Analysis of Aceclofenac, Ketorolac, and Sulindac in Human Urine Using the Microemulsion Electrokinetic Chromatography Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Buffer and Microemulsion Preparation
2.2.2. CE Instrumentation
2.2.3. Sample Collection and Pretreatment
2.2.4. Solid Phase Extraction (SPE)
2.2.5. Solid Phase Membrane Tip Extraction (SPMTE)
3. Results and Discussion
3.1. Optimization of the MEEKC
3.1.1. Borate Buffer pH
3.1.2. Borate Concentration
3.1.3. Surfactant Concentration
3.1.4. Co-Surfactant Concentration
3.1.5. Organic Modifier Concentration
3.1.6. Oil Droplet Concentration
3.1.7. Temperature
3.1.8. Applied Voltage
3.2. Analytical Performance of the MEEKC Method
3.3. Analysis of Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gwenzi, W.; Selvasembian, R.; Offiong, N.A.O.; Mahmoud, A.E.D.; Sanganyado, E.; Mal, J. COVID-19 drugs in aquatic systems: A review. Environ. Chem. Let. 2022, 20, 1275–1294. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, T.J.; Fudin, J. Nonsteroidal antiinflammatory drugs for acute and chronic pain. Phys. Med. Rehabil. Clin. N. Am. 2020, 31, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Avino, P.; Notardonato, I.; Passarela, S.; Russo, M.V. Determination of non-steroidal anti-inflammatory drugs in animal urine samples by ultrasound vortex-assisted dispersive liquid–liquid microextraction and gas chromatography coupled to ion trap-mass spectrometry. Appl. Sci. 2020, 10, 5441. [Google Scholar] [CrossRef]
- Sandrin, V.S.S.; Oliveira, G.M.; Weckwerth, G.M.; Polanco, N.L.D.H.; Faria, F.A.C.; Santos, C.F. Analysis of different methods of extracting NSAIDs in biological fluid samples for LC-MS/MS assays: Scoping review. Metabolites 2022, 12, 751. [Google Scholar] [CrossRef] [PubMed]
- Reinholds, I.; Pugajeva, I.; Zacs, D.; Lundanes, E.; Rusko, J.; Perkons, I.; Bartkevics, V. Determination of acidic non-steroidal anti-inflammatory drugs in aquatic samples by liquid chromatography-triple quadrupole mass spectrometry combined with carbon nanotubes-based solid-phase extraction. Env. Mon. Assess. 2017, 189, 568. [Google Scholar] [CrossRef] [PubMed]
- Alatawi, H.; Hogan, A.; Albalawi, I.; Alsefri, S.; Moore, E. Efficient determination of non-steroidal anti-inflammatory drugs by micellar electrokinetic chromatography in wastewater. Anal. Methods 2023, 15, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.L.; Liu, W.L.; Hsieh, S.H.; Huang, H.Y. Analyses of non-steroidal anti-inflammatory drugs in environmental water samples with microemulsion electrokinetic chromatography. Anal. Sci. 2010, 26, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Semail, N.F.; Abdul Keyon, A.S.; Saad, B.; Kamaruzaman, S.; Zain, N.N.M.; Lim, V.; Miskam, M.; Wan Abdullah, W.N.; Raoov, M.; Yahaya, N. Induced sample via transient isotachophoresis mediated with sweeping in micellar electrokinetic chromatography for the dual-stacking strategy of non-steroidal anti-inflammatory drugs in environmental water samples. J. Chrom. A 2022, 1685, 463616. [Google Scholar] [CrossRef] [PubMed]
- Reminek, F.; Foret, F. Capillary electrophoretic methods for quality control analyses of pharmaceuticals: A review. Electrophoresis 2021, 42, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Finja, K.; Holger, Z.; Matthias, S.; Ratih, R.; Robert, M.; Mais, O.; Sophie, H.; Christin, S.; Blanca, H.; Lapizco, E.; et al. Strategies for capillary electrophoresis: Method development and validation for pharmaceutical and biological applications Updated and completely revised edition. Electrophoresis 2023, 44, 1279–1341. [Google Scholar] [CrossRef]
- Zeid, A.M.; El-Masry, A.A.; El-Wasseef, D.R.; Eid, M.; Shehata, I.A. Green microemulsion electrokinetic chromatographic method for simultaneous determination of azelastine and budesonide. Sust. Chem. Pharm. 2022, 29, 100795. [Google Scholar] [CrossRef]
- Yan, T.C.; Zhang, H.H.; Cao, J.; Ye, L.H. Analysis of iodinated amino acids by microemulsion electrokinetic chromatography using aliphatic amine as a cosurfactant. J. Chrom. A 2022, 1685, 463644. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.W.; Chen, Y.C.; Liu, C.Y. Separation and on-line preconcentration of nonsteroidal anti-inflammatory drugs by microemulsion electrokinetic chromatography. Electrophoresis 2015, 36, 2745–2753. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.T.; Chen, Y.; Yang, J.; Dong, X.; Zheng, H.; Cao, J. On-line concentration of triazine herbicides in microemulsion electrokinetic chromatography by electrokinetic injection assisted micelle to cyclodextrin stacking. J. Chrom. A 2020, 1628, 461438. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, W. Microemulsion electrokinetic chromatography. In Capillary Electrophoresis. Methods and Protocols, 2nd ed.; Schmitt-Kopplin, P., Ed.; Humana: New York, NY, USA, 2016; Volume 1483, pp. 91–109. [Google Scholar] [CrossRef]
- Medina, G.S.; Acquaviva, A.; Reta, R. Development of monolithic sorbent cartridges (m-SPE) for the extraction of non-steroidal anti-inflammatory drugs from surface waters and their determination by HPLC. Microchem. J. 2020, 159, 105447. [Google Scholar] [CrossRef]
- See, H.H.; Sanagi, M.M.; Wan Ibrahim, W.A.; Naim, A.A. Determination of triazine herbicides using membrane-protected carbon nanotubes solid phase membrane tip extraction prior to micro-liquid chromatography. J. Chrom. A 2010, 1217, 1767–1772. [Google Scholar] [CrossRef] [PubMed]
- Kamaruzaman, S.; Sanagi, M.M.; Endud, S.; Wan Ibrahim, W.A.; Yahaya, N. MCM-41 solid phase membrane tip extraction combined with liquid chromatography for the determination of non-steroidal anti-inflammatory drugs in human urine. J. Chrom. B. 2013, 940, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Rahim, K.A.; Sanagi, M.M.; Hermawan, D.; Wan Ibrahim, W.A.; Abdul Keyon, A.S. Microemulsion electrokinetic chromatography coupled with dispersive micro-solid phase extraction for determination of isoflavones in soybean products. Malay. J. Anal. Sci. 2018, 22, 54–63. [Google Scholar] [CrossRef]
- Paíga, P.; Lolić, A.; Hellebuyck, F.; Santos, L.H.; Correia, M.; Delerue-Matos, C. Development of a SPE–UHPLC–MS/MS methodology for the determination of non-steroidal anti-inflammatory and analgesic pharmaceuticals in seawater. J. Pharm. Biomed. Anal. 2015, 106, 61–70. [Google Scholar] [CrossRef] [PubMed]
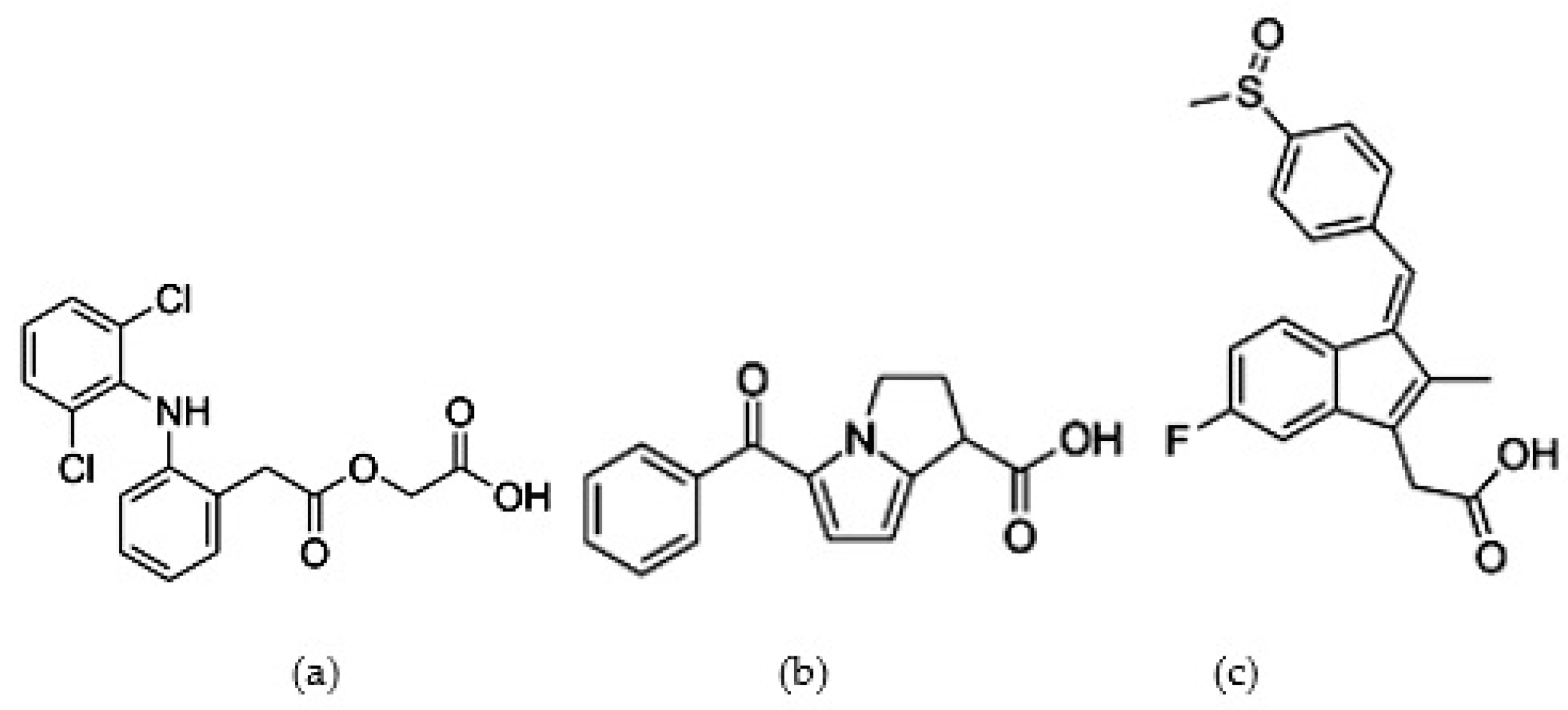
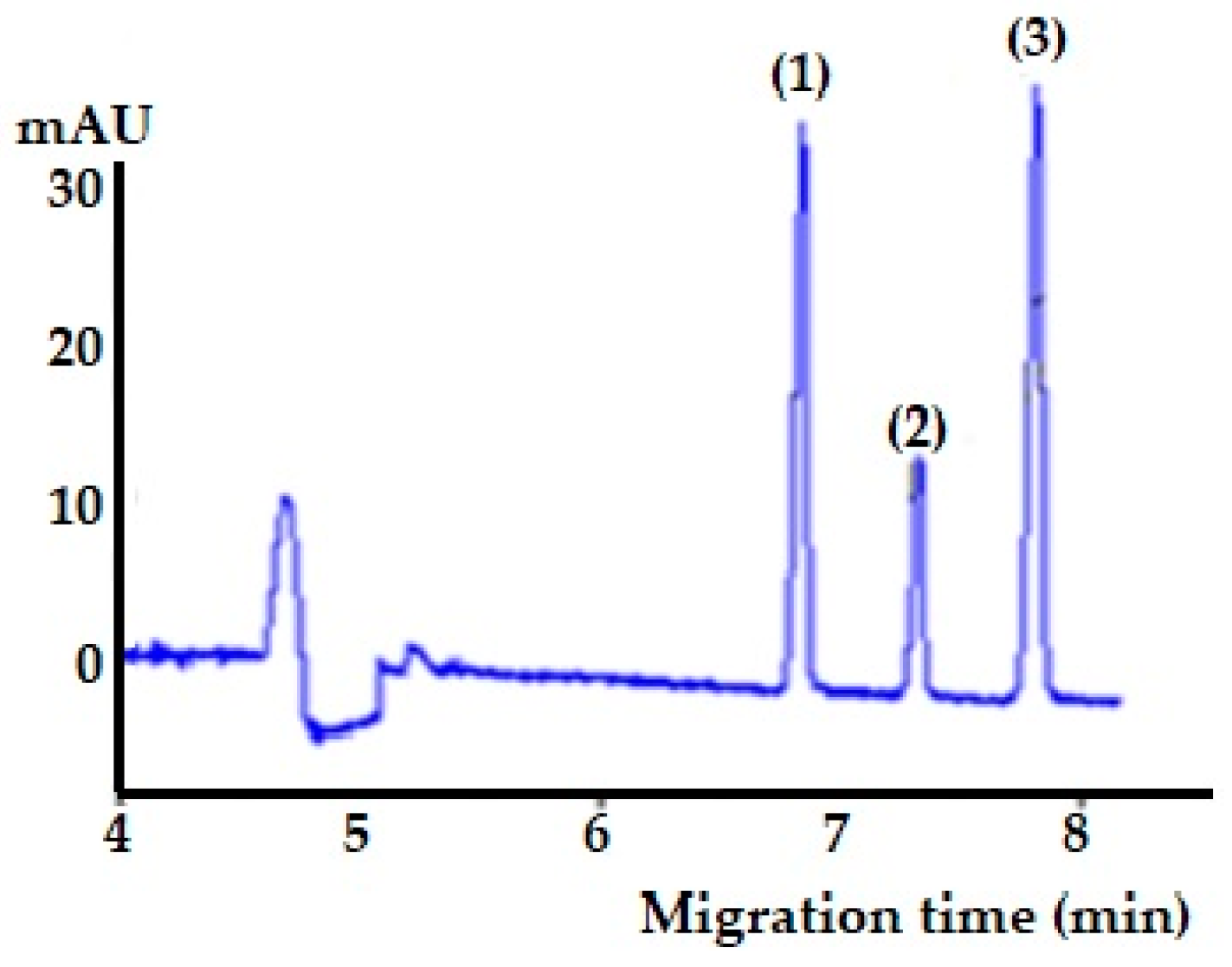

| Peak * | Buffer pH | Peak Area * | Rs ** | N ** |
|---|---|---|---|---|
| 1 | 7 | 81.14 | - | 33,157 |
| 8 | 103.21 | - | 44,168 | |
| 9 | 101.78 | - | 88,576 | |
| 10 | 77.85 | - | 55,300 | |
| 2 | 7 | 42.99 | 1.51 | 39,996 |
| 8 | 53.68 | 2.06 | 54,699 | |
| 9 | 56.98 | 4.40 | 97,422 | |
| 10 | 61.04 | 2.79 | 66,220 | |
| 3 | 7 | 103.90 | 6.90 | 25,785 |
| 8 | 146.64 | 8.94 | 33,289 | |
| 9 | 137.24 | 8.18 | 69,081 | |
| 10 | 158.79 | 11.13 | 42,349 |
| Peak | Borate Concentration (mM) | Peak Area | Rs | N |
|---|---|---|---|---|
| 1 | 2.5 | 103.15470 | - | 87,656 |
| 7.5 | 106.29944 | - | 73,044 | |
| 10.0 | 117.34855 | - | 64,902 | |
| 12.5 | 112.99209 | - | 58,637 | |
| 2 | 2.5 | 55.11003 | 3.74 | 96,642 |
| 7.5 | 60.55586 | 3.23 | 81,250 | |
| 10.0 | 65.75339 | 3.22 | 72,622 | |
| 12.5 | 61.11577 | 3.30 | 70,557 | |
| 3 | 2.5 | 136.20381 | 9.86 | 68,994 |
| 7.5 | 149.40866 | 9.95 | 55,371 | |
| 10.0 | 157.86875 | 9.35 | 45,543 | |
| 12.5 | 150.25440 | 8.40 | 44,674 |
| Peak | SDS (%) | Peak Area | Rs | N |
|---|---|---|---|---|
| 1 | 0.25 | 98.32646 | - | 81,842 |
| 0.50 | 110.29612 | - | 74,069 | |
| 1.00 | 111.29242 | - | 58,456 | |
| 1.25 | 90.98324 | - | 41,461 | |
| 2 | 0.25 | 35.99672 | 5.19 | 94,659 |
| 0.50 | 40.22963 | 4.74 | 85,577 | |
| 1.00 | 59.73791 | 3.15 | 68,264 | |
| 1.25 | 30.02828 | 1.59 | 52,886 | |
| 3 | 0.25 | 122.89176 | 3.37 | 76,173 |
| 0.50 | 140.46258 | 4.78 | 64,713 | |
| 1.00 | 150.52068 | 8.54 | 42,739 | |
| 1.25 | 125.62084 | 10.98 | 30,540 |
| Peak | Temperature (°C) | Migration Time (min) | Peak Area | Rs | N |
|---|---|---|---|---|---|
| 1 | 20 | 11.888 | 94.05359 | - | 112,363 |
| 25 | 10.411 | 97.88517 | - | 97,228 | |
| 30 | 8.451 | 90.45343 | - | 48,154 | |
| 2 | 20 | 12.777 | 35.32412 | 6.25 | 140,632 |
| 25 | 11.165 | 37.61095 | 5.63 | 114,177 | |
| 30 | 8.781 | 33.54822 | 3.45 | 45,076 | |
| 3 | 20 | 13.647 | 131.10137 | 5.71 | 103,746 |
| 25 | 11.811 | 135.42228 | 4.42 | 88,639 | |
| 30 | 9.596 | 126.35067 | 3.62 | 45,076 |
| Parameter | Peak 1 | Peak 2 | Peak 3 |
|---|---|---|---|
| Linearity range (ppm) | 25–200 | 25–200 | 25–200 |
| r2 | 0.9999 | 0.9996 | 0.9998 |
| RSD (%, n = 3) | |||
| Migration time | 0.70 | 1.65 | 0.97 |
| Peak Area | 2.03 | 2.09 | 2.30 |
| LOD | 3.51 | 4.75 | 2.72 |
| LOQ | 11.72 | 15.85 | 9.08 |
| Method | Parameter | Peak 1 | Peak 2 | Peak 3 |
|---|---|---|---|---|
| SPE-MEEKC | Recovery (%) | 91 | 98 | 103 |
| RSD (%, n = 3); (intra-day) | 1.33 | 2.33 | 1.26 | |
| RSD (%, n = 9); (inter-day) | 3.26 | 4.03 | 2.73 | |
| SPMTE-MEEKC | Recovery (%) | 87 | 79 | 96 |
| RSD (%, n = 3); (intra-day) | 2.04 | 3.15 | 3.66 | |
| RSD (%, n = 9); (inter-day) | 5.33 | 5.80 | 4.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hermawan, D.; Mohd Yatim, I.; Wan Ibrahim, W.A.; Abdul Keyon, A.S.; Cacu; Riswoko, A.; Laksmono, J.A. Analysis of Aceclofenac, Ketorolac, and Sulindac in Human Urine Using the Microemulsion Electrokinetic Chromatography Method. Analytica 2024, 5, 431-439. https://doi.org/10.3390/analytica5030028
Hermawan D, Mohd Yatim I, Wan Ibrahim WA, Abdul Keyon AS, Cacu, Riswoko A, Laksmono JA. Analysis of Aceclofenac, Ketorolac, and Sulindac in Human Urine Using the Microemulsion Electrokinetic Chromatography Method. Analytica. 2024; 5(3):431-439. https://doi.org/10.3390/analytica5030028
Chicago/Turabian StyleHermawan, Dadan, Izdiani Mohd Yatim, Wan Aini Wan Ibrahim, Aemi Syazwani Abdul Keyon, Cacu, Asep Riswoko, and Joddy Arya Laksmono. 2024. "Analysis of Aceclofenac, Ketorolac, and Sulindac in Human Urine Using the Microemulsion Electrokinetic Chromatography Method" Analytica 5, no. 3: 431-439. https://doi.org/10.3390/analytica5030028
APA StyleHermawan, D., Mohd Yatim, I., Wan Ibrahim, W. A., Abdul Keyon, A. S., Cacu, Riswoko, A., & Laksmono, J. A. (2024). Analysis of Aceclofenac, Ketorolac, and Sulindac in Human Urine Using the Microemulsion Electrokinetic Chromatography Method. Analytica, 5(3), 431-439. https://doi.org/10.3390/analytica5030028







