Evaluation of the Anti-Inflammatory Activity of Microwave Extracts of Thymus algeriensis: In Vitro, In Vivo, and In Silico Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Preparation of Extracts
2.2. Profile of Bioactive Compounds
2.3. In Vitro Anti-Inflammatory Activity
2.4. Inflammatory Paw Edema Test in Rats
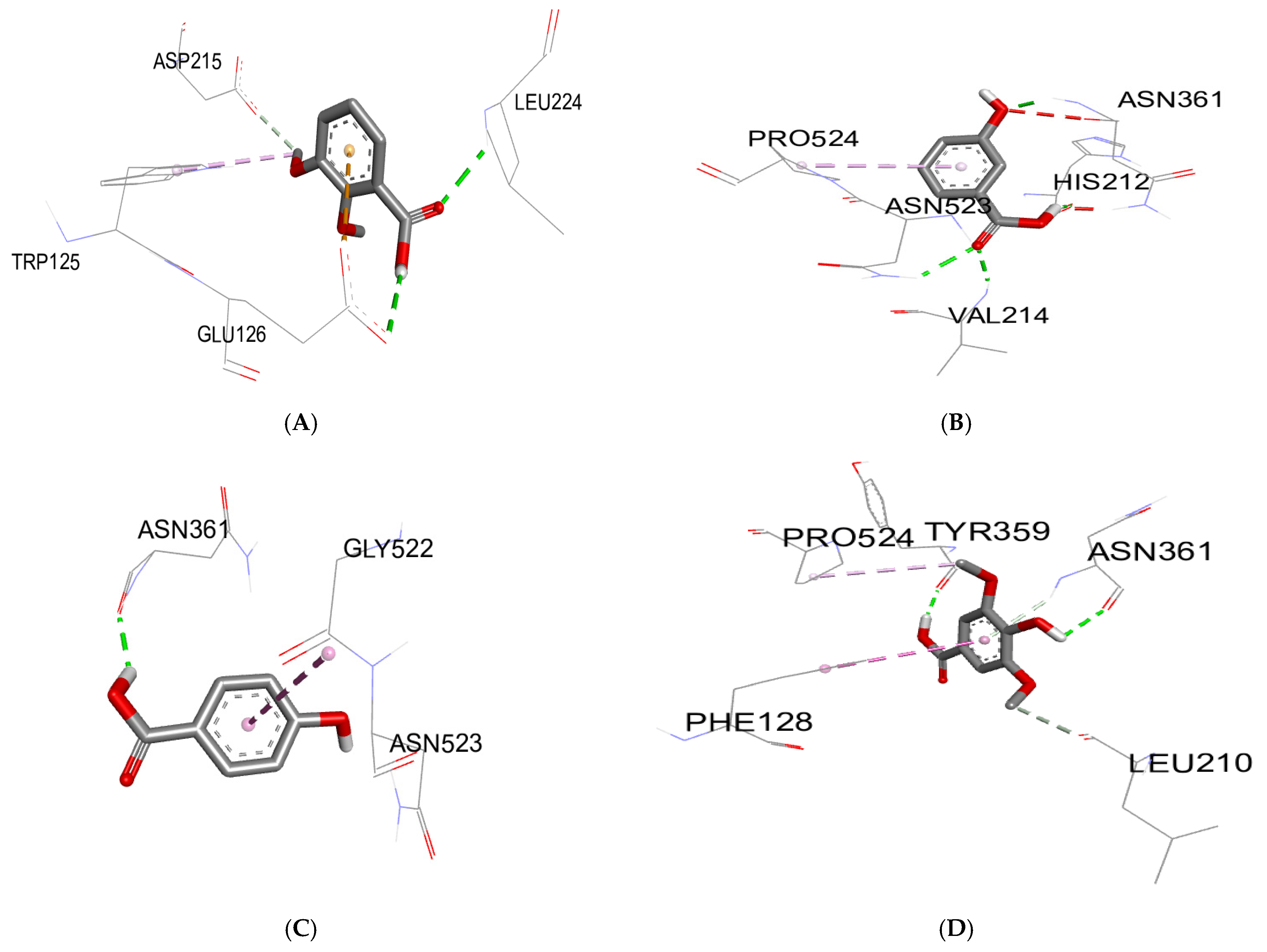
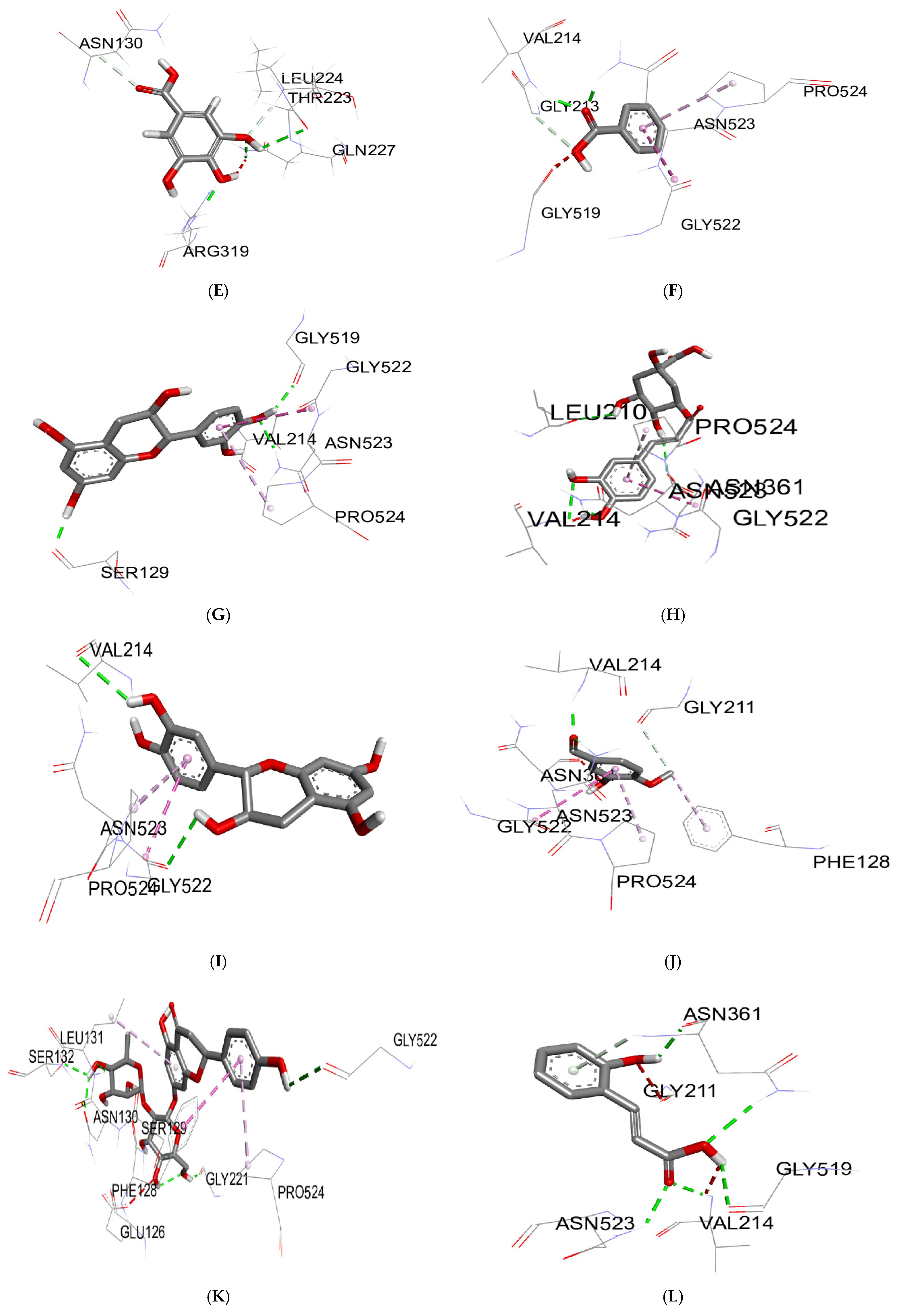
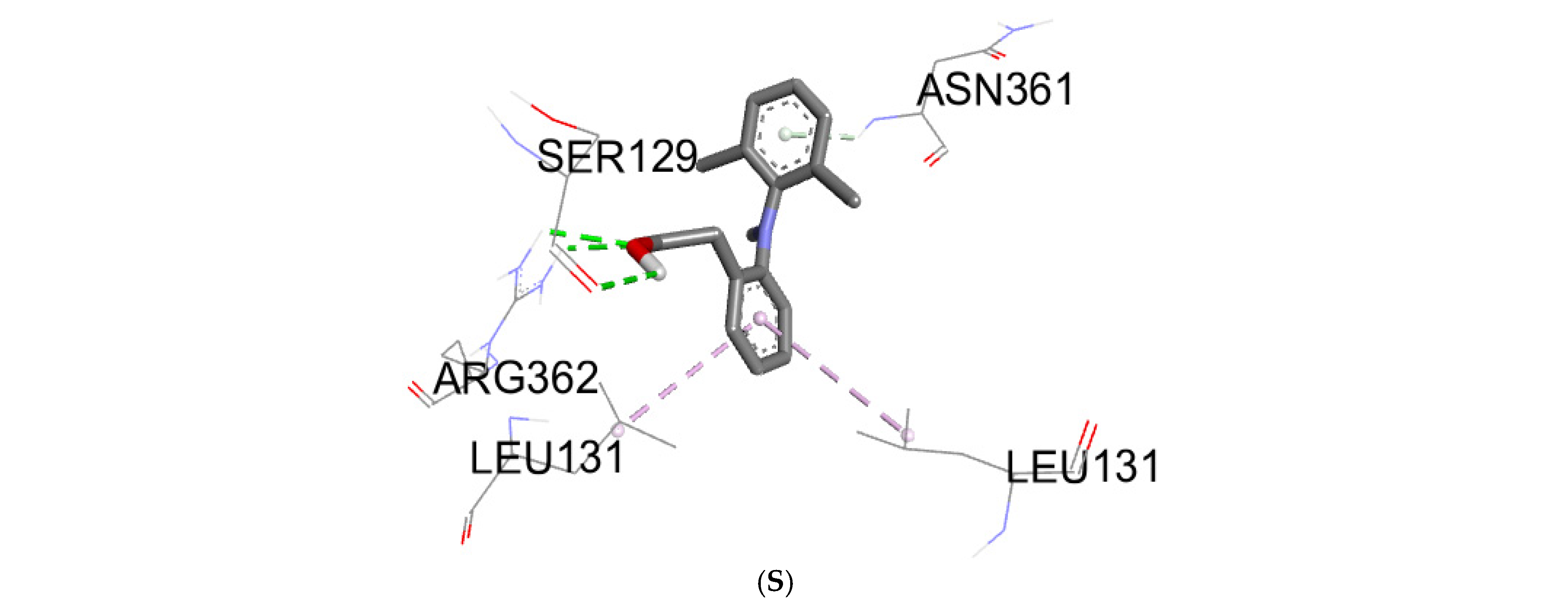
2.5. Molecular Docking Study
2.6. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Anti-Inflammatory Activity in Human RBCs (HBRCs)
3.2. Carrageenan-Induced Paw Oedema
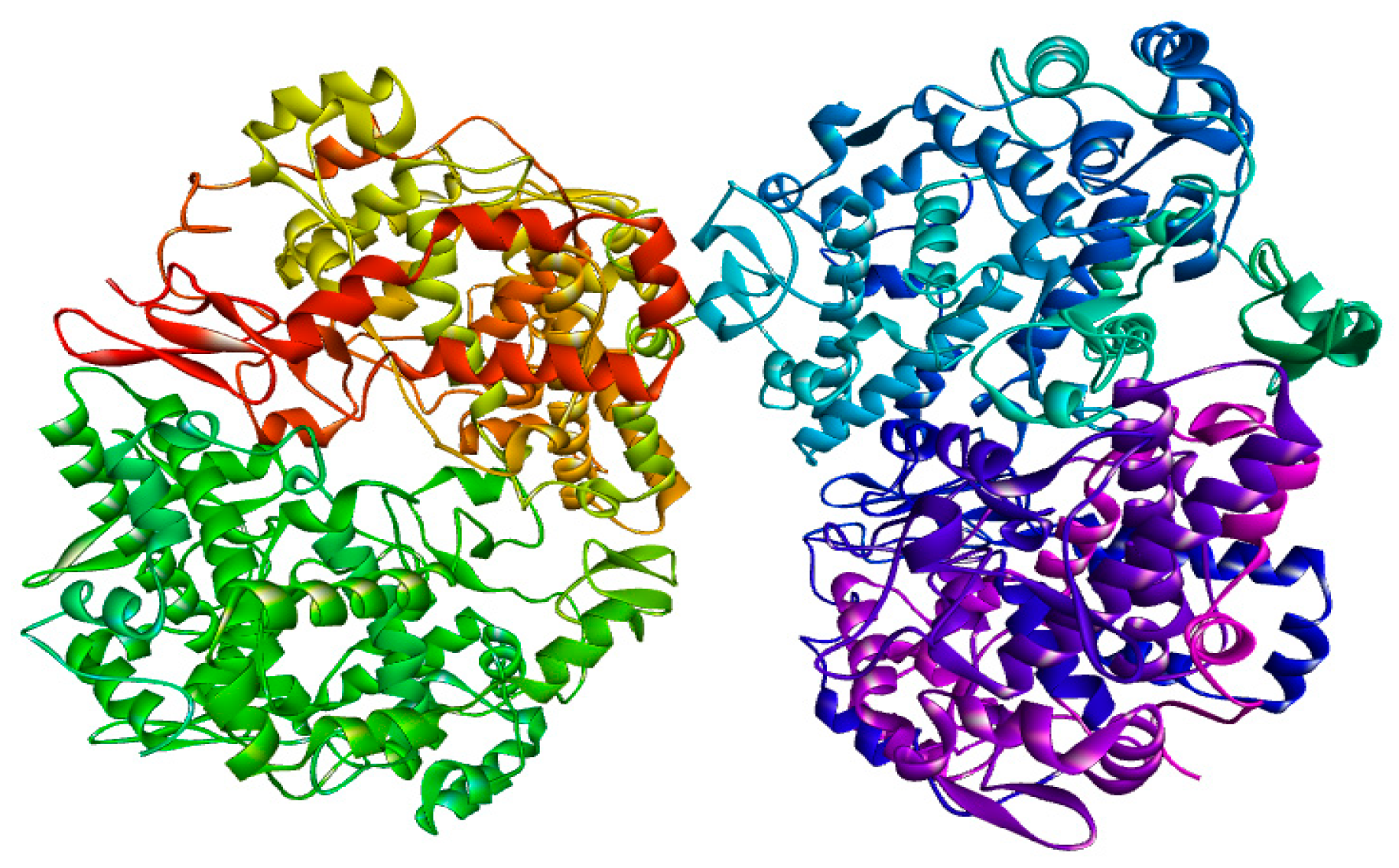
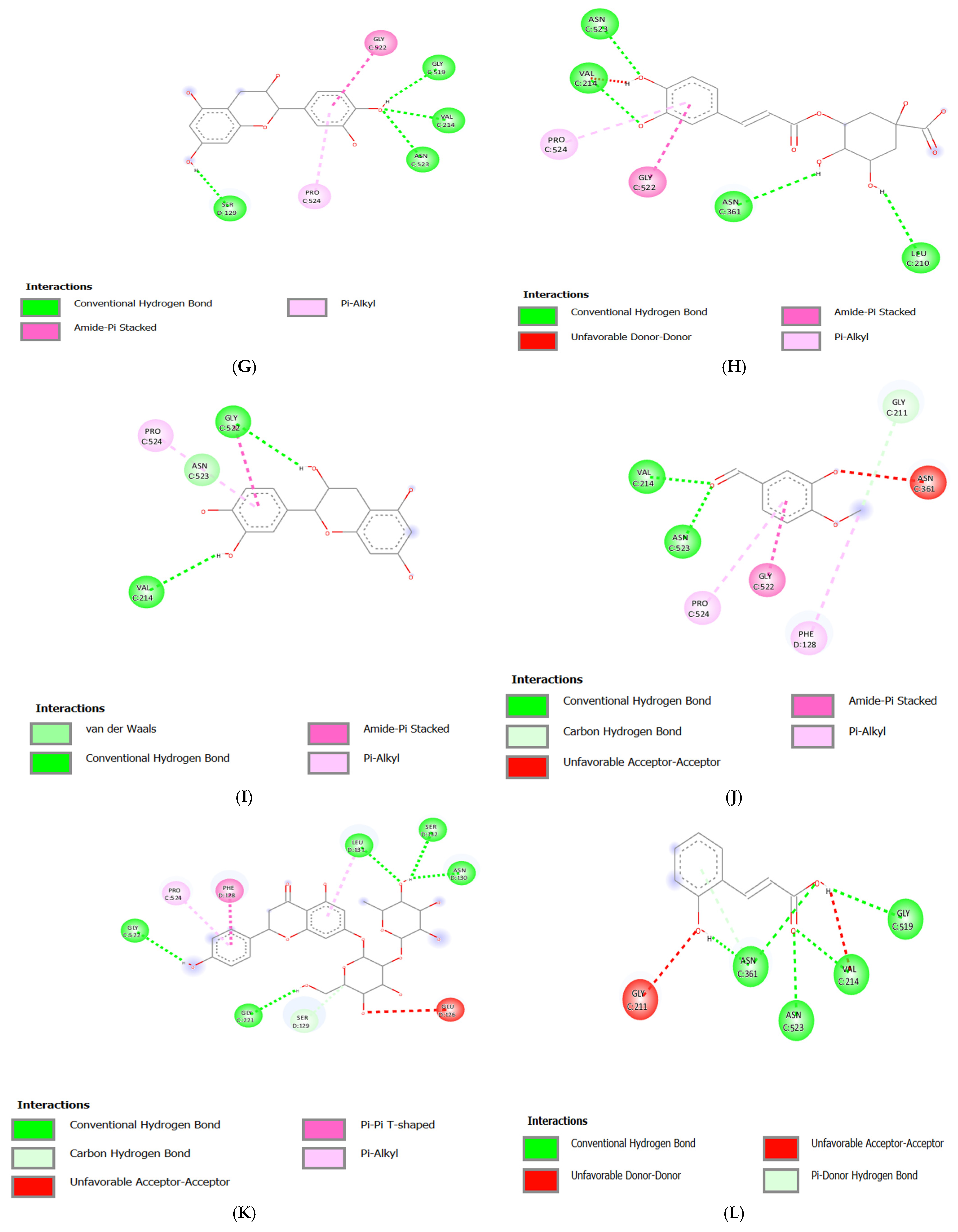
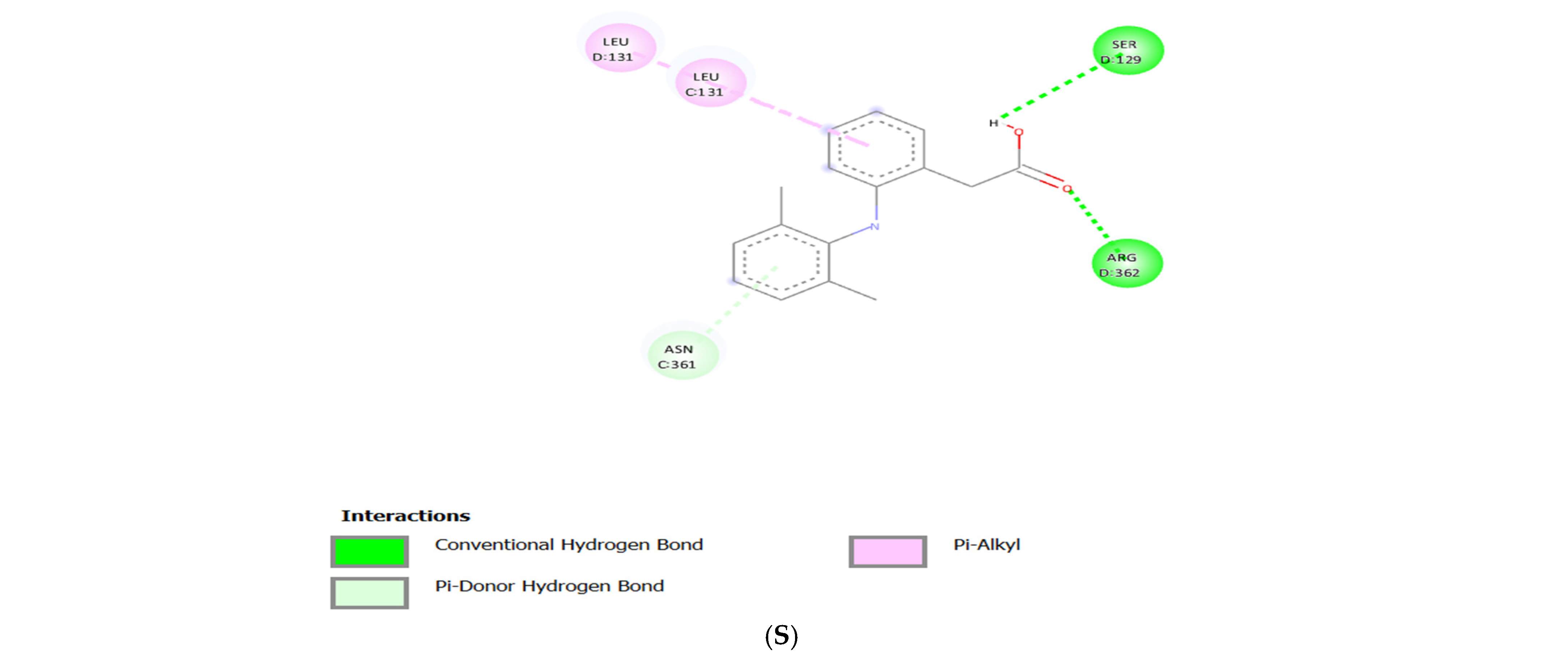
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MW | Microwave extracts |
| RBC | Red blood cell |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
References
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.-B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A Systematic Screening of Total Antioxidants in Dietary Plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef]
- Lindsay, D.G.; Astley, S.B. European research on the functional effects of dietary antioxidants—EUROFEDA. Mol. Asp. Med. 2002, 23, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Fioranelli, M.; Roccia, M.G.; Flavin, D.; Cota, L. Regulation of inflammatory reaction in health and disease. Int. J. Mol. Sci. 2021, 22, 5277. [Google Scholar] [CrossRef] [PubMed]
- Pezone, A.; Olivieri, F.; Napoli, M.V.; Procopio, A.; Avvedimento, E.V.; Gabrielli, A. Inflammation and DNA damage: Cause, effect or both. Nat. Rev. Rheumatol. 2023, 19, 200–211. [Google Scholar] [CrossRef]
- Cronquist, A. The Evolution and Classification of Fowering Plants; New York Botanical Garden: Bronx, NY, USA, 1988; p. 556. [Google Scholar]
- Bourlière, F.; Mabberley, D.J. The Plant Book. A Portable Dictionary of the Higher Plants; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Benjilali, B.; Hammoumi, M.; M’Hamedi, A.; Richard, H. Essential oil composition of different Moroccan thyme varieties. 2. Principal component analysis. Sci. Des Aliment. 1987, 7, 275–299. [Google Scholar]
- Guesmi, F.; Prasad, S.; Ben Ali, M.; Ismail, I.A.; Landoulsi, A. Thymus hirtus sp. algeriensis Boiss. and Reut. volatile oil enhances TRAIL/Apo2L induced apoptosis and inhibits colon carcinogenesis through upregulation of death receptor pathway. Aging 2021, 13, 21975–21990. [Google Scholar] [CrossRef]
- Guesmi, F.; Khantouche, L.; Mehrez, A.; Bellamine, H.; Landoulsi, A. Histopathological and biochemical effects of thyme essential oil on H2O2 stress in heart tissues. Heart Lung Circ. 2020, 29, 308–314. [Google Scholar] [CrossRef]
- Bukvicki, D.; Giweli, A.; Stojkovic, D.; Vujisic, L.; Tesevic, V.; Nikolic, M.; Sokovic, M.; Marin, P.D. Short communication: Cheese supplemented with Thymus algeriensis oil, a potential natural food preservative. J. Dairy Sci. 2018, 101, 3859–3865. [Google Scholar] [CrossRef]
- Zairi, A.; Nouir, S.; Khalifa, M.A.; Ouni, B.; Haddad, H.; Khelifa, A.; Achour, L.; Trabelsi, M. Phytochemical analysis and assessment of biological properties of essential oils obtained from Thyme and Rosmarinus species. Curr. Pharm. Biotechnol. 2020, 21, 414–424. [Google Scholar] [CrossRef]
- Fatma, G.; Sami, B.H.A.; Ahmed, L. Investigation of extracts from Tunisian ethnomedicinal plants as antioxidants, cytotoxins, and antimicrobials. Biomed. Environ. Sci. 2017, 30, 811–824. [Google Scholar]
- Ouakouak, H.; Benarfa, A.; Messaoudi, M.; Begaa, S.; Sawicka, B.; Benchikha, N.; Simal-Gandara, J. Biological properties of essential oils from Thymus algeriensis Boiss. Plants 2021, 10, 786. [Google Scholar] [CrossRef] [PubMed]
- Zouari, N.; Ayadi, I.; Fakhfakh, N.; Rebai, A.; Zouari, S. Variation of chemical composition of essential oils in wild populations of Thymus algeriensis Boiss. et Reut., a North African endemic Species. Lipids Health Dis. 2012, 11, 28. [Google Scholar] [CrossRef]
- Maissa, B.J.; Walid, H. Antifungal activity of chemically different essential oils from wild Tunisian Thymus spp. Nat. Prod. Res. 2015, 29, 869–873. [Google Scholar] [CrossRef]
- Guesmi, F.; Ben Ali, M.; Barkaoui, T.; Tahri, W.; Mejri, M.; Ben-Attia, M.; Bellamine, H.; Landoulsi, A. Effects of Thymus hirtus sp. algeriensis Boiss. et Reut. (Lamiaceae) essential oil on healing gastric ulcers according to sex. Lipids Health Dis. 2014, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Ait-Ouazzou, A.; Lorán, S.; Bakkali, M.; Laglaoui, A.; Rota, C.; Herrera, A.; Pagán, R.; Conchello, P. Chemical composition and antimicrobial activity of essential oils of Thymus algeriensis, Eucalyptus globulus and Rosmarinus officinalis from Morocco. J. Sci. Food Agric. 2011, 91, 2643–2651. [Google Scholar] [CrossRef]
- Ahmed, S.B.H.; Sghaier, R.; Guesmi, F.; Kaabi, B.; Mejri, M.; Attia, H.; Laouini, D.; Smaali, I. Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from the leishmaniasis-endemic region of Sned (Tunisia). Nat. Prod. Res. 2011, 25, 1195–1201. [Google Scholar] [CrossRef]
- Rezq, S.; Alsemeh, A.E.; D’elia, L.; El-Shazly, A.M.; Monti, D.M.; Sobeh, M.; Mahmoud, M.F. Thymus algeriensis and Thymus fontanesii exert neuroprotective effect against chronic constriction injury-induced neuropathic pain in rats. Sci. Rep. 2020, 10, 20559. [Google Scholar] [CrossRef]
- Sobeh, M.; Rezq, S.; Cheurfa, M.; Abdelfattah, M.A.; Rashied, R.M.; El-Shazly, A.M.; Yasri, A.; Wink, M.; Mahmoud, M.F. Thymus algeriensis and Thymus fontanesii: Chemical Composition, in vivo antiinflammatory, pain killing and antipyretic activities: A comprehensive comparison. Biomolecules 2020, 10, 599. [Google Scholar] [CrossRef]
- Guesmi, F.; Bellamine, H.; Landoulsi, A. H2O2-Induced Oxidative Stress, AChE inhibition and mediated brain injury attenuated by Thymus algeriensis. Appl. Physiol. Nutr. Metab. 2018, 43, 12. [Google Scholar] [CrossRef] [PubMed]
- Kouache, B.; Brada, M.; Saadi, A.; Fauconnier, M.L.; Lognay, G.; Heuskin, S. Chemical composition and acaricidal activity of Thymus algeriensis essential oil against Varroa destructor. Nat. Prod. Commun. 2017, 12, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Guesmi, F.; Tyagi, A.K.; Bellamine, H.; Landoulsi, A. Antioxidant machinery related to decreased MDA generation by Thymus algeriensis essential oil-induced liver and kidney regeneration. Biomed. Environ. Sci. 2016, 29, 639–649. [Google Scholar]
- Guesmi, F.; Beghalem, H.; Tyagi, A.K.; Ben Ali, M.; Ben Mouhoub, R.; Bellamine, H.; Landoulsi, A. Prevention of H2O2 induced oxidative damages of rat testis by Thymus algeriensis. Biomed. Environ. Sci. 2016, 29, 275–285. [Google Scholar] [CrossRef]
- Fatma, G.; Mouna, B.F.; Mondher, M.; Ahmed, L. In-vitro assessment of antioxidant and antimicrobial activities of methanol extracts and essential oil of Thymus hirtus sp. algeriensis. Lipids Health Dis. 2014, 13, 114. [Google Scholar] [CrossRef]
- Jayari, A.; Donsì, F.; Ferrari, G.; Maaroufi, A. Nanoencapsulation of thyme essential oils: Formulation, characterization, storage stability, and biological activity. Foods 2022, 11, 1858. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Trace element quantification in two algerian thymes (Thymus algeriensis Boiss & Reut. and Thymus capitatus (L.) Hoffm. & Link) using EDXRF spectrometry. Biol. Trace Element Res. 2022, 201, 455–463. [Google Scholar] [CrossRef]
- El Ouahdani, K.; Es-Safi, I.; Mechchate, H.; Al-Zahrani, M.; Qurtam, A.A.; Aleissa, M.; Bari, A.; Bousta, D. Thymus algeriensis and Artemisia herba-alba essential oils: Chemical analysis, antioxidant potential and in vivo anti-inflammatory, analgesic activities, and acute toxicity. Molecules 2021, 26, 6780. [Google Scholar] [CrossRef]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Guido, F.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S.K. Chemical composition and bioactivity of essential oils and Ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. & Reut. from the Algerian Saharan Atlas. BMC Complement. Altern. Med. 2019, 19, 146. [Google Scholar] [CrossRef]
- Mahdi, I.; Ben Bakrim, W.; Bitchagno, G.T.M.; Annaz, H.; Mahmoud, M.F.; Sobeh, M. Unraveling the phytochemistry, traditional uses, and biological and pharmacological activities of Thymus algeriensis Boiss. & Reut. Oxidative Med. Cell. Longev. 2022, 2022, 6487430. [Google Scholar] [CrossRef]
- Bouafia, M.; Amamou, F.; Gherib, M.; Benaissa, M.; Azzi, R.; Nemmiche, S. Ethnobotanical and ethnomedicinal analysis of wild medicinal plants traditionally used in Naâma, southwest Algeria. Vegetos 2021, 34, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Righi, N.; Boumerfeg, S.; Fernandes, P.A.; Deghima, A.; Baali, F.; Coelho, E.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Thymus algeriensis Bioss & Reut: Relationship of phenolic compounds composition with in vitro/in vivo antioxidant and antibacterial activity. Food Res. Int. 2020, 136, 109500. [Google Scholar] [CrossRef] [PubMed]
- Jaouadi, R.; Silva, A.M.S.; Boussaid, M.; Yahia, I.B.H.; Cardoso, S.M.; Zaouali, Y. Differentiation of phenolic composition among Tunisian thymus algeriensis boiss. Et reut.(lamiaceae) populations: Correlation to bioactive activities. Antioxidants 2019, 8, 515. [Google Scholar] [CrossRef]
- Ziani, B.E.; Heleno, S.A.; Bachari, K.; Dias, M.I.; Alves, M.J.; Barros, L.; Ferreira, I.C.F.R. Phenolic compounds characterization by LC-DAD-ESI/MSn and bioactive properties of Thymus algeriensis Boiss. & Reut. and Ephedra alata Decne. Food Res. Int. 2019, 116, 312–319. [Google Scholar]
- Zairi, A.; Nouir, S.; M`hamdi, N.; Bennani, M.; Bergaoui, I.; Mtiraoui, A.; Chaouachi, M.; Trabelsi, M. Antioxidant, antimicrobial and the phenolic content of infusion, decoction and methanolic extracts of Thyme and Rosmarinus species. Curr. Pharm. Biotechnol. 2018, 19, 590–599. [Google Scholar] [CrossRef]
- Boutaoui, N.; Zaiter, L.; Benayache, F.; Benayache, S.; Carradori, S.; Cesa, S.; Giusti, A.M.; Campestre, C.; Menghini, L.; Innosa, D.; et al. Qualitative and quantitative phytochemical analysis of different extracts from Thymus algeriensis aerial parts. Molecules 2018, 23, 463. [Google Scholar] [CrossRef]
- Herowati, R.; Widodo, G.P. Molecular Docking Analysis: Interaction Studies of Natural Compounds to Anti-Inflammatory Targets Quantitative Structure-Activity Relationship; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Thamaraiselvi, L.; Selvankumar, T.; Wesely, E.; Kumar, N.V. In-silico molecular docking analysis of some plant derived molecules for anti-inflammatory inhibitory activity. Curr. Bot. 2021, 12, 22–27. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, X.; Li, J.; Hu, Y.; Liu, J.; Wang, F.; Zhang, W.; Chang, F. Mechanism of anti-inflammatory and antibacterial effects of QingXiaoWuWei decoction based on network pharmacology, molecular docking and in vitro experiments. Front. Pharmacol. 2021, 12, 678685. [Google Scholar] [CrossRef]
- Laloo, D.; Kalita, J.M.; Prasad, S.K. Molecular docking studies of plant-derived bioactive compounds: A comprehensive in silico standardization approach. In Evidence Based Validation of Traditional Medicines: A Comprehensive Approach; Springer: Singapore, 2021; pp. 371–404. [Google Scholar]
- Brown, J.H.; Mackey, H.K.; Riggilo, D.A. A novel in vitro assay for anti-inflammatory agents based on stabilization of erythrocytes. Exp. Biol. Med. 1967, 125, 837–843. [Google Scholar] [CrossRef]
- Trovato, A.; Raneri, E.; Kouladis, M.; Tzakou, O.; Taviano, M.F.; Galati, E.M. Anti-inflammatory and analgesic activity of Hypericum empetrifolium Willd. (Guttiferae). Il Farm. 2001, 56, 455–457. [Google Scholar] [CrossRef]
- Wang, J.L.; Limburg, D.; Graneto, M.J.; Springer, J.; Hamper, J.R.B.; Liao, S.; Pawlitz, J.L.; Kurumbail, R.G.; Maziasz, T.; Talley, J.J.; et al. The novel benzopyran class of selective cyclooxygenase-2 inhibitors. Part 2: The second clinical candidate having a shorter and favorable human half-life. Bioorganic Med. Chem. Lett. 2010, 20, 7159–7163. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-T. The Antiinflammatory Effect of an Extract of Tripterygium wilfordii Hook F on Adjuvant-induced Paw Oedema in Rats and Inflammatory Mediators Release. Phytotherapy Res. 1997, 11, 152–154. [Google Scholar] [CrossRef]
- Murugesh, N.; Vembar, S.; Damodaran, C. Studies on erythrocyte membrane IV: In vitro haemolytic activity of oleander extract. Toxicol. Lett. 1981, 8, 33–38. [Google Scholar] [CrossRef]
- David, S. Studies force new view on biology of flavonoids. Biol. Med. 2007, 541, 737–787. [Google Scholar]
- González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S.; Tuñón, M.J. Fruit polyphenols, immunity and inflammation. Br. J. Nutr. 2010, 104, S15–S27. [Google Scholar] [CrossRef]
- Rosa, M.D.; Willoughby, D. Screens for anti-inflammatory drugs. J. Pharm. Pharmacol. 1971, 23, 297–298. [Google Scholar] [CrossRef]
- Salvemini, D.; Wang, Z.-Q.; Bourdon, D.M.; Stern, M.K.; Currie, M.G.; Manning, P.T. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur. J. Pharmacol. 1996, 303, 217–220. [Google Scholar] [CrossRef]
- Rayego-Mateos, S.; Morgado-Pascual, J.L.; Opazo-Ríos, L.; Guerrero-Hue, M.; García-Caballero, C.; Vázquez-Carballo, C.; Mas, S.; Sanz, A.B.; Herencia, C.; Mezzano, S.; et al. Pathogenic pathways and therapeutic approaches targeting inflammation in diabetic nephropathy. Int. J. Mol. Sci. 2020, 21, 3798. [Google Scholar] [CrossRef]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol. 2003, 225, 115–121. [Google Scholar] [CrossRef]
- Tai, F.W.D.; McAlindon, M.E. Non-steroidal anti-inflammatory drugs and the gastrointestinal tract. Clin. Med. 2021, 21, 131–134. [Google Scholar] [CrossRef]
- Srivastava, S.; Singh, P.; Jha, K.K.; Mishra, G.; Srivastava, S.; Khosa, R.L. Antiinflammatory, analgesic and antipyretic activities of aerial parts of Costus speciosus Koen. Indian J. Pharm. Sci. 2013, 75, 83. [Google Scholar]
- Silva, G.N.; Martins, F.R.; Matheus, M.E.; Leitão, S.G.; Fernandes, P.D. Investigation of anti-inflammatory and antinociceptive activities of Lantana trifolia. J. Ethnopharmacol. 2005, 100, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S. Molecular docking: A structure-based approach for drug repurposing. In In Silico Drug Design; Elsevier: Amsterdam, The Netherlands, 2019; pp. 161–189. [Google Scholar]
- Cheng, Y.-C.; Prusoff, W.H. Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, S.D.; Zaric, S.D. Hydrogen bonds and hydrophobic interactions of porphyrins in porphyrin-containing proteins. Open Struct. Biol. J. 2009, 3, 34–41. [Google Scholar] [CrossRef]
- Omoboyowa, D.A.; Balogun, T.A.; Omomule, O.M.; Saibu, O.A. Identification of terpenoids from Abrus precatorius against parkinson’s disease proteins using in silico approach. Bioinform. Biol. Insights 2021, 15, 11779322211050757. [Google Scholar] [CrossRef]
- Mohapatra, S.; Prasad, A.; Haque, F.; Ray, S.; De, B.; Ray, S.S. In silico investigation of black tea components on α-amylase, α-glucosidase and lipase. J. Appl. Pharm. Sci. 2015, 5, 42–47. [Google Scholar] [CrossRef]
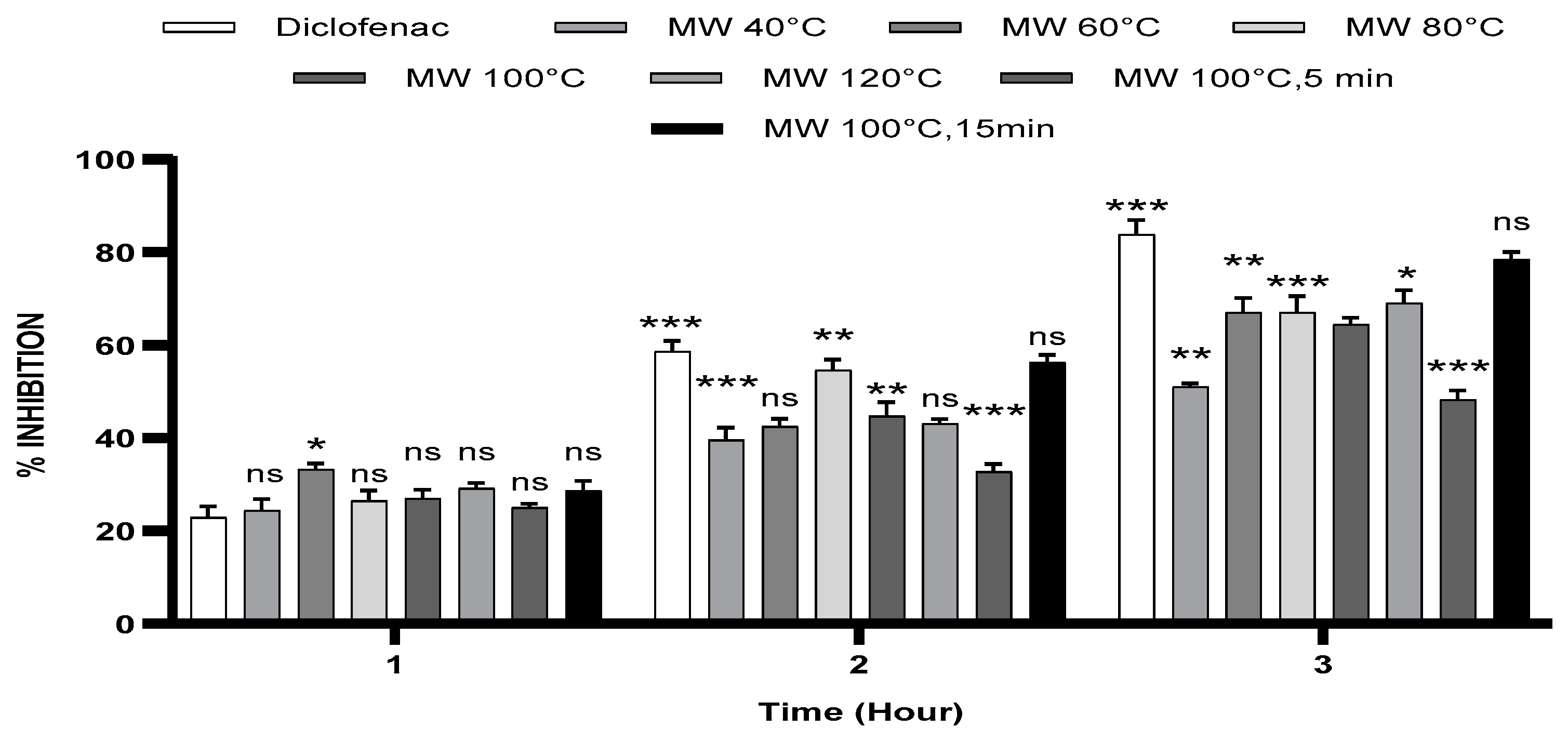



| Percentage Inhibition (%) | |||
|---|---|---|---|
| 250 µg/mL | 500 µg/mL | 1000 µg/mL | |
| MW 40 °C | 77.18 ± 0.17 *** | 84.92 ± 0.30 *** | 89.28 ± 0.20 *** |
| MW 60 °C | 78.59 ± 0.18 *** | 87.43 ± 0.22 *** | 90.67 ± 0.10 *** |
| MW 80 °C | 80.53 ± 0.20 *** | 88.12 ± 0.13 *** | 91.97 ± 0.15 *** |
| MW 100 °C | 77.06 ± 3.04 *** | 88.99 ± 0.31 *** | 94.94 ± 0.25 *** |
| MW 120 °C | 86.25 ± 0.12 ns | 91.13 ± 0.25 ns | 96.88 ± 0.15 ns |
| MW 100 °C, 5 min | 76.92 ± 0.20 *** | 85.09 ± 0.22 *** | 88.64 ± 0.18 *** |
| MW 100 °C, 15 min | 88.96± 0.17 ns | 90.95 ± 0.15 ns | 96.85 ± 0.17 ns |
| Diclofenac | 89.91 ± 0.17 | 91.91 ± 0.2 | 96.67 ± 0.15 |
| Mean Increase in Paw Thickness (mm) ± SD | |||
|---|---|---|---|
| Group | 1 h | 2 h | 3 h |
| Control | 1.92 ± 0.04 | 1.74 ± 0.09 | 1.49 ± 0.07 |
| Diclofenac | 1.48 ± 0.04 *** | 0.72 ± 0.04 *** | 0.24 ± 0.04 *** |
| MW 40 °C | 1.45 ± 0.04 *** | 1.05 ± 0.04 *** | 0.73 ± 0.01 *** |
| MW 60 °C | 1.28 ± 0.02 *** | 1 ± 0.02 *** | 0.49 ± 0.04 *** |
| MW 80 °C | 1.41 ± 0.04 *** | 0.79 ± 0.04 *** | 0.49 ± 0.05 *** |
| MW 100 °C | 1.4 ± 0.03 *** | 0.96 ± 0.05 *** | 0.53 ± 0.02 *** |
| MW 120 °C | 1.36 ± 0.02 *** | 0.99 ± 0.01 *** | 0.46 ± 0.04 *** |
| MW 100 °C 5 min | 1.44 ± 0.01 *** | 1.17 ± 0.02 *** | 0.77 ± 0.02 *** |
| MW 100 °C 15 min | 1.37 ± 0.04 *** | 0.76 ± 0.02 *** | 0.32 ± 0.02 *** |
| Biomolecules | Anti-Inflammatory Activity | |
|---|---|---|
| Protein: COX-2 (3LN0) | ||
| ΔG (Kcal/mol) | Ki (µmol) | |
| 2,3-dimethoxybenzoic acid | −6.0 | 39.95 |
| 3-hydroxybenzoic acid | −6.1 | 33.74 |
| 4-hydroxybenzoic acid | −5.7 | 66.28 |
| syringic acid | −5.9 | 47.29 |
| gallic acid | −6.4 | 20.33 |
| benzoic acid | −5.5 | 92.90 |
| catechin | −9.2 | 0.18 |
| chlorogenic acid | −8.5 | 0.58 |
| epicatechin | −8.9 | 0.29 |
| isovanillin | −5.9 | 47.29 |
| naringin | −10.3 | 0.028 |
| o-coumaric acid | −6.3 | 24.07 |
| p-coumaric acid | −6.1 | 33.74 |
| quercetin | −7.9 | 1.61 |
| rutin | −8.8 | 0.35 |
| sinapinic acid | −5.8 | 55.99 |
| t-ferulic acid | −6.1 | 33.74 |
| vanillic acid | −6.2 | 28.50 |
| dichlofenac | −7.1 | 6.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutaoui, N.; Acila, M.; Lariche, N.; Lemoui, R.; Khellafi, A.; Campestre, C.; Melfi, F.; Locatelli, M. Evaluation of the Anti-Inflammatory Activity of Microwave Extracts of Thymus algeriensis: In Vitro, In Vivo, and In Silico Studies. Analytica 2025, 6, 16. https://doi.org/10.3390/analytica6020016
Boutaoui N, Acila M, Lariche N, Lemoui R, Khellafi A, Campestre C, Melfi F, Locatelli M. Evaluation of the Anti-Inflammatory Activity of Microwave Extracts of Thymus algeriensis: In Vitro, In Vivo, and In Silico Studies. Analytica. 2025; 6(2):16. https://doi.org/10.3390/analytica6020016
Chicago/Turabian StyleBoutaoui, Nassima, Meryem Acila, Nesrine Lariche, Redouane Lemoui, Asma Khellafi, Cristina Campestre, Francesco Melfi, and Marcello Locatelli. 2025. "Evaluation of the Anti-Inflammatory Activity of Microwave Extracts of Thymus algeriensis: In Vitro, In Vivo, and In Silico Studies" Analytica 6, no. 2: 16. https://doi.org/10.3390/analytica6020016
APA StyleBoutaoui, N., Acila, M., Lariche, N., Lemoui, R., Khellafi, A., Campestre, C., Melfi, F., & Locatelli, M. (2025). Evaluation of the Anti-Inflammatory Activity of Microwave Extracts of Thymus algeriensis: In Vitro, In Vivo, and In Silico Studies. Analytica, 6(2), 16. https://doi.org/10.3390/analytica6020016









