.; Bround, M.J.; Khalil, H.; Sargent, M.A.; Latchman, N.; Terada, N.; Peixoto, P.M.; Molkentin, J.D. Inhibition of mitochondrial permeability transition by deletion of the ANT family and CypD. Sci. Adv. 2019, 5, eaaw4597. [Google Scholar] [CrossRef] [PubMed]
- Bround, M.J.; Havens, J.R.; York, A.J.; Sargent, M.A.; Karch, J.; Molkentin, J.D. ANT-dependent MPTP underlies necrotic myofiber death in muscular dystrophy. Sci. Adv. 2023, 9, eadi2767. [Google Scholar] [CrossRef] [PubMed]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabo, I.; et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef] [PubMed]
- Carraro, M.; Giorgio, V.; Šileikytė, J.; Sartori, G.; Forte, M.; Lippe, G.; Zoratti, M.; Szabò, I.; Bernardi, P. Channel formation by yeast F-ATP synthase and the role of dimerization in the mitochondrial permeability transition. J. Biol. Chem. 2014, 289, 15980–15985. [Google Scholar] [CrossRef] [PubMed]
- Alavian, K.N.; Beutner, G.; Lazrove, E.; Sacchetti, S.; Park, H.A.; Licznerski, P.; Li, H.; Nabili, P.; Hockensmith, K.; Graham, M.; et al. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA 2014, 111, 10580–10585. [Google Scholar] [CrossRef]
- He, J.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Permeability transition in human mitochondria persists in the absence of peripheral stalk subunits of ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 9086–9091. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ford, H.C.; Carroll, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Persistence of the mitochondrial permeability transition in the absence of subunit c of human ATP synthase. Proc. Natl. Acad. Sci. USA 2017, 114, 3409–3414. [Google Scholar] [CrossRef]
- Carroll, J.; He, J.; Ding, S.; Fearnley, I.M.; Walker, J.E. Persistence of the permeability transition pore in human mitochondria devoid of an assembled ATP synthase. Proc. Natl. Acad. Sci. USA 2019, 116, 12816–12821. [Google Scholar] [CrossRef] [PubMed]
- Pekson, R.; Liang, F.G.; Axelrod, J.L.; Lee, J.; Qin, D.; Wittig, A.J.H.; Paulino, V.M.; Zheng, M.; Peixoto, P.M.; Kitsis, R.N. The mitochondrial ATP synthase is a negative regulator of the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci. USA 2023, 120, e2303713120. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lemasters, J.J. Regulated and unregulated mitochondrial permeability transition pores: A new paradigm of pore structure and function? FEBS Lett. 2002, 512, 1–7. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lemasters, J.J. Heat shock suppresses the permeability transition in rat liver mitochondria. J. Biol. Chem. 2003, 278, 16755–16760. [Google Scholar] [CrossRef] [PubMed]
- Neginskaya, M.A.; Solesio, M.E.; Berezhnaya, E.V.; Amodeo, G.F.; Mnatsakanyan, N.; Jonas, E.A.; Pavlov, E.V. ATP Synthase C-Subunit-Deficient Mitochondria Have a Small Cyclosporine A-Sensitive Channel, but Lack the Permeability Transition Pore. Cell Rep. 2019, 26, 11–17.e12. [Google Scholar] [CrossRef]
- Neginskaya, M.A.; Morris, S.E.; Pavlov, E.V. Both ANT and ATPase are essential for mitochondrial permeability transition but not depolarization. iScience 2022, 25, 105447. [Google Scholar] [CrossRef] [PubMed]
- Carrer, A.; Tommasin, L.; Šileikytė, J.; Ciscato, F.; Filadi, R.; Urbani, A.; Forte, M.; Rasola, A.; Szabò, I.; Carraro, M.; et al. Defining the molecular mechanisms of the mitochondrial permeability transition through genetic manipulation of F-ATP synthase. Nat. Commun. 2021, 12, 4835. [Google Scholar] [CrossRef] [PubMed]
- Connern, C.P.; Halestrap, A.P. Recruitment of mitochondrial cyclophilin to the mitochondrial inner membrane under conditions of oxidative stress that enhance the opening of a calcium-sensitive non-specific channel. Biochem. J. 1994, 302 Pt 2, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Waldmeier, P.C.; Zimmermann, K.; Qian, T.; Tintelnot-Blomley, M.; Lemasters, J.J. Cyclophilin D as a drug target. Curr. Med. Chem. 2003, 10, 1485–1506. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Ramshesh, V.K.; Theruvath, T.P.; Kim, I.; Currin, R.T.; Giri, S.; Lemasters, J.J.; Zhong, Z. NIM811 (N-methyl-4-isoleucine cyclosporine), a mitochondrial permeability transition inhibitor, attenuates cholestatic liver injury but not fibrosis in mice. J. Pharmacol. Exp. Ther. 2008, 327, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Theruvath, T.P.; Zhong, Z.; Pediaditakis, P.; Ramshesh, V.K.; Currin, R.T.; Tikunov, A.; Holmuhamedov, E.; Lemasters, J.J. Minocycline and N-methyl-4-isoleucine cyclosporin (NIM811) mitigate storage/reperfusion injury after rat liver transplantation through suppression of the mitochondrial permeability transition. Hepatology 2008, 47, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.; Sun, J.; Shi, Y.; Ramshesh, V.K.; Liu, Q.; Currin, R.T.; Lemasters, J.J.; Zhong, Z. NIM811 prevents mitochondrial dysfunction, attenuates liver injury, and stimulates liver regeneration after massive hepatectomy. Transplantation 2011, 91, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Theruvath, T.P.; Currin, R.T.; Waldmeier, P.C.; Lemasters, J.J. NIM811, a mitochondrial permeability transition inhibitor, prevents mitochondrial depolarization in small-for-size rat liver grafts. Am. J. Transplant. 2007, 7, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Suda, C.; Horie, T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J. Hepatol. 2005, 42, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.B.; Kurten, R.C.; McCullough, S.S.; Brock, R.W.; Hinson, J.A. Mechanisms of acetaminophen-induced hepatotoxicity: Role of oxidative stress and mitochondrial permeability transition in freshly isolated mouse hepatocytes. J. Pharmacol. Exp. Ther. 2005, 312, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J. Dying a Thousand Deaths: Redundant Pathways From Different Organelles to Apoptosis and Necrosis. Gastroenterology 2005, 129, 351–360. [Google Scholar] [CrossRef]
- Kon, K.; Ikejima, K.; Okumura, K.; Aoyama, T.; Arai, K.; Takei, Y.; Lemasters, J.J.; Sato, N. Role of apoptosis in acetaminophen hepatotoxicity 19. J. Gastroenterol. Hepatol. 2007, 22 (Suppl. S1), S49–S52. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Qian, T.; Lemasters, J.J. Mitochondrial permeability transition in the switch from necrotic to apoptotic cell death in ischemic rat hepatocytes. Gastroenterology 2003, 124, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Lemasters, J.J.; Nieminen, A.L.; Qian, T.; Trost, L.C.; Elmore, S.P.; Nishimura, Y.; Crowe, R.A.; Cascio, W.E.; Bradham, C.A.; Brenner, D.A.; et al. The mitochondrial permeability transition in cell death: A common mechanism in necrosis, apoptosis and autophagy. Biochim. Biophys. Acta 1998, 1366, 177–196. [Google Scholar] [CrossRef] [PubMed]
- Gujral, J.S.; Knight, T.R.; Farhood, A.; Bajt, M.L.; Jaeschke, H. Mode of cell death after acetaminophen overdose in mice: Apoptosis or oncotic necrosis? Toxicol. Sci. 2002, 67, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Williams, C.D.; Farhood, A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology 2011, 53, 718–719. [Google Scholar] [CrossRef]
- Possamai, L.A.; McPhail, M.J.; Quaglia, A.; Zingarelli, V.; Abeles, R.D.; Tidswell, R.; Puthucheary, Z.; Rawal, J.; Karvellas, C.J.; Leslie, E.M.; et al. Character and temporal evolution of apoptosis in acetaminophen-induced acute liver failure*. Crit. Care Med. 2013, 41, 2543–2550. [Google Scholar] [CrossRef] [PubMed]
- Gunawan, B.K.; Liu, Z.X.; Han, D.; Hanawa, N.; Gaarde, W.A.; Kaplowitz, N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology 2006, 131, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Han, D.; Petrovic, L.M.; Kaplowitz, N. c-Jun N-terminal kinase (JNK)-dependent acute liver injury from acetaminophen or tumor necrosis factor (TNF) requires mitochondrial Sab protein expression in mice. J. Biol. Chem. 2011, 286, 35071–35078. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Kaplowitz, N. Mitochondrial P-JNK target, SAB (SH3BP5), in regulation of cell death. Front. Cell Dev. Biol. 2024, 12, 1359152. [Google Scholar] [CrossRef]
- Win, S.; Than, T.A.; Min, R.W.; Aghajan, M.; Kaplowitz, N. c-Jun N-terminal kinase mediates mouse liver injury through a novel Sab (SH3BP5)-dependent pathway leading to inactivation of intramitochondrial Src. Hepatology 2016, 63, 1987–2003. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Fernandez-Checa, J.C.; Kaplowitz, N. JNK interaction with Sab mediates ER stress induced inhibition of mitochondrial respiration and cell death. Cell Death Dis. 2014, 5, e989. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Zhang, J.; Oo, C.; Min, R.W.M.; Kaplowitz, N. New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases. Hepatology 2018, 67, 2013–2024. [Google Scholar] [CrossRef] [PubMed]
- Samuvel, D.J.; Nguyen, N.T.; Jaeschke, H.; Lemasters, J.J.; Wang, X.; Choo, Y.M.; Hamann, M.T.; Zhong, Z. Platanosides, a Potential Botanical Drug Combination, Decrease Liver Injury Caused by Acetaminophen Overdose in Mice. J. Nat. Prod. 2022, 85, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Bajt, M.L. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006, 89, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bajt, M.L.; Knight, T.R.; Lemasters, J.J.; Jaeschke, H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: Protection by N-acetyl cysteine. Toxicol. Sci. 2004, 80, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: The protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990, 255, 935–941. [Google Scholar] [PubMed]
- Hu, J.; Kholmukhamedov, A.; Lindsey, C.C.; Beeson, C.C.; Jaeschke, H.; Lemasters, J.J. Translocation of iron from lysosomes to mitochondria during acetaminophen-induced hepatocellular injury: Protection by starch-desferal and minocycline. Free Radic. Biol. Med. 2016, 97, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Du, K.; Akakpo, J.Y.; Umbaugh, D.S.; Jaeschke, H.; Ramachandran, A. Mitochondrial protein adduct and superoxide generation are prerequisites for early activation of c-jun N-terminal kinase within the cytosol after an acetaminophen overdose in mice. Toxicol. Lett. 2021, 338, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P.; Klotz, L.O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Aust, S.D. Redox cycling of iron and lipid peroxidation. Lipids 1992, 27, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Galy, B.; Conrad, M.; Muckenthaler, M. Mechanisms controlling cellular and systemic iron homeostasis. Nat. Rev. Mol. Cell Biol. 2024, 25, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.J.; Merlot, A.M.; Huang, M.L.; Bae, D.H.; Jansson, P.J.; Sahni, S.; Kalinowski, D.S.; Richardson, D.R. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim. Biophys. Acta 2015, 1853, 1130–1144. [Google Scholar] [CrossRef] [PubMed]
- McKie, A.T.; Barrow, D.; Latunde-Dada, G.O.; Rolfs, A.; Sager, G.; Mudaly, E.; Mudaly, M.; Richardson, C.; Barlow, D.; Bomford, A.; et al. An iron-regulated ferric reductase associated with the absorption of dietary iron. Science 2001, 291, 1755–1759. [Google Scholar] [CrossRef] [PubMed]
- Gunshin, H.; Mackenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Jenkitkasemwong, S.; Wang, C.Y.; Mackenzie, B.; Knutson, M.D. Physiologic implications of metal-ion transport by ZIP14 and ZIP8. Biometals 2012, 25, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, R.S.; Campagna, D.R.; Greer, E.L.; Antiochos, B.; McDonald, A.; Chen, J.; Sharp, J.J.; Fujiwara, Y.; Barker, J.E.; Fleming, M.D. Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat. Genet. 2005, 37, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, R.S.; Campagna, D.R.; McDonald, A.; Fleming, M.D. The Steap proteins are metalloreductases. Blood 2006, 108, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, A.; Kim, J.S.; Kon, K.; Jaeschke, H.; Ikejima, K.; Watanabe, S.; Lemasters, J.J. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology 2008, 48, 1644–1654. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Gustafsson, B.; Brunk, U.T. Lysosomes in iron metabolism, ageing and apoptosis. Histochem. Cell Biol. 2008, 129, 389–406. [Google Scholar] [CrossRef]
- Lill, R. Function and biogenesis of iron-sulphur proteins. Nature 2009, 460, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Lill, R.; Hoffmann, B.; Molik, S.; Pierik, A.J.; Rietzschel, N.; Stehling, O.; Uzarska, M.A.; Webert, H.; Wilbrecht, C.; Muhlenhoff, U. The role of mitochondria in cellular iron-sulfur protein biogenesis and iron metabolism. Biochim. Biophys. Acta 2012, 1823, 1491–1508. [Google Scholar] [CrossRef] [PubMed]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118863. [Google Scholar] [CrossRef] [PubMed]
- Kořený, L.; Oborník, M.; Horáková, E.; Waller, R.F.; Lukeš, J. The convoluted history of haem biosynthesis. Biol. Rev. Camb. Philos. Soc. 2022, 97, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Sheftel, A.D.; Zhang, A.S.; Brown, C.; Shirihai, O.S.; Ponka, P. Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 2007, 110, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Komatsu, M.; Yamaguchi-Iwai, Y.; Ishikawa, F.; Mizushima, N.; Iwai, K. Distinct mechanisms of ferritin delivery to lysosomes in iron-depleted and iron-replete cells. Mol. Cell Biol. 2011, 31, 2040–2052. [Google Scholar] [CrossRef]
- De Domenico, I.; Vaughn, M.B.; Li, L.; Bagley, D.; Musci, G.; Ward, D.M.; Kaplan, J. Ferroportin-mediated mobilization of ferritin iron precedes ferritin degradation by the proteasome. EMBO J. 2006, 25, 5396–5404. [Google Scholar] [CrossRef] [PubMed]
- De Domenico, I.; Ward, D.M.; Kaplan, J. Specific iron chelators determine the route of ferritin degradation. Blood 2009, 114, 4546–4551. [Google Scholar] [CrossRef] [PubMed]
- Flatmark, T.; Romslo, I. Energy-dependent accumulation of iron by isolated rat liver mitochondria. Requirement of reducing equivalents and evidence for a unidirectional flux of Fe(II) across the inner membrane. J. Biol. Chem. 1975, 250, 6433–6438. [Google Scholar] [CrossRef] [PubMed]
- Matlib, M.A.; Zhou, Z.; Knight, S.; Ahmed, S.; Choi, K.M.; Krause-Bauer, J.; Phillips, R.; Altschuld, R.; Katsube, Y.; Sperelakis, N.; et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J. Biol. Chem. 1998, 273, 10223–10231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lemasters, J.J. Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic. Biol. Med. 2013, 63, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.C.; Cope, J.J.; Li, L.; Corson, K.; Hersey, C.; Ackermann, G.E.; Gwynn, B.; Lambert, A.J.; Wingert, R.A.; Traver, D.; et al. Mitoferrin is essential for erythroid iron assimilation. Nature 2006, 440, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Troadec, M.B.; Warner, D.; Wallace, J.; Thomas, K.; Spangrude, G.J.; Phillips, J.; Khalimonchuk, O.; Paw, B.H.; Ward, D.M.; Kaplan, J. Targeted deletion of the mouse Mitoferrin1 gene: From anemia to protoporphyria. Blood 2011, 117, 5494–5502. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, A.L.; Schwartz, J.; Hung, H.I.; Blocker, E.R.; Gooz, M.; Lemasters, J.J. Mitoferrin-2 (Mfrn2) regulates the electrogenic mitochondrial calcium uniporter and inter-acts physically with MCU. Biophys. J. 2014, 106, 581a–582a. [Google Scholar] [CrossRef]
- Dietz, J.V.; Fox, J.L.; Khalimonchuk, O. Down the Iron Path: Mitochondrial Iron Homeostasis and Beyond. Cells 2021, 10, 2198. [Google Scholar] [CrossRef] [PubMed]
- Aust, S.D.; Morehouse, L.A.; Thomas, C.E. Role of metals in oxygen radical reactions. J. Free Radic. Biol. Med. 1985, 1, 3–25. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Masaki, N.; Kyle, M.E.; Farber, J.L. tert-Butyl hydroperoxide kills cultured hepatocytes by peroxidizing membrane lipids. Arch. Biochem. Biophys. 1989, 269, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, A.L.; Byrne, A.M.; Herman, B.; Lemasters, J.J. Mitochondrial permeability transition in hepatocytes induced by t-BuOOH: NAD(P)H and reactive oxygen species. Am. J. Physiol. 1997, 272, C1286–C1294. [Google Scholar] [CrossRef] [PubMed]
- Adel, N.; Mantawy, E.M.; El-Sherbiny, D.A.; El-Demerdash, E. Iron chelation by deferasirox confers protection against concanavalin A-induced liver fibrosis: A mechanistic approach. Toxicol. Appl. Pharmacol. 2019, 382, 114748. [Google Scholar] [CrossRef] [PubMed]
- Gerson, R.J.; Casini, A.; Gilfor, D.; Serroni, A.; Farber, J.L. Oxygen-mediated cell injury in the killing of cultured hepatocytes by acetaminophen. Biochem. Biophys. Res. Commun. 1985, 126, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Byrne, A.M.; Lemasters, J.J.; Nieminen, A.L. Contribution of increased mitochondrial free Ca2+ to the mitochondrial permeability transition induced by tert-butylhydroperoxide in rat hepatocytes. Hepatology 1999, 29, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Kon, K.; Kim, J.S.; Uchiyama, A.; Jaeschke, H.; Lemasters, J.J. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol. Sci. 2010, 117, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lemasters, J.J. Suppression of iron mobilization from lysosomes to mitochondria attenuates liver injury after acetaminophen overdose in vivo in mice: Protection by minocycline. Toxicol. Appl. Pharmacol. 2020, 392, 114930. [Google Scholar] [CrossRef] [PubMed]
- Adamson, G.M.; Harman, A.W. Oxidative stress in cultured hepatocytes exposed to acetaminophen. Biochem. Pharmacol. 1993, 45, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Schnellmann, J.G.; Pumford, N.R.; Kusewitt, D.F.; Bucci, T.J.; Hinson, J.A. Deferoxamine delays the development of the hepatotoxicity of acetaminophen in mice. Toxicol. Lett. 1999, 106, 79–88. [Google Scholar] [CrossRef]
- Kyle, M.E.; Miccadei, S.; Nakae, D.; Farber, J.L. Superoxide dismutase and catalase protect cultured hepatocytes from the cytotoxicity of acetaminophen. Biochem. Biophys. Res. Commun. 1987, 149, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Kyle, M.E.; Nakae, D.; Serroni, A.; Farber, J.L. 1,3-(2-Chloroethyl)-1-nitrosourea potentiates the toxicity of acetaminophen both in the phenobarbital-induced rat and in hepatocytes cultured from such animals. Mol. Pharmacol. 1988, 34, 584–589. [Google Scholar] [PubMed]
- Moon, M.S.; Richie, J.P.; Isom, H.C. Iron potentiates acetaminophen-induced oxidative stress and mitochondrial dysfunction in cultured mouse hepatocytes. Toxicol. Sci. 2010, 118, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Epsztejn, S.; Kakhlon, O.; Glickstein, H.; Breuer, W.; Cabantchik, I. Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 1997, 248, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kholmukhamedov, A.; Li, L.; Lindsey, C.C.; Hu, J.; Nieminen, A.L.; Takemoto, K.; Beeson, G.C.; Beneker, C.M.; McInnes, C.; Beeson, C.C.; et al. A new fluorescent sensor mitoferrofluor indicates the presence of chelatable iron in polarized and depolarized mitochondria. J. Biol. Chem. 2022, 298, 102336. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Nieminen, A.L.; Weemhoff, J.L.; Jaeschke, H.; Murphy, L.G.; Dent, J.A.; Lemasters, J.J. The mitochondrial calcium uniporter mediates mitochondrial Fe2+ uptake and hepatotoxicity after acetaminophen. Toxicol. Appl. Pharmacol. 2023, 479, 116722. [Google Scholar] [CrossRef] [PubMed]
- Sukhbaatar, N.; Weichhart, T. Iron Regulation: Macrophages in Control. Pharmaceuticals 2018, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H.; Farhood, A. Neutrophil and Kupffer cell-induced oxidant stress and ischemia-reperfusion injury in rat liver. Am. J. Physiol. 1991, 260, G355–G362. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.L.; Pumford, N.R.; Mayeux, P.R.; Niesman, M.R.; Hinson, J.A. Pretreatment of mice with macrophage inactivators decreases acetaminophen hepatotoxicity and the formation of reactive oxygen and nitrogen species. Hepatology 1999, 30, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ju, C.; Reilly, T.P.; Bourdi, M.; Radonovich, M.F.; Brady, J.N.; George, J.W.; Pohl, L.R. Protective role of Kupffer cells in acetaminophen-induced hepatic injury in mice. Chem. Res. Toxicol. 2002, 15, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, E.; Pop, O.T.; Possamai, L.A.; Wilhelm, A.; Liaskou, E.; Singanayagam, A.; Bernsmeier, C.; Khamri, W.; Petts, G.; Dargue, R.; et al. MerTK expressing hepatic macrophages promote the resolution of inflammation in acute liver failure. Gut 2018, 67, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Pan, Y.; Huang, W.; Li, M.; Yan, X.; Zhou, Z.; Qi, J. CXCL5 Promotes Acetaminophen-Induced Hepatotoxicity by Activating Kupffer Cells. Int. J. Mol. Sci. 2023, 24, 12180. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Umbaugh, D.S.; Sanchez-Guerrero, G.; Ramachandran, A.; Jaeschke, H. Kupffer cells regulate liver recovery through induction of chemokine receptor CXCR2 on hepatocytes after acetaminophen overdose in mice. Arch. Toxicol. 2022, 96, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Starke, P.E.; Farber, J.L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J. Biol. Chem. 1985, 260, 10099–10104. [Google Scholar] [CrossRef] [PubMed]
- Gores, G.J.; Flarsheim, C.E.; Dawson, T.L.; Nieminen, A.L.; Herman, B.; Lemasters, J.J. Swelling, reductive stress, and cell death during chemical hypoxia in hepatocytes. Am. J. Physiol. 1989, 257, C347–C354. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.L.; Leonard, T.B.; Kyle, M.E.; Nakae, D.; Serroni, A.; Rogers, S.A. Peroxidation-dependent and peroxidation-independent mechanisms by which acetaminophen kills cultured rat hepatocytes. Arch. Biochem. Biophys. 1988, 267, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Karasawa, T.; Kimura, H.; Watanabe, S.; Komada, T.; Kamata, R.; Sampilvanjil, A.; Ito, J.; Nakagawa, K.; Kuwata, H.; et al. Ferroptosis driven by radical oxidation of n-6 polyunsaturated fatty acids mediates acetaminophen-induced acute liver failure. Cell Death Dis. 2020, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xu, N.; Liu, B.; Ma, Y.; Fu, X.; Shang, Y.; Huang, Q.; Yao, Q.; Chen, J.; Li, H. Mifepristone protects acetaminophen induced liver injury through NRF2/GSH/GST mediated ferroptosis suppression. Free Radic. Biol. Med. 2024, 222, 229–243. [Google Scholar] [CrossRef]
- Tao, J.; Xue, C.; Wang, X.; Chen, H.; Liu, Q.; Jiang, C.; Zhang, W. GAS1 Promotes Ferroptosis of Liver Cells in Acetaminophen-Induced Acute Liver Failure. Int. J. Med. Sci. 2023, 20, 1616–1630. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.A.; Pike, S.L.; Pumford, N.R.; Mayeux, P.R. Nitrotyrosine-protein adducts in hepatic centrilobular areas following toxic doses of acetaminophen in mice. Chem. Res. Toxicol. 1998, 11, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.R.; Kurtz, A.; Bajt, M.L.; Hinson, J.A.; Jaeschke, H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: Role of mitochondrial oxidant stress. Toxicol. Sci. 2001, 62, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Cover, C.; Mansouri, A.; Knight, T.R.; Bajt, M.L.; Lemasters, J.J.; Pessayre, D.; Jaeschke, H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005, 315, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Campolo, N.; Bartesaghi, S.; Radi, R. Metal-catalyzed protein tyrosine nitration in biological systems. Redox Rep. 2014, 19, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Farhood, A.; Jaeschke, H. Mitochondria-targeted antioxidant Mito-Tempo protects against acetaminophen hepatotoxicity. Arch. Toxicol. 2017, 91, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Eckl, P.; Ortner, A. Possible mutagens derived from lipids and lipid precursors. Mutat. Res. 1990, 238, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Cheeseman, K.H. Determination of aldehydic lipid peroxidation products: Malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990, 186, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A.; Feuerstein, S.; Konz, K.H. Acute paracetamol intoxication of starved mice leads to lipid peroxidation in vivo. Biochem. Pharmacol. 1979, 28, 2051–2055. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A.; Feuerstein, S. Drug-induced lipid peroxidation in mice--I. Modulation by monooxygenase activity, glutathione and selenium status. Biochem. Pharmacol. 1981, 30, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Wendel, A.; Jaeschke, H.; Gloger, M. Drug-induced lipid peroxidation in mice--II. Protection against paracetamol-induced liver necrosis by intravenous liposomally entrapped glutathione. Biochem. Pharmacol. 1982, 31, 3601–3605. [Google Scholar] [CrossRef] [PubMed]
- Knight, T.R.; Fariss, M.W.; Farhood, A.; Jaeschke, H. Role of lipid peroxidation as a mechanism of liver injury after acetaminophen overdose in mice. Toxicol. Sci. 2003, 76, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Younes, M.; Cornelius, S.; Siegers, C.P. Ferrous ion supported in vivo lipid peroxidation induced by paracetamol--its relation to hepatotoxicity. Res. Commun. Chem. Pathol. Pharmacol. 1986, 51, 89–99. [Google Scholar] [PubMed]
- Albano, E.; Poli, G.; Chiarpotto, E.; Biasi, F.; Dianzani, M.U. Paracetamol-stimulated lipid peroxidation in isolated rat and mouse hepatocytes. Chem. Biol. Interact. 1983, 47, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Minamide, Y.; Horie, T.; Tomaru, A.; Awazu, S. Spontaneous chemiluminescence production, lipid peroxidation, and covalent binding in rat hepatocytes exposed to acetaminophen. J. Pharm. Sci. 1998, 87, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Wimborne, H.J.; Hu, J.; Takemoto, K.; Nguyen, N.T.; Jaeschke, H.; Lemasters, J.J.; Zhong, Z. Aldehyde dehydrogenase-2 activation decreases acetaminophen hepatotoxicity by prevention of mitochondrial depolarization. Toxicol. Appl. Pharmacol. 2020, 396, 114982. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Budas, G.R.; Churchill, E.N.; Disatnik, M.H.; Hurley, T.D.; Mochly-Rosen, D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science 2008, 321, 1493–1495. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, J.; Nieminen, A.-L.; Zhong, Z.; Lemasters, J.J. Role of Mitochondrial Iron Uptake in Acetaminophen Hepatotoxicity. Livers 2024, 4, 333-351. https://doi.org/10.3390/livers4030024
Hu J, Nieminen A-L, Zhong Z, Lemasters JJ. Role of Mitochondrial Iron Uptake in Acetaminophen Hepatotoxicity. Livers. 2024; 4(3):333-351. https://doi.org/10.3390/livers4030024
Chicago/Turabian StyleHu, Jiangting, Anna-Liisa Nieminen, Zhi Zhong, and John J. Lemasters. 2024. "Role of Mitochondrial Iron Uptake in Acetaminophen Hepatotoxicity" Livers 4, no. 3: 333-351. https://doi.org/10.3390/livers4030024
APA StyleHu, J., Nieminen, A.-L., Zhong, Z., & Lemasters, J. J. (2024). Role of Mitochondrial Iron Uptake in Acetaminophen Hepatotoxicity. Livers, 4(3), 333-351. https://doi.org/10.3390/livers4030024






