Spectroscopic Analysis of Bioactive Compounds from Latex of Calotropis gigantea L. and an Evaluation of Its Biological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Latex, Preparation, and Phytochemical Screening
2.2. UV–Visible Spectrophotometric Analysis
2.3. Analysis of Functional Groups
2.4. Analysis of Phytoconstituents by Gas Chromatography/Mass Spectrometry
3. Determination of Biological Activities
3.1. Analysis of Antioxidant Properties
3.2. Analysis of Antibacterial Activity Using Well Diffusion Method
4. Result and Discussion
4.1. Analysis of Phytochemicals
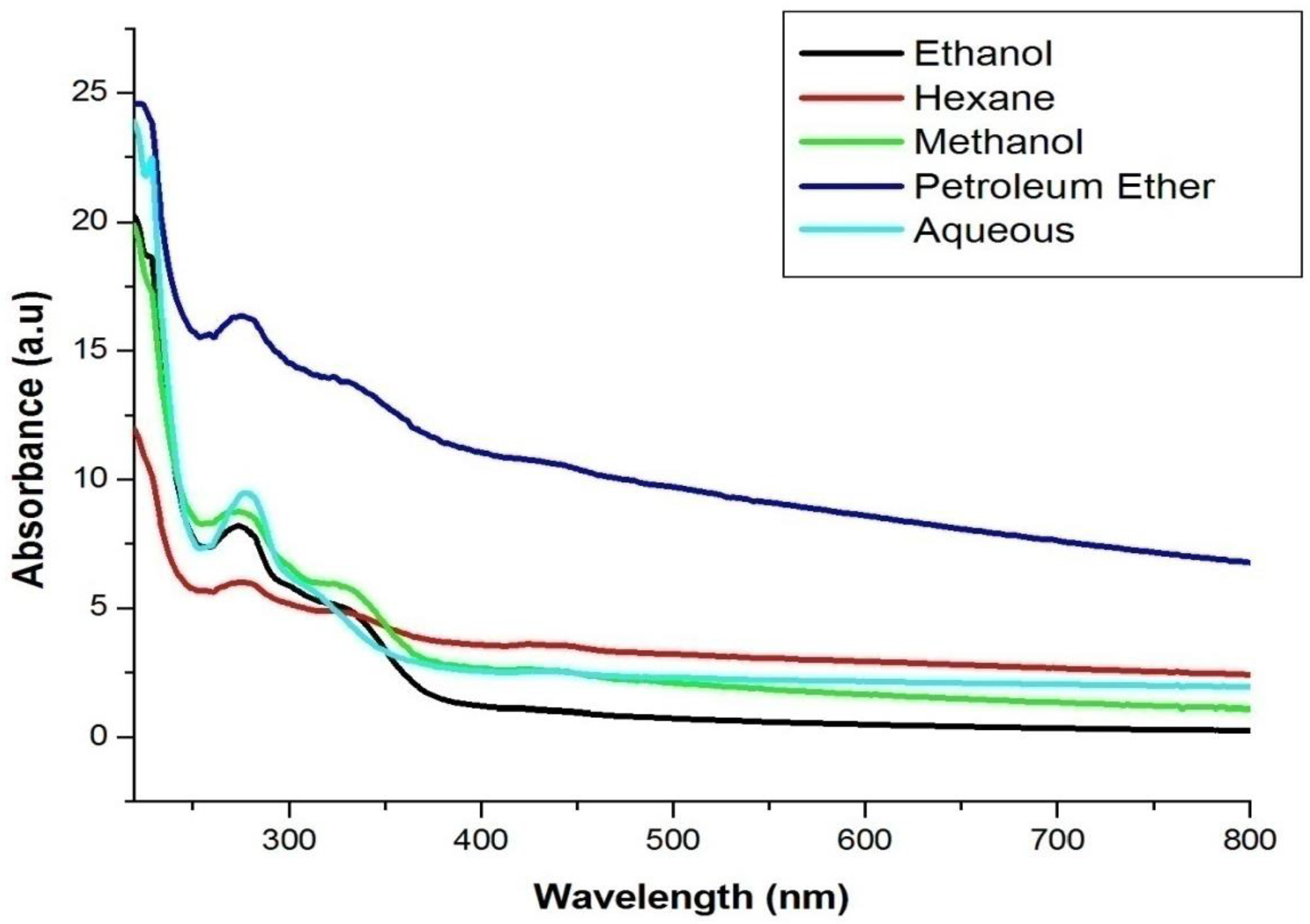
4.2. UV–Visible Spectrophotometric Analysis
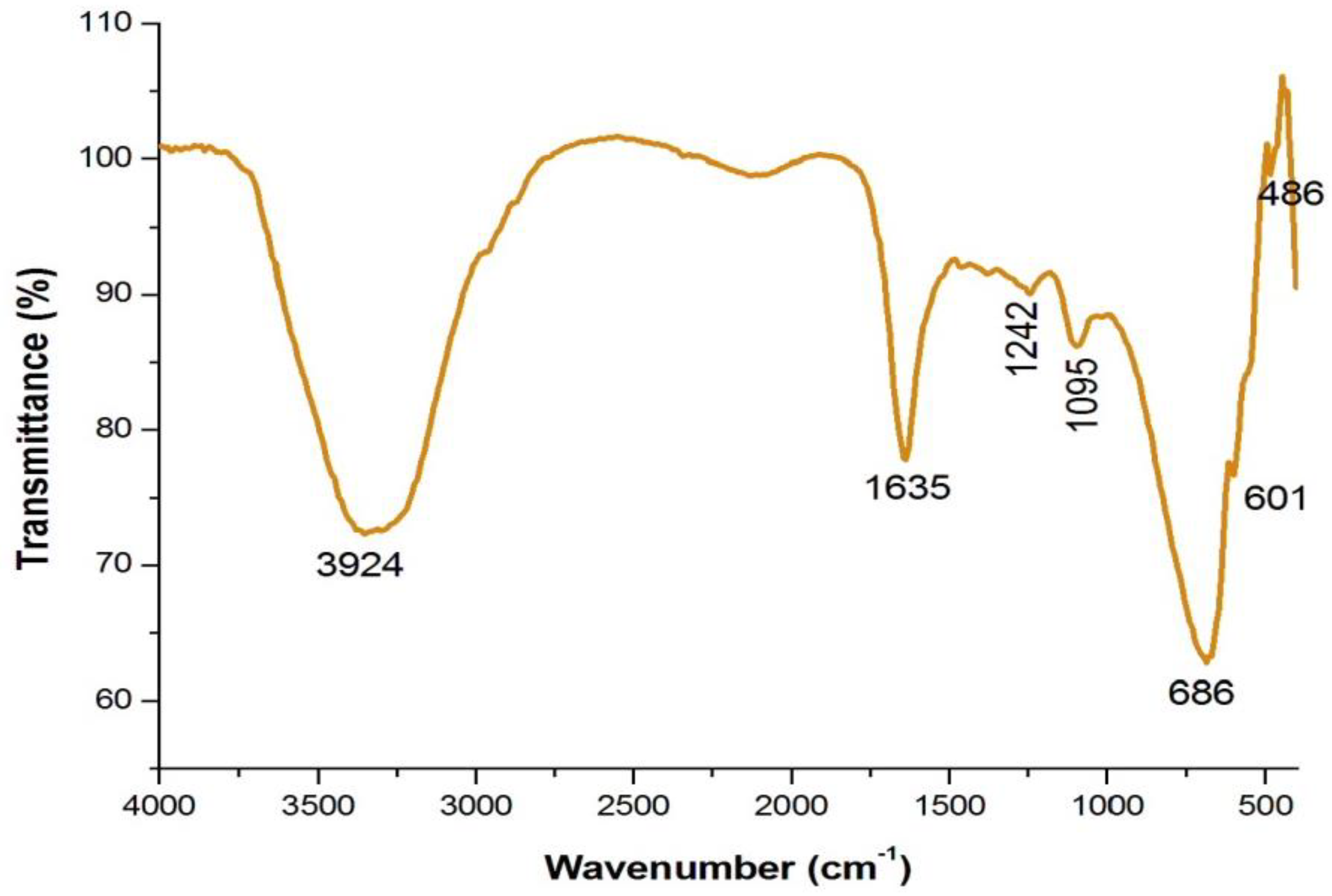
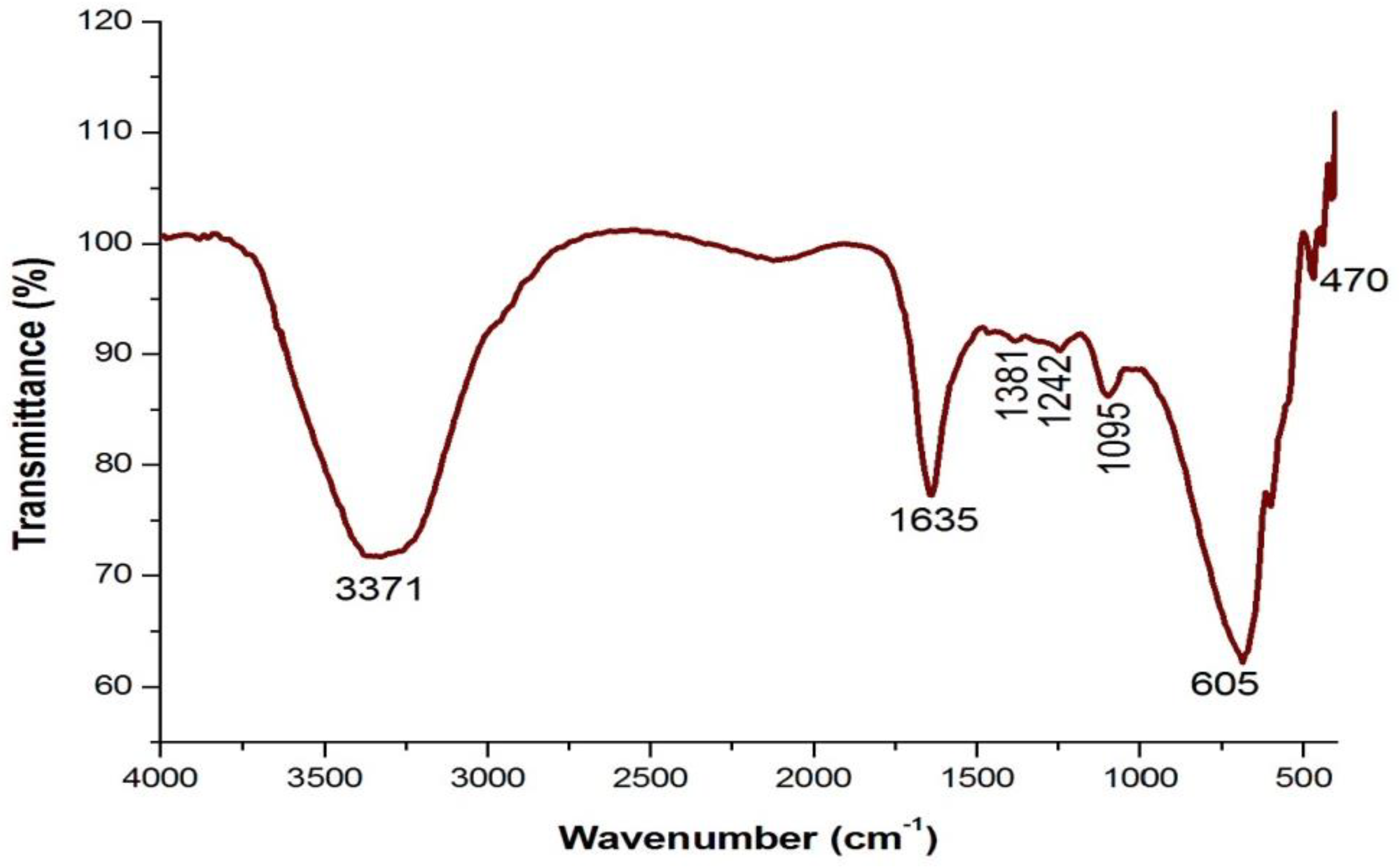
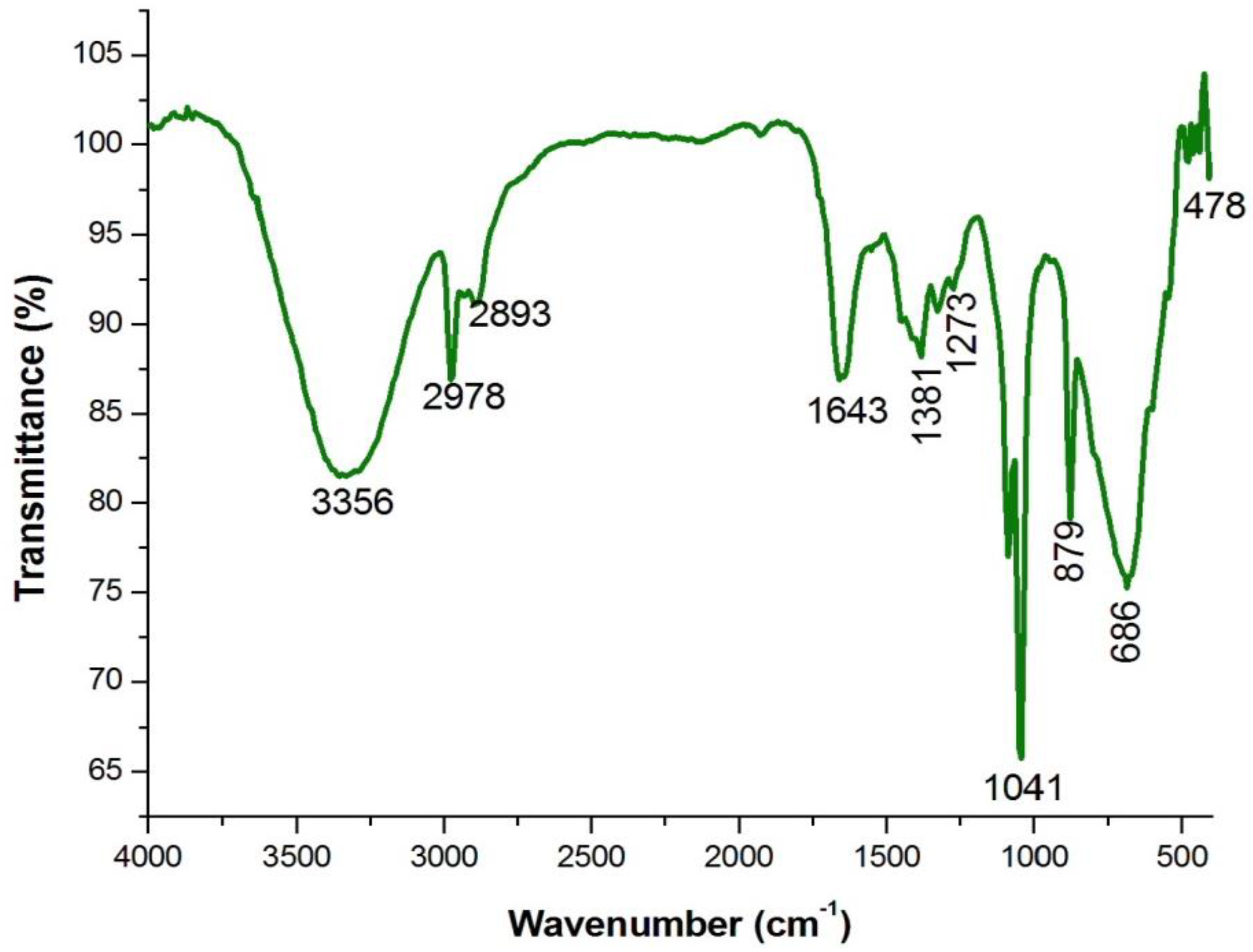
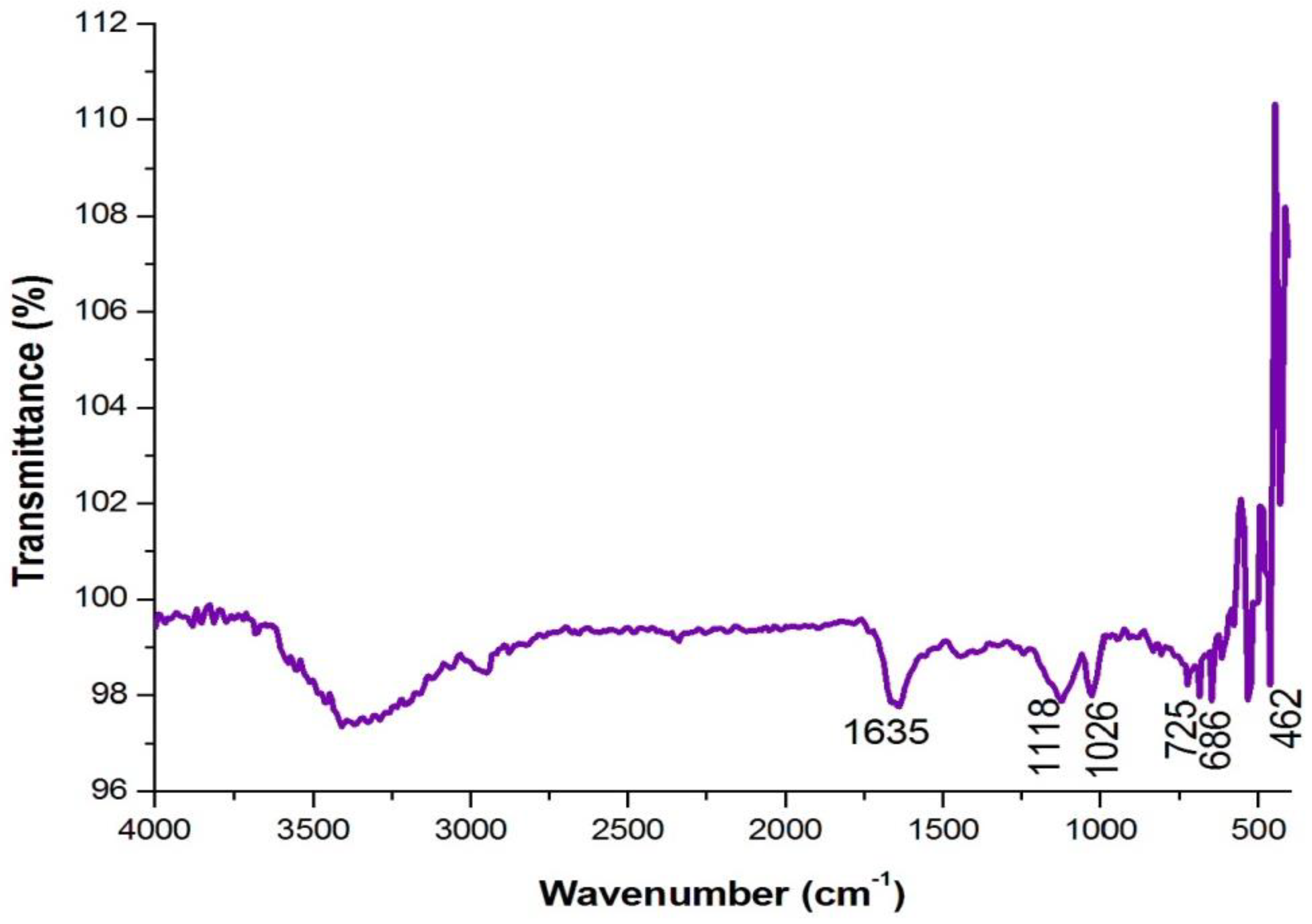
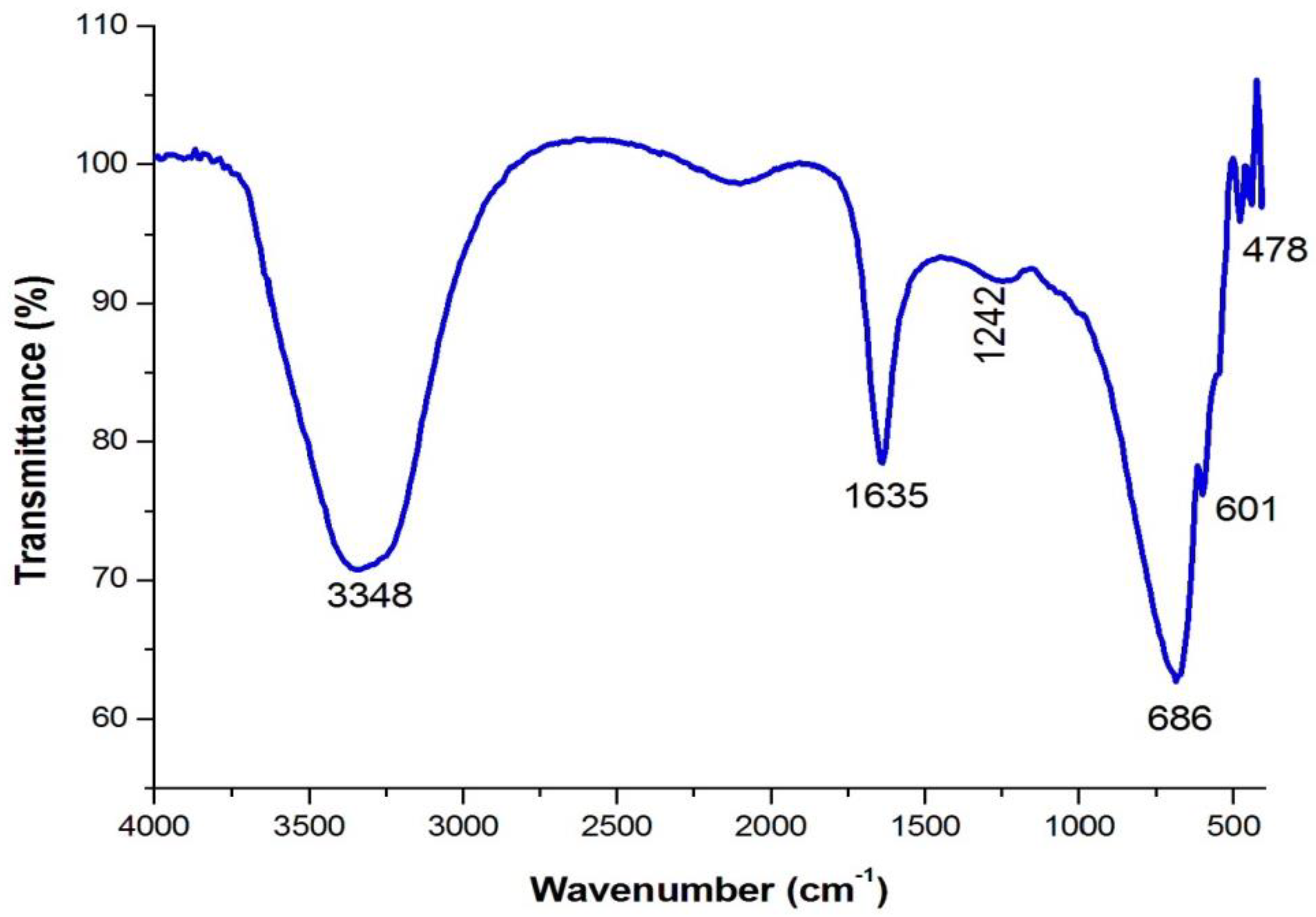
4.3. Analysis of Functional Groups
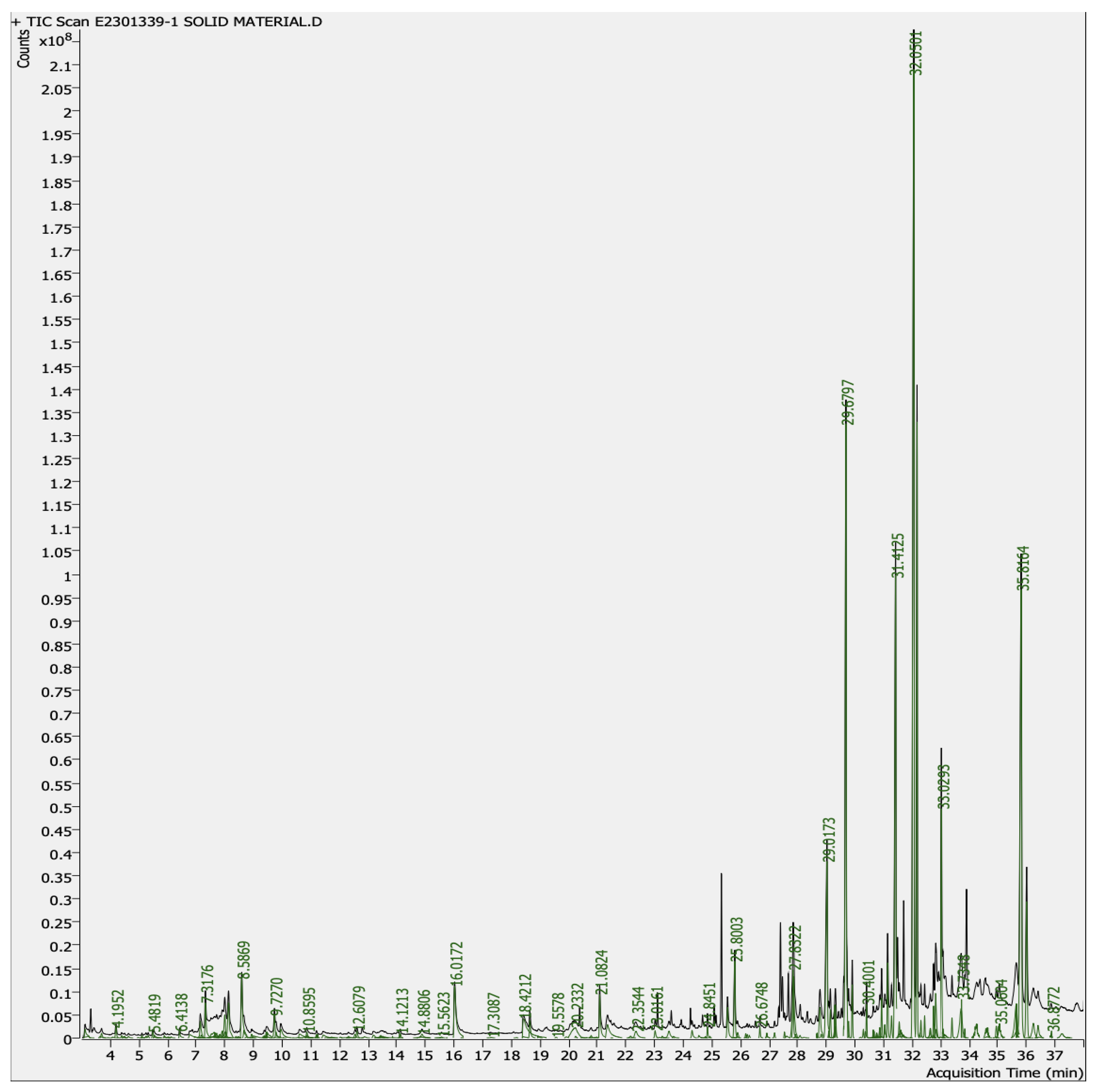
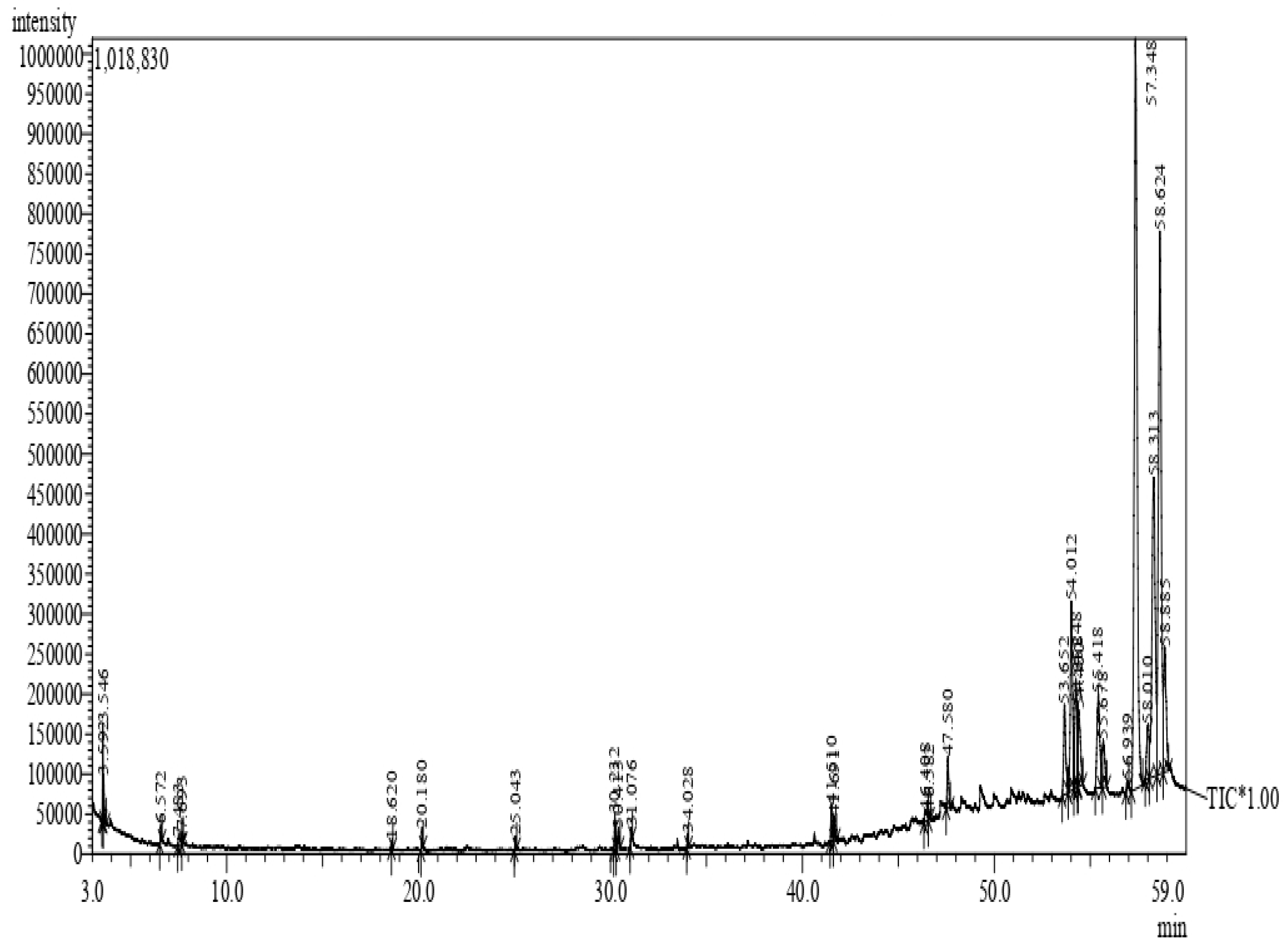
4.4. Analysis of Phytoconstituents by GC–MS
4.4.1. GC–MS Analysis of Aqueous-Mixed Latex of C. gigantea
4.4.2. GC–MS Analysis of Methanol-Mixed Latex of C. gigantea
4.5. Antioxidant Activity
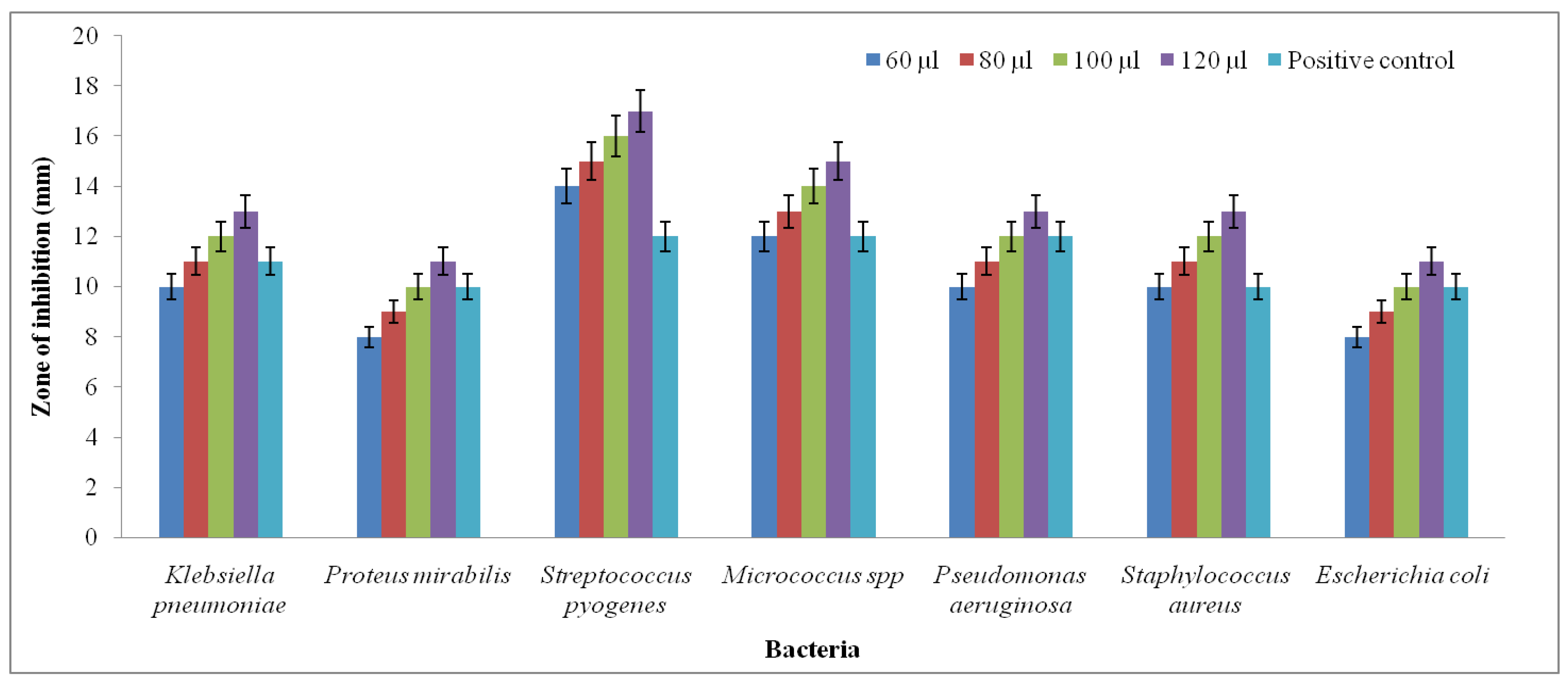
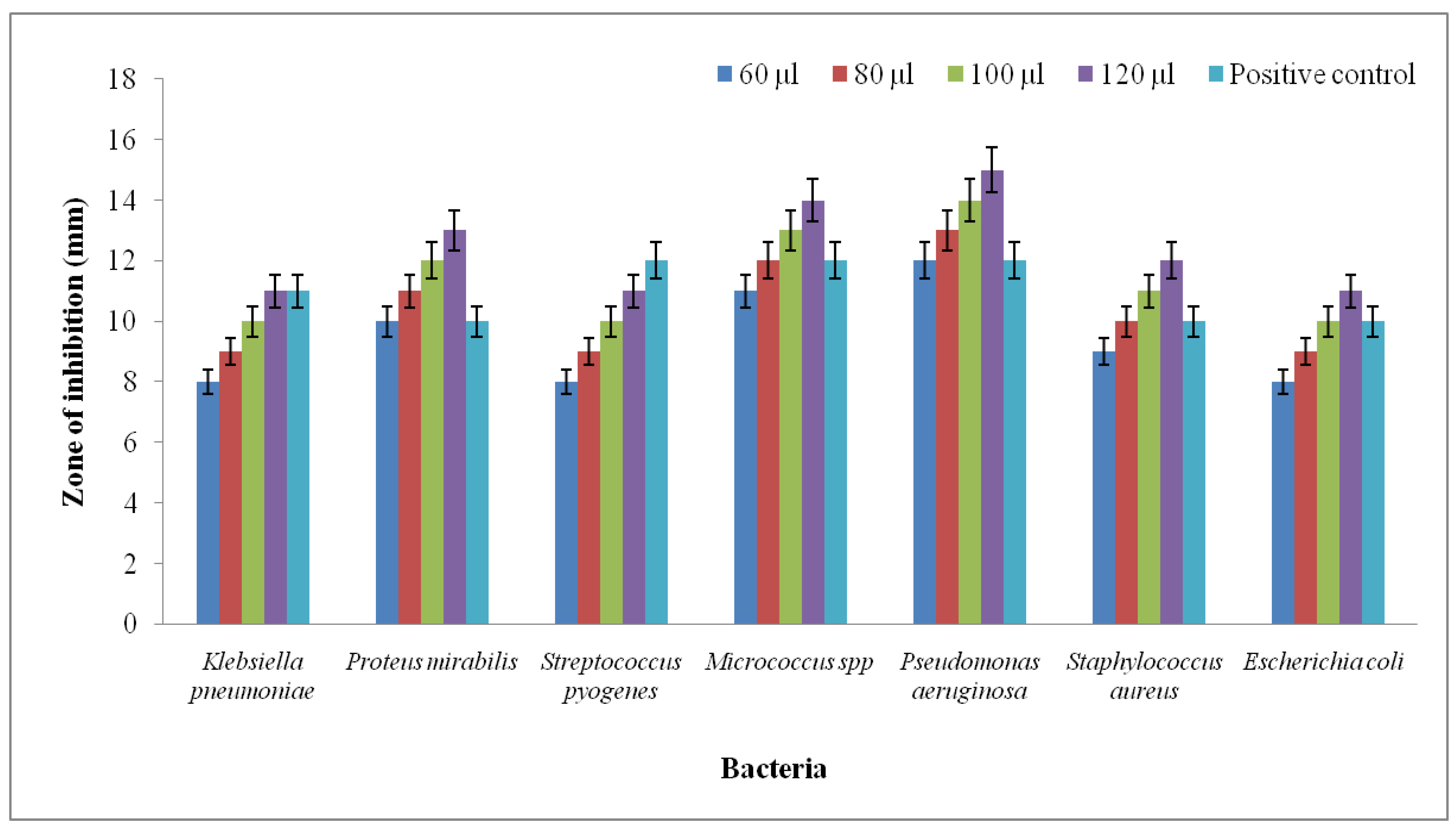
4.6. Antibacterial Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mishra, A.; Parida, S. Latex of plants: Wonders of nature for its therapeutic potentials and a valuable resource towards new drug development. Int. J. Bot. Stud. 2020, 5, 334–338. [Google Scholar]
- Samrot, A.V.; Mun, C.Y.; Qi, N.X. Plant latex: Phytochemistry, medicinal properties and application—A review. J. Pharm. Negat. Results 2022, 13, 5351–5375. [Google Scholar]
- Aref, H.L.; Salah, K.B.; Chaumont, J.P.; Fekih, A.; Aouni, M.; Said, K. In vitro antimicrobial activity of four Ficus carica latex fractions against resistant human pathogens (antimicrobial activity of Ficus carica latex). Pak. J. Pharm. Sci. 2010, 23, 53–58. [Google Scholar] [PubMed]
- Ramos, M.V.; Freitas, A.P.F.; Leitão, R.F.; Costa, D.V.; Cerqueira, G.S.; Martins, D.S.; Martins, C.S.; Alencar, N.M.; Freitas, L.B.N.; Brito, G.A.C. Anti-inflammatory latex proteins of the medicinal plant Calotropis procera: A promising alternative for oral mucositis treatment. Inflamm. Res. 2020, 69, 951–966. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Hernandez, A.B.; Alarcon-Aguilar, F.J.; Almanza-Perez, J.C.; Nieto-Yañez, O.; Olivares-Sanchez, J.M.; Duran-Diaz, A.; Rodriguez-Monroy, M.A.; Canales-Martinez, M.M. Antimicrobial and anti-inflammatory activities, wound-healing effectiveness and chemical characterization of the latex of Jatropha neopauciflora Pax. J. Ethnopharmacol. 2017, 204, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sumathi, S.; Malathy, N.; Dharani, B.; Sivaprabha, J.; Hamsa, D.; Radha, P.; Padma, P.R. Antibacterial and antifungal activity of latex of Euphorbia antiquorum. Afr. J. Microbiol. Res. 2011, 5, 4753–4756. [Google Scholar] [CrossRef]
- Prastiyanto, M.E.; Tama, P.D.; Ananda, N.; Wilson, W.; Mukaromah, A.H. Antibacterial potential of Jatropha sp. latex against multidrug-resistant bacteria. Int. J. Microbiol. 2020, 2020, 8509650. [Google Scholar] [CrossRef]
- Guerra, N.B.; Pegorin, G.S.A.; Boratto, M.H.; de Barros, N.R.; de Oliveira Graeff, C.F.; Herculano, R.D. Biomedical applications of natural rubber latex from the rubber tree Hevea brasiliensis. Mater. Sci. Eng. C 2021, 126, 112126. [Google Scholar] [CrossRef]
- Sivapalan, S.; Dharmalingam, S.; Venkatesan, V.; Angappan, M.; Ashokkumar, V. Phytochemical analysis, anti-inflammatory, antioxidant activity of Calotropis gigantea and its therapeutic applications. J. Ethnopharmacol. 2023, 303, 115963. [Google Scholar] [CrossRef]
- Mushir, A.; Jahan, N.; Ahmed, A. A review on phytochemical and biological properties of Calotropis gigantea (Linn) R.Br. Discov. Phytomed. 2016, 3, 15. [Google Scholar]
- Harborne, A.J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Shaikh, J.R.; Patil, M. Qualitative tests for preliminary phytochemical screening: An overview. Int. J. Chem. Stud. 2020, 8, 603–608. [Google Scholar] [CrossRef]
- Thomas, N.M.; Sathasivam, V.; Thirunavukarasu, M.; Muthukrishnan, A.; Muthukrishnan, S.; Rajkumar, V.; Velusamy, G.; Packiaraj, G. Influence of Borassus flabellifer Endocarps Hydrolysate on Fungal Biomass and Fatty Acids Production by the Marine Fungus Aspergillus sp. Appl. Biochem. Biotechnol. 2023, 196, 932–948. [Google Scholar] [CrossRef] [PubMed]
- Shahinuzzaman, M.; Yaakob, Z.; Anuar, F.H.; Akhtar, P.; Kadir, N.H.A.; Hasan, A.M.; Sobayel, K.; Nour, M.; Sindi, H.; Amin, N.; et al. In vitro antioxidant activity of Ficus carica L. latex from 18 different cultivars. Sci. Rep. 2020, 10, 10852. [Google Scholar] [CrossRef] [PubMed]
- Saratha, V.; Subramanian, S.P. Evaluation of antifungal activity of Calotropis gigantea latex extract: An in vitro study. Int. J. Pharm. Sci. Res. 2010, 1, 88–96. [Google Scholar]
- Jain, P.K.; Soni, A.; Jain, P.; Bhawsar, J. Phytochemical analysis of Mentha spicata plant extract using UV-VIS, FTIR and GC/MS technique. J. Chem. Pharm. Res. 2016, 8, 1–6. [Google Scholar]
- Pradeepkumar, P.; Govindaraj, D.; Jeyaraj, M.; Munusamy, M.A.; Rajan, M. Assembling of multifunctional latex-based hybrid nanocarriers from Calotropis gigantea for sustained (doxorubicin) DOX releases. Biomed. Pharmacother. 2017, 87, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Sonia Angeline, M.; Bhattacharjee, S.; Lotjem, K. Anti-proliferative activity of Calotropis gigantea (Flower Extract) on colorectal cancer cell line. Int. J. Life Sci. Pharma Res. 2020, 2250-0480. [Google Scholar]
- Spano, D.; Pintus, F.; Mascia, C.; Scorciapino, M.A.; Casu, M.; Floris, G.; Medda, R. Extraction and characterization of a natural rubber from Euphorbia characias latex. Biopolymers 2012, 97, 589–594. [Google Scholar] [CrossRef]
- Lahmar, I.; Ben Nasri-Ayachi, M.; Belghith, K. Laticifer identification, rubber characterization, phenolic content, and antioxidant activity of Pergularia tomentosa latex extract. BioMed Res. Int. 2022, 2022, 7158905. [Google Scholar] [CrossRef]
- Sharma, S.; Kumari, A.; Sharma, M. Comparative GC-MS analysis of bioactive compounds in methanolic extract of Calotropis gigantea (L) WT Aiton leaf and latex. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1823–1827. [Google Scholar]
- De Marino, S.; Gala, F.; Zollo, F.; Vitalini, S.; Fico, G.; Visioli, F.; Iorizzi, M. Identification of minor secondary metabolites from the latex of Croton lechleri (Muell-Arg) and evaluation of their antioxidant activity. Molecules 2008, 13, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.P.; Saratha, V. Evaluation of antibacterial activity of Calotropis gigantea latex extract on selected pathogenic bacteria. J. Pharm. Res. 2010, 3, 517–521. [Google Scholar]
- Kumar, G.; Karthik, L.; Rao, K.B. Antimicrobial activity of latex of Calotropis gigantea against pathogenic microorganisms—An in vitro study. Pharmacologyonline 2010, 3, 155–163. [Google Scholar]
| S. No | Metabolites | Solvents | ||||
|---|---|---|---|---|---|---|
| Petroleum-Ether-Mixed Latex | Hexane-Mixed Latex | Ethanol Mixed-Latex | Methanol-Mixed Latex | Aqueous-Mixed Latex | ||
| 1. | Alkaloids | − | − | + | + | − |
| 2. | Flavonoids | − | − | − | − | − |
| 3. | Sterols | − | − | − | − | − |
| 4. | Terpenoids | − | − | + | + | − |
| 5. | Anthraquinone | + | + | + | + | + |
| 6. | Anthocyanin | − | − | − | − | − |
| 7. | Proteins | − | − | − | − | − |
| 8. | Phenolic compounds | − | − | − | − | − |
| 9. | Quinones | − | − | − | − | − |
| 10. | Carbohydrates | + | + | + | + | + |
| 11. | Tannin | − | − | − | − | − |
| 12. | Saponins | + | + | − | − | + |
| 13. | Cardiac glycosides | − | − | + | + | − |
| 14. | Glycoside’s test | − | − | − | − | − |
| 15. | Lignin | − | − | − | − | − |
| 16. | Coumarins | − | − | − | − | − |
| 17. | Volatile oils | − | − | − | − | − |
| S. No | Retention Time | Compound |
|---|---|---|
| 1. | 3.1164 | Acetic acid |
| 2. | 3.2222 | Diisopropylamine |
| 3. | 3.6468 | n-Propyl acetate |
| 4. | 3.6943 | Paraldehyde |
| 5. | 3.9246 | Benzoic acid, 4-(1-methylpropyl)oxy-, methyl ester |
| 6. | 4.0701 | 4-Chloro-5-[[2 diethylaminoethyl]amino]pyridazin-3[2H]-one |
| 7. | 4.0773 | 2-Propenamide, N-[2-(diethylamino)ethyl]-N,2-dimethyl- |
| 8. | 4.1952 | 3(2H)-Furanone, dihydro-5-methyl- |
| 9. | 4.3936 | Thiocyanic acid, ethyl ester |
| 10. | 4.4954 | 3-Methyl-6-ethyl--2,4-dioxadecane |
| 11. | 4.4957 | 2-Butenedioic acid (Z)-, dimethyl ester |
| 12. | 4.6320 | Butanoic acid, 3-methyl- |
| 13. | 4.9677 | 2-Propenal |
| 14. | 5.0975 | 3,5,7,8-Tetrahydro-4,6-pteridinedione |
| 15. | 5.1014 | Silane, (2-methoxyphenyl)trimethyl- |
| 16. | 5.2457 | 2-Heptenoic acid, 4-nitrophenyl ester |
| 17. | 5.2464 | Bicyclo[2.2.1]heptan-2-ol, 2-(2-cyclopenten-1-yl)- |
| 18. | 5.3218 | 3-Hepten-2-one, 3-methyl- |
| 19. | 5.4819 | Oxime-, methoxy-phenyl-_ |
| 20. | 5.5294 | Thiazole, tetrahydro- |
| 21. | 5.5387 | 2,2′-Bithiazolidine |
| 22. | 5.6710 | 1-Pentamethyldisilanyl-4-trimethylsiloxybenzene |
| 23. | 5.8823 | Nitroguanidine |
| 24. | 5.8836 | Thiomorpholine |
| 25. | 5.9934 | Methyl vinyl ketone |
| 26. | 6.0348 | Cyclobutanone, 2,3,3,4-tetramethyl- |
| 27. | 6.1198 | 3-Pentenoic acid, 4-methyl-, methyl ester |
| 28. | 6.4138 | Pyridine, 2-(1-methylethyl)- |
| 29. | 6.7693 | 2-Thiophenethiol |
| 30. | 6.9591 | 2,4-Dihydroxy-2,5-dimethyl-3(2H)-furan-3-one |
| 31. | 7.0853 | 2-Hydroxypentanediamide |
| 32. | 7.1416 | Bicyclo[3.1.0]hexan-3-ol, 4-methyl-1-(1-methylethyl)- |
| 33. | 7.1760 | alpha.-Phellandrene |
| 34. | 7.2121 | 2-Furancarboxylic acid, 2-methoxyethyl ester |
| 35. | 7.2822 | Cyclotetrasiloxane, octamethyl- |
| 36. | 7.3176 | 6-Hepten-1-ol, 2-methyl- |
| 37. | 7.5330 | 1,4-Butanediamine, 2,3-dimethoxy-N,N,N′,N′-tetramethyl-, [S-R*,R*)]- |
| 38. | 7.6244 | Propanoic acid, ethenyl ester |
| 39. | 7.6246 | 2-Pentenoic acid, 4-methyl- |
| 40. | 7.6250 | 2-Butenoic acid, ethyl ester, (E)- |
| 41. | 7.6500 | Pentane, 2,2,4,4-tetramethyl-3-methoxy- |
| 42. | 7.7127 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- |
| 43. | 7.7757 | Hydrazine, 1,1-dibutyl- |
| 44. | 7.8171 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- |
| 45. | 7.9027 | Benzene, 1-methyl-3-(1-methylethyl)- |
| 46. | 7.9923 | 2-Hydroxyquinine |
| 47. | 7.9936 | D-Limonene |
| 48. | 8.0039 | Hydroxylamine, O-(phenylmethyl)- |
| 49. | 8.0363 | m-Aminophenylacetylene |
| 50. | 8.0411 | Benzene, 1,2,4,5-tetramethyl- |
| 51. | 8.1266 | D-Limonene |
| 52. | 8.1698 | 3-Acetoxy-3-hydroxypropionic acid, methyl ester |
| 53. | 8.3868 | Bicyclo[3.1.0]hex-2-ene, 2-methyl-5-(1-methylethyl)- |
| 54. | 8.4197 | Furan, 2-methyl-5-(methylthio)- |
| 55. | 8.4611 | Bicyclo[3.1.0]hex-2-ene, 2-methyl-5-(1-methylethyl)- |
| 56. | 8.4768 | 2H-1,2,4-Oxadiazine-3,6(4H,5H)-dione, 4,5-dimethyl-2-phenyl- |
| 57. | 8.5869 | 1-Amino-2,6-dimethylpiperidine |
| 58. | 8.6649 | 3-Carene |
| 59. | 8.7552 | Thymine |
| 60. | 8.8170 | Cyclotrisiloxane, hexamethyl- |
| 61. | 8.8774 | Benzofuran-5,6-diol-3-one, 2-benzylidene- |
| 62. | 8.9324 | .gamma.-Terpinene |
| 63. | 8.9842 | 2-Isopropenylthiazole |
| 64. | 9.3303 | Cyclotrisiloxane, hexamethyl- |
| 65. | 9.3745 | 2-Furanmethanol, 5-ethenyltetrahydro-.alpha.,.alpha.,5-trimethyl-, cis- |
| 66. | 9.3746 | trans-Linalool oxide (furanoid) |
| 67. | 9.4544 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- |
| 68. | 9.4594 | 1,3-Cyclohexadiene, 1-methyl-4-(1-methylethyl)- |
| 69. | 9.5043 | Benzene, 1-ethenyl-3,5-dimethyl- |
| 70. | 9.5073 | Benzaldehyde, 4-(1-phenyl-2-propenyloxy)- |
| 71. | 9.7270 | (+)-4-Carene |
| 72. | 9.7498 | Cyclotrisiloxane, hexamethyl- |
| 73. | 9.7837 | Benzene, 4-ethenyl-1,2-dimethyl- |
| 74. | 9.9367 | 3-Methyl-5-nonylpyrrolizidine |
| 75. | 9.9521 | Dideoxy-dimethylene-iditol |
| 76. | 10.0442 | 1,3-Benzenediol, monobenzoate |
| 77. | 10.3802 | 1H-Imidazo[4,5-b]pyridine |
| 78. | 10.5562 | 4-Ethylbenzamide |
| 79. | 10.6038 | Maltol |
| 80. | 10.7661 | Methyl 2-O-methyl-.beta.-D-xylopyranoside |
| 81. | 10.8566 | Benzoic acid, hydrazide |
| 82. | 10.8595 | 1,3-Cyclohexadiene, 1,3,5,5-tetramethyl- |
| 83. | 10.8951 | Benzene, 1-ethyl-3,5-dimethyl- |
| 84. | 10.9589 | trans-(2-Chlorovinyl)dimethylethoxysilane |
| 85. | 11.1955 | 1,3-Cyclopentadiene, 1,2,3,4,5-pentamethyl- |
| 86. | 11.1976 | N-Formyl-1-phenylethylamine, N- (pentafluoropropionyl)- |
| 87. | 11.2112 | 1,3-Cyclohexadiene, 1,3,5,5-tetramethyl- |
| 88. | 11.2344 | 2,4,6-Octatriene, 2,6-dimethyl-, (E,Z)- |
| 89. | 11.4400 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
| 90. | 11.5297 | L-Alanyl-L-norleucine, N-dimethylaminomethylene-,methylester |
| 91. | 11.7208 | 1-Methyl-5-fluorouracil |
| 92. | 11.7286 | Ethyl hydrogen succinate |
| 93. | 12.0331 | 2,4-Pentanedione, 1,1,1,5,5,5-hexafluoro- |
| 94. | 12.1718 | Bis(hexahydro-7a-methyl-3-oxo-3H-pyrrolizin-5-yl)ether |
| 95. | 12.2723 | 3,4-Hexanedione, 1 (phenylmethoxy)- |
| 96. | 12.2892 | 1-(Hexahydropyrrolizin-3-ylidene)-3,3-dimethyl-butan-2-one |
| 97. | 12.3125 | Thiazolidine, 2-isobutyl- |
| 98. | 12.5429 | 1,5-Heptadiene, 3,3,6-trimethyl- |
| 99. | 12.6079 | L-.alpha.-Terpineol |
| 100. | 12.8687 | 1,3,2-Oxazaborolidine-4-carboxylic acid, 2-butyl-5-methyl-, methyl ester, cis- |
| 101. | 13.1899 | 1,4:3,6-Dianhydro-.alpha.-d-glucopyranose |
| 102. | 13.2456 | Ethanol, 2-phenoxy- |
| 103. | 13.3101 | Dimethyl sulfone |
| 104. | 13.3444 | 6-Chlorohexanoic acid, 4-isopropylphenyl ester |
| 105. | 13.3724 | 1,3-Benzenediacetonitrile |
| 106. | 13.4215 | Cyclohexanecarboxylic acid, 4-propyl-, 4-ethoxyphenyl ester, trans- |
| 107. | 13.4450 | Bicyclo[2.2.1]heptane, 2-chloro-1,7,7-trimethyl-,exo- |
| 108. | 13.4452 | Bicyclo[2.2.1]heptane, 2-chloro-1,7,7-trimethyl-,exo- |
| 109. | 13.5029 | 2-Methoxy-3-methyl-butyric acid, methyl ester |
| 110. | 13.5817 | 2-Chloro-4,6-difluoro-pyrimidine |
| 111. | 13.6006 | Dinocap |
| 112. | 13.8528 | 1-Bromopyrene |
| 113. | 14.1003 | 2,6-Octadiene, 4,5-dimethyl- |
| 114. | 14.1213 | Geranyl bromide |
| 115. | 14.6500 | l-Norvaline, N-allyloxycarbonyl-, isobutyl ester |
| 116. | 14.8145 | Benzoic acid, 3-methyl-, methyl ester |
| 117. | 14.8153 | Benzeneacetic acid |
| 118. | 14.8806 | (-)-O-Acetylmalic anhydride |
| 119. | 15.0060 | l-Leucine. N-(3-fluorobenzoyl)-, hexyl ester |
| 120. | 15.4630 | 1,3-Benzodioxole, 5-propyl- |
| 121. | 15.4732 | 3,4-(Methylenedioxy)toluene |
| 122. | 15.5623 | Cyclobutane, 1,3-diisopropenyl-, trans |
| 123. | 15.7477 | Thymol |
| 124. | 16.0172 | 2-Methoxy-4-vinylphenol |
| 125. | 16.2591 | 1-Naphthoic acid, 4-chloro-2-methylphenyl ester |
| 126. | 17.3087 | Succinic acid, di(2-fluoroethyl) ester |
| 127. | 18.1799 | Cyclopentasiloxane, decamethyl- |
| 128. | 18.4063 | Cyclopropanecarboxylic acid, pent-2-en-4-ynyl ester |
| 129. | 18.4212 | 1,4-Benzenediol, 2-methoxy- |
| 130. | 18.6433 | Isoquinoline, 1,2,3,4-tetrahydro-6,7-dihydroxy-1-methyl- |
| 131. | 18.9219 | 3-Acetoxy-2-methyl-pyran-4-one |
| 132. | 18.9566 | Benzenemethanol, .alpha.-methyl-.alpha.-(1-methyl-2-propenyl)- |
| 133. | 19.5578 | 2-tert-Butyl-3,4,5,6-tetrahydropyridine |
| 134. | 19.9121 | Ethanone, 1-(3-hydroxy-4-methoxyphenyl)- |
| 135. | 20.2332 | beta.-D-Glucopyranose, 1,6-anhydro- |
| 136. | 20.2439 | Pentanoic acid, 5-hydroxy-, 2,4-di-t-butylphenyl esters |
| 137. | 20.4522 | Benzoic acid, 4-ethoxy-, ethyl ester |
| 138. | 20.4815 | 2,5-Dihydroxy-4-methoxyacetophenone |
| 139. | 20.6307 | Propan-2-one, 1-(4-isopropoxy-3-methoxyphenyl)- |
| 140. | 20.7563 | 4′-(Trifluoromethyl)acetophenone |
| 141. | 20.7858 | Nonanedioic acid, dimethyl ester |
| 142. | 20.9204 | Pyrido[2,3-b]indole, 6-amino- |
| 143. | 20.9761 | Glutaric acid, 2-pentyl propyl ester |
| 144. | 21.0824 | Phenol, 4-ethenyl-2,6-dimethoxy- |
| 145. | 21.3600 | 3-Hydroxy-4-methoxybenzoic acid |
| 146. | 21.5168 | Butamben |
| 147. | 22.1123 | 3-Phenyl-4-hydroxyacetophenone |
| 148. | 22.1584 | 4-Pyridinol, 2,6-dimethyl-3-[methylthio]- |
| 149. | 22.3544 | 2,5-Piperazinedione, 3-methyl-6-(1-methylethyl)- |
| 150. | 23.0161 | 4′-(1-Pyrroyl)acetophenone |
| 151. | 23.1652 | L-Proline, N-(hexanoyl)-, pentyl ester |
| 152. | 23.2674 | 2H-1-Benzopyran-3-carbonitrile, 4-methyl-2-oxo- |
| 153. | 23.3523 | 9H-Xanthene |
| 154. | 23.5004 | (3S,6S)-3-Butyl-6-methylpiperazine-2,5-dione |
| 155. | 23.6610 | 3,6-Diisopropylpiperazin-2,5-dione |
| 156. | 24.3079 | Cyclo(L-prolyl-L-valine) |
| 157. | 24.5477 | 2-Propenoic acid, 3-(4-hydroxy-3-methoxyphenyl)-,methyl ester |
| 158. | 24.6445 | Valeric acid, 2-biphenyl ester |
| 159. | 24.7733 | Phthalic acid, cyclohexyl isohexyl ester |
| 160. | 24.7733 | Phthalic acid, propyl tridec-2-yn-1-yl ester |
| 161. | 24.8451 | Cyclotetradecane |
| 162. | 24.9312 | Tetradecanoic acid, 10,13-dimethyl-, methyl ester |
| 163. | 25.5461 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl)- |
| 164. | 25.7591 | 4-Methylumbelliferyl laurate |
| 165. | 25.7692 | Phenol, 4-[(5,6,7,8-tetrahydro-1,3-dioxolo[4,5-g]isoquinolin-5-yl)methyl]-, (R)- |
| 166. | 25.8003 | n-Hexadecanoic acid |
| 167. | 25.8927 | Propane-1,1-diol dipropanoate |
| 168. | 25.8976 | Heptadecanoic acid, methyl ester |
| 169. | 26.1829 | Hexadecanoic acid, 14-methyl-, methyl ester |
| 170. | 26.2367 | Methyl 8-heptadecenoate |
| 171. | 26.3061 | Methyl 8-heptadecenoate |
| 172. | 26.6748 | Dodecanamide, N-(2-hydroxyethyl)- |
| 173. | 26.9179 | 1,13-Tetradecadiene |
| 174. | 26.9181 | 5-Cyclohexyl-1-pentene |
| 175. | 27.1865 | 1-Hexadecanol |
| 176. | 27.5357 | Phytol |
| 177. | 27.6157 | 2-Furancarboxamide, N-ethyl- |
| 178. | 27.8322 | Dodecanamide, N-(2-hydroxyethyl)- |
| 179. | 27.8570 | cis-13-Octadecenoic acid |
| 180. | 27.8887 | 2-Cyano-5-(3-fluorophenyl)pyrimidine |
| 181. | 27.9256 | Benzene, (1-methylbutyl)- |
| 182. | 28.0760 | 2-Propanamine, N,N′-methanetetraylbis- |
| 183. | 28.2097 | 2-Phenylacetic acid, 2,2,2-trifluoroethyl ester |
| 184. | 28.6594 | (Z)-2-(pentadec-8-en-1-yl)-4,5-dihydrooxazole |
| 185. | 28.6741 | (Z)-2-(pentadec-8-en-1-yl)-4,5-dihydrooxazole |
| 186. | 28.7725 | Cyclohexanethiol, 2,5-dimethyl-, acetate |
| 187. | 28.8193 | Pivalic acid, 2-methylpropyl ester |
| 188. | 29.0173 | Cyclohexanethiol, 2,5-dimethyl-, acetate |
| 189. | 29.0827 | Cyclohexanepropanol- |
| 190. | 29.0831 | 5-Methyl-4-hexene-1-yl acetate |
| 191. | 29.1350 | Cyclohexanethiol, 2,5-dimethyl-, acetate |
| 192. | 29.2850 | Cyclohexanethiol, 2,5-dimethyl-, acetate |
| 193. | 29.3103 | 3-Chloropropionic acid, heptadecyl ester |
| 194. | 29.3207 | Cyclohexane, tetracosyl- |
| 195. | 29.6797 | Oxazole, 2-(8Z)-8-heptadecen-1-yl-4,5-dihydro- |
| 196. | 29.7709 | Octyl tiglate |
| 197. | 29.9002 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-,(E,E)- |
| 198. | 30.1936 | Benzene, 5-(2-isothiocyanatoethyl)-1,2,3-trimethoxy- |
| 199. | 30.2872 | 1-Heneicosanol |
| 200. | 30.3570 | Pyrrolo[1,2-a]pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)- |
| 201. | 30.4001 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-,(E,E)- |
| 202. | 30.6341 | (E)-1-(6,10-Dimethylundec-5-en-2-yl)-4-methylbenzene |
| 203. | 30.6343 | (E)-1-(6,10-Dimethylundec-5-en-2-yl)-4-methylbenzene |
| 204. | 30.7528 | p-Camphorene |
| 205. | 30.7534 | (E)-1-(6,10-Dimethylundec-5-en-2-yl)-4-methylbenzene |
| 206. | 30.7593 | 1-Ethyl-1,3,3-trimethyl-5-(1-methyl-3-propanal)indan |
| 207. | 30.8610 | Squalene |
| 208. | 30.8621 | Supraene |
| 209. | 30.8721 | Hexane, 3,4-bis(1,1-dimethylethyl)-2,2,5,5-tetramethyl- |
| 210. | 30.9253 | 1,6,10,14-Hexadecatetraen-3-ol, 3,7,11,15-tetramethyl-,(E,E)- |
| 211. | 31.0366 | (E,E,E)-3,7,11,15-Tetramethylhexadeca- 1,3,6,10,14-pentaene |
| 212. | 31.0368 | (E)-1-(6,10-Dimethylundec-5-en-2-yl)-4-methylbenzene |
| 213. | 31.1347 | Palmitoyl chloride |
| 214. | 31.2706 | Glycidyl palmitate |
| 215. | 31.4125 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester |
| 216. | 31.5403 | Isobutyl 2-(4-methylcyclohex-3-enyl)propan-2-ylcarbonate |
| 217. | 31.5760 | Fenoterol, N-trifluoroacetyl-O,O,O,O-tetrakis(trimethylsilyl)deriv. |
| 218. | 31.5859 | Tetracosamethyl-cyclododecasiloxane |
| 219. | 32.0501 | trans-Geranylgeraniol |
| 220. | 32.1611 | trans-Geranylgeraniol |
| 221. | 32.2627 | Benzoic acid, hexadecyl ester |
| 222. | 32.2976 | Supraene |
| 223. | 32.4260 | (2E,6E,10E)-3,7,11,15-Tetramethylhexadeca-2,6,10,14-tetraen-1-yl formate |
| 224. | 32.6000 | L-Valine, N-(2-fluoro-5-trifluoromethylbenzoyl)-,isohexyl ester |
| 225. | 32.6258 | Oleoyl chloride |
| 226. | 32.6261 | 9-Octadecenoic acid (Z)-, 2,3-dihydroxypropyl ester |
| 227. | 32.6605 | 6H-Dibenzo[b,d]pyran-6-one, 7,9-dihydroxy-3-methoxy-1-methyl- |
| 228. | 32.6607 | 9,10-Dihydro-10-phenyl-9,10-ethano-9-phosphaanthracene-11,12-dicarboxylic anhydride |
| 229. | 32.7174 | 1,2,3,4-Tetrahydro-3-(phenylacetamido)quinoline |
| 230. | 32.7381 | Squalene |
| 231. | 32.7873 | Isonipecotic acid, N-(3-phenylpropionyl)-, undecyl ester |
| 232. | 32.8123 | Stearic acid chloride |
| 233. | 32.8338 | Petasitene |
| 234. | 32.9963 | Isophthalic acid, di(2-fluorophenyl) ester |
| 235. | 33.0156 | Acetamide, 2-(1-benzimidazolyl)- |
| 236. | 33.0293 | Octadecanoic acid, 2,3-dihydroxypropyl ester |
| 237. | 33.0835 | Methyl-methoxy-hydroxymethyl-amine |
| 238. | 33.0864 | 5H-Tetrazol-5-amine |
| 239. | 33.1417 | DL-Lactamide, N,N-dimethyl-, methyl ether |
| 240. | 33.4009 | (2E,6E,10E)-3,7,11,15-Tetramethylhexadeca-2,6,10,14-tetraen-1-yl formate |
| 241. | 33.7179 | Pentyl palmitoleate |
| 242. | 33.7348 | 9-Hexadecenoic acid, octadecyl ester, (Z)- |
| 243. | 33.8473 | (E,E)-7,11,15-Trimethyl-3-methylene-hexadeca-1,6,10,14-tetraene |
| 244. | 34.2625 | Tetradecanoic acid, octadecyl ester |
| 245. | 34.6215 | Pyrido[2,3-d]pyrimidin-5(8H)-one, 2-methyl-4-phenyl- |
| 246. | 34.6353 | 9-Hexadecenoic acid, octadecyl ester, (Z)- |
| 247. | 34.8413 | Benzimidazole, 1-(4-chloro-1-methyl-3-pyrazolyl)carbonyl- |
| 248. | 34.9489 | Squalene |
| 249. | 35.0604 | Squalene |
| 250. | 35.6490 | 2-Fluorobenzylamine, N,N-diheptyl |
| 251. | 35.6490 | 9-Hexadecenoic acid, octadecyl ester, (Z)- |
| 252. | 35.6538 | 9-Hexadecenoic acid, octadecyl ester, (Z)- |
| 253. | 35.8164 | trans-Geranylgeraniol |
| 254. | 36.0085 | 1,6,10,14,18,22-Tetracosahexaen-3-ol, 2,6,10,15,19,23-hexamethyl-, (all-E)-(.+/−.)- |
| 255. | 36.0566 | Silane, diethylheptyloxyoctadecyloxy- |
| 256. | 36.2410 | Oxirane, 2-dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11,15,19-heneicosapentaenyl)-, (all-E)- |
| 257. | 36.4041 | Oxirane, 2-dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11,15,19-heneicosapentaenyl)-, (all-E)- |
| 258. | 36.4347 | Benzalphthalide |
| 259. | 36.8772 | (2E,6E,10E)-3,7,11,15-Tetramethylhexadeca-2,6,10,14-tetraen-1-yl formate |
| 260. | 36.8830 | 2,6,10,14-Hexadecatetraen-1-ol, 3,7,11,15-tetramethyl-, acetate, (E,E,E)- |
| 261. | 37.2283 | Urs-12-en-3-ol, acetate, (3.beta.)- |
| 262. | 37.4453 | Vitamin E |
| 263. | 37.4462 | 9,9′-Bi-9H-fluorene |
| 264. | 37.4947 | Ethane, 1,2-diiodo- |
| S. No | Retention Time | Compound |
|---|---|---|
| 1. | 3.546 | 2-methylbutyl acetate |
| 2. | 3.592 | 1-Butanol, 2-Methyl-,Acetate |
| 3. | 6.572 | CIS-Sesquilavandulol |
| 4. | 7.483 | Propene-3,3,3-D3 |
| 5. | 7.693 | 4-Heptyn-2-ol |
| 6. | 18.620 | Furo[3,4-D]-1,3-Dioxole-3A(4H)-Car |
| 7. | 20.180 | Decane, 1-Chloro |
| 8. | 25.043 | (4.Alpha.,10.Beta.,15s)-4-Carbomeo |
| 9. | 30.232 | Tetradecanoic Acid, 12-Methyl |
| 10. | 30.413 | Phthalic acid, 4-bromophenyl heptyl ester |
| 11. | 31.076 | 3,4-Hexanediol, 2,5-Dimethyl |
| 12. | 34.028 | Tetradecanoic Acid, 12-Methyl-, |
| 13. | 41.510 | 1,5-Heptadiene, 2,6-Dimethyl- |
| 14. | 41.691 | 2z,6e-Farnesol |
| 15. | 46.408 | 1,9-Nonanediol, Dimethanesulfonate |
| 16. | 46.582 | N-(4-Methylcyclohexyl)Acetamide, Cis- |
| 17. | 47.580 | 6,11-Dimethyl-2,6,10-Dodecatrien |
| 18. | 53.652 | Pseduosarsasapogenin-5,20-Dien |
| 19. | 54.012 | Cholest-5-En-3-Ol, 24-Propylidene-, (3.Beta |
| 20. | 54.248 | 1,6,10-Dodecatrien-3-Ol, 3,7,11-Tri |
| 21. | 54.358 | Urs-12-En-28-Ol |
| 22. | 54.400 | Cholesta-8,24-Dien-3-Ol, 4-Methyl-, (3.Beta |
| 23. | 55.418 | 1,6,10-Dodecatrien-3-Ol, 3,7,11-Trim |
| 24. | 55.678 | Dehydrolinalool |
| 25. | 56.939 | 3-Decyn-2-Ol |
| 26. | 57.348 | 5H-3,5a-Epoxynaphth[2,1-C]Oxepin, Dodeca |
| 27. | 58.010 | 1-Heptatriacotanol |
| 28. | 58.313 | Lup-20(29)-Ene-3,28-Diol,(3.Beta.)- |
| 29. | 58.624 | Veridiflorol |
| 30. | 58.885 | Lanosta-8,24-dien-3-ol, acetate,(3.beta.)- |
| Concentrations (µL) | DPPH Assay (% of Inhibition) | |
|---|---|---|
| MC | AC | |
| 10 | 40.98 ± 1.47 | 65.57 ± 1.12 |
| 50 | 52.46 ± 1.69 | 68.03 ± 1.73 |
| 150 | 59.02 ± 1.92 | 81.15 ± 1.15 |
| 250 | 60.84 ± 1.78 | 82.75 ± 1.36 |
| 350 | 64.75 ± 1.04 | 83.61 ± 1.79 |
| 500 | 65.57 ± 1.84 | 85.25 ± 1.92 |
| 750 | 68.85 ± 1.23 | 86.35 ± 1.67 |
| IC50 | 32.15 µg/mL | 30.16 µg/mL |
| Concentrations (µL) | DPPH Assay (% of Inhibition) | |
|---|---|---|
| MC | AC | |
| 10 | 42.23 ± 1.01 | 50.46 ± 2.89 |
| 50 | 50.35 ± 1.56 | 65.92 ± 3.58 |
| 150 | 57.90 ± 1.62 | 70.24 ± 2.13 |
| 250 | 59.73 ± 1.81 | 72.86 ± 1.45 |
| 350 | 60.64 ± 1.25 | 75.72 ± 2.54 |
| 500 | 63.46 ± 1.04 | 80.37 ± 2.12 |
| 750 | 65.74 ± 1.66 | 83.46 ± 1.45 |
| IC50 | 40.14 µg/mL | 38.22 µg/mL |
| Concentrations (µL) | DPPH Assay (% of Inhibition) | |
|---|---|---|
| MC | AC | |
| 10 | 31.12 ± 1.89 | 49.35 ± 1.48 |
| 50 | 39.24 ± 1.71 | 54.81 ± 1.40 |
| 150 | 46.02 ± 1.33 | 59.13 ± 1.29 |
| 250 | 48.62 ± 1.67 | 61.75 ± 1.32 |
| 350 | 49.53 ± 1.29 | 64.61 ± 1.74 |
| 500 | 50.35 ± 1.57 | 69.59 ± 1.87 |
| 750 | 54.63 ± 1.05 | 73.14 ± 1.96 |
| IC50 | 33.59 µg/mL | 31.28 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
C, J.; Periakaruppan, R.; Vijai Selvaraj, K.S.; Al-Dayan, N. Spectroscopic Analysis of Bioactive Compounds from Latex of Calotropis gigantea L. and an Evaluation of Its Biological Activities. Analytica 2024, 5, 384-401. https://doi.org/10.3390/analytica5030024
C J, Periakaruppan R, Vijai Selvaraj KS, Al-Dayan N. Spectroscopic Analysis of Bioactive Compounds from Latex of Calotropis gigantea L. and an Evaluation of Its Biological Activities. Analytica. 2024; 5(3):384-401. https://doi.org/10.3390/analytica5030024
Chicago/Turabian StyleC, Jayalekshmi, Rajiv Periakaruppan, Karungan Selvaraj Vijai Selvaraj, and Noura Al-Dayan. 2024. "Spectroscopic Analysis of Bioactive Compounds from Latex of Calotropis gigantea L. and an Evaluation of Its Biological Activities" Analytica 5, no. 3: 384-401. https://doi.org/10.3390/analytica5030024
APA StyleC, J., Periakaruppan, R., Vijai Selvaraj, K. S., & Al-Dayan, N. (2024). Spectroscopic Analysis of Bioactive Compounds from Latex of Calotropis gigantea L. and an Evaluation of Its Biological Activities. Analytica, 5(3), 384-401. https://doi.org/10.3390/analytica5030024







