1. The Pancreas, the “Internal Secretions”, and the Discovery of Insulin
In the pre-1900s, what we now call type 1 or autoimmune diabetes mellitus was almost universally fatal. Most presented in childhood or adolescence with classic symptoms of diabetes became cachectic and emaciated from significant glycosuria as well as catabolic changes causing muscle wasting, weakness, and loss of body fat, severe weight loss reflecting acute insulin deficiency, and often, there was diabetic ketoacidosis, decompensation, coma, and death. Diabetes was recognized as the “wasting” disease or the “honey wasting” disease, and hence, its Latin and Greek nomenclature, diabetes mellitus. In financially distressed parts of the world where medical care is minimally available or just too expensive or too far away, not much has changed about diabetes mellitus for children and adolescents until programs such as Changing Diabetes in Children and Life for a Child as well as Insulin for Life offered hope and insulin—sometimes also offering trained diabetes staff, glucose meters, test strips, and lancets—in recent years [
1]. Instead of dying at rates exceeding 95%, such children now are living relatively normal lives with their families and friends, attending schools, and thriving.
It took a very long time, however, for medical specialists to determine that it was the pancreas that was the site of the problem [
2]. Oskar Minkowski, working with Joseph Von Mering in 1889 in Strasbourg, identified that the pancreas was the likely source of the problem after pancreatectomized dogs in experiments concerning digestive effects of the pancreas became diabetic and died in their laboratories. Prior to that, Paul Langerhans, a medical student working with Rudolph Virchow in Berlin, had identified the islands of pancreatic tissue that seemed different from the rest of the pancreas, but there was no identification of these “islands” as having anything to do with diabetes until many years later when Gustave-Edourt Laguesse later named them the “islets of Langerhans” and there appeared suspicions, without proof, that there was some type of pancreatic secretion involved with diabetes. In fact, TA Cawley, in 1788, was the first to propose in writing that the pancreas was the culprit anatomically involved with diabetes. In 1845, Boucherdat suspected pancreatic “juices”, and in 1883, Claudio Ulesko in Russia wrote about “internal pancreas secretions”, but none had been identified.
Michael Bliss, in his amazing historical research (
Figure 1) published his book, “The Discovery of Insulin” [
3], suggests about 400 citations in the medical literature that he has uncovered talking about the forerunner of insulin and the pancreatic secretions with various degrees of scientific guess-work, research, lectures, and written work to back up these proponents before the awarding of the Nobel Prize in Medicine to Macleod and Banting in 1923, 18 months after the first successful treatment of a human for more than a few hours with what they called insulin. Fourteen-year-old Leonard Thompson was dying at Toronto General Hospital of diabetes, cachectic but not in acute diabetic ketoacidosis or coma when he was brought “back to life” with first relatively unpure beef insulin extract and then with a more purified ethanol extract following the success of Collip, Banting, Best, and Macleod experiments with dogs the previous 6 months. However, the search for the elusive “extract” from the pancreas did not begin in Toronto or Bucharest but perhaps more than 50 years before the work that led to manufacturing success and production of insulin in Canada. For instance, in 1900, George Zuelzer in Berlin patented pancreatic extraction procedures after treating several dogs and then five humans, but complications with fever and excessive hypoglycemia, as well as his described inability to remove the “impurities” from his preparations, caused him to cease his research. Before doing so, he enlisted the resources of several large German pharmaceutical companies, notably Hoechst, Schering, and Hoffmann-La Roche, and the work was discontinued. In 1905, Eugen Gley presented a paper at the French Society of Biology in which he described extracts of degenerated pancreas given to pancreatectomized dogs, in whom decreased urinary sugar and alleviation of diabetes symptoms were noted. Once again, follow-up research was discontinued for lack of support. De Meyer in Belgium in 1906 and then, independently and without apparent knowledge of de Meyer’s proposal, Schafer in 1916 proposed “insuline” as the name for the internal pancreatic secretions still remaining elusive but felt to be the source of the problem with diabetes. Ernest Scott wrote his master’s thesis that was published in 1912 [
4], and in 1919, Israel Kleiner, working at the Rockefeller Institute in New York City, published his work on pancreas extracts abler to decrease both blood sugar and urinary sugar readings [
5].
In the late 1800s and the early 1900s, treatment of diabetes was very primitive by today’s standards. Understanding was limited, urinary glucose measurements were tedious and not always available, and blood glucose measurements were imprecise, plus required large amounts of blood to be processed in specialized research facilities. JR Macleod, a Scottish physician who had moved to Toronto, Canada, to lead the Department of Physiology at the Medical School, was one of the world’s experts on carbohydrate metabolism and had helped create such a way for measuring blood glucose in relatively small quantities of blood. Clinical treatment, however, was promoted by many, such as renowned American physicians Elliot Joslin in Boston and Frederick Allen in Morristown, New Jersey, to include strict avoidance of sugars and carbohydrates with a diet loaded with fat to attempt to provide calories to youngsters starving from the caloric losses of high volumes of glycosuria and accompanied by protein and fat catabolism, ketosis, and general cachexia. For those who did not succumb to diabetic ketoacidosis and its attendant metabolic decompensation, coma, and death, life could sometimes be prolonged for several months with such a starvation regimen.
During this same time period, a Romanian physician and physiologist Nicolae Paulescu (
Figure 2) worked and studied in France and rose to become Professor of Medicine in Bucharest. In the late 1890s, he conducted studies on diabetes that he continued off and on with resumed efforts around 1916 and thereafter. He became convinced that the diabetes factor from the pancreas, which he called either “pancreine” or “pancreatine”, could be produced as an extract, but his experiments were inconclusive and not always reproducible [
6]. He had some success and read a paper at an international meeting in France, which was published in 1921 [
7] with follow-up research that same year [
8], but the events in Toronto coupled with the success of Collip, Connaught, and eventually, Eli Lilly and Company overshadowed his work until years later.
At about the same time, and presumably without a good deal of preparation and reading of the available medical literature (but perhaps having heard Paulescu’s paper in France), an unemployed young Canadian surgeon Frederick Grant Banting (
Figure 3) had an idea about ligating the ducts of the pancreas in dogs so that he could either then transplant the remaining pancreatic tissue back to the dogs to “cure” their surgically induced diabetes or, alternatively, if that did not work, he could figure out a way to produce the elusive pancreatic secretions from the remnant pancreases and then give this extract to reverse diabetes [
3]. He went to Macleod to present his idea, and Macleod generously provided free laboratory space for the summer of 1921 to Banting, a medical student, Charles Best, to serve as his laboratory assistant and several dogs for the experiments. Macleod presumably also made available his methodology for measuring blood glucose from relatively small quantities of blood to complement the known measurements of glycosuria and ketonuria so that the biochemical effects of these experiments could be undertaken. Macleod then went on vacation back to Scotland, while Banting and Best began their work otherwise unsupervised. Attempts at pancreatectomy killed several of their dogs, and those that did not die from the transplant or the surgery/anesthesia probably died from the second surgical attempt at transplantation of residual pancreatic tissue post-pancreatic duct ligation. While there are different descriptions of these events from the four main participants from 1921 to 1922, Banting, Best, [
9] Macleod [
10], and J.B. Collip [
11], something along those lines took place, and the surgical approach with ligation of the pancreatic ducts was abandoned in favor of attempting to produce a pancreatic extract with the internal secretions. These were first recorded as being called “isletin” and then only later changed to insulin.
With some success in bringing the blood glucose, urine glucose, and urinary ketone levels down in some of their dog, and after some trial and error with various methods of extracts, attempts at purification to remove fever-producing infections in their animals, abscess formation, and death, J.B. Collip was brought in as a Visiting Professor of Pharmacology to assist with the extraction procedures and their success with the dogs was presented 14 November 1921 at the University of Toronto Physiological Journal Club and in a paper published in February 1922 [
12].
Eventually, high-dose ethanol was used as an extraction processor with some success. Fourteen-year-old Leonard Thompson, a patient at Toronto General Hospital, was near death; the team prepared the pancreatic extracts and injected Leonard Thompson on 21 January 1922—a point in time when his blood glucose was approximately 580 mg/dL (approximately 32 mmol), and his glucose levels dropped about 100 mg/dL (approximately 5.5 mmol) (
Figure 4 and
Figure 5). Soon afterward, a sterile abscess occurred, and the extract was halted. Sugar levels rose, and several days later, a higher potency pancreas extract was prepared so that on 23–25 January 1922, Leonard Thompson’s glucose levels normalized, his glycosuria decreased significantly, and his ketonuria cleared. This was well documented in a paper presented by Macleod on 3 May 1922 in Washington DC and published that same year [
13].
Patents were submitted, and Connaught Laboratories in Toronto was authorized to begin production of what would later be called “Toronto insulin” or regular insulin elsewhere in the world. Production problems and removal of impurities continued to be problematic, and Eli Lilly, an American pharmaceutical company in Indianapolis, became involved in the problem, solving it successfully in 1922 so that insulin in sufficient volume to treat not only the patient in Toronto but also elsewhere became possible. Lilly officials agreed to pay royalties to the University of Toronto to support research in exchange for manufacturing rights for North and South America, while Connaught retained manufacturing rights for Canada. Lilly’s insulin was named Iletin, and beef, as well as pig pancreas, was collected for processing.
In 1922, August Krogh (
Figure 6), a Danish physician and physiologist, having heard about their discoveries, visited Macleod and Banting. With a physician wife who also had diabetes, he returned home to Copenhavn with manufacturing rights to produce insulin at the Nordisk Laboratories, working closely with his wife, Marie Krogh, and another physician pharmacologist, Hans Christian (HC) Hagedorn (
Figure 7). The Medical Research Council in the UK (eventually Wellcome) obtained rights for British manufacturing, as did Hoechst in Germany. In 1925, Novo Laboratories, started by two former employees of Krogh and Hagedorn, also began producing insulin in direct competition with Nordisk; Nordisk and Novo remained in highly competitive mode through the next several decades [
14] until finally merging and ending “hostilities” as Novo Nordisk Laboratories in 1989.
Stories of the miracle of insulin were published in the Toronto newspapers and in other newspapers around the world. Physicians from around the world heard of these successes, and patients came to Toronto to receive insulin until it became more widely available throughout Canada, the USA, and Western Europe. Within 18 months of this discovery, an incredibly short time, Banting and Macleod were awarded the Nobel Prize in Medicine and Physiology in 1923 principally for bringing the extraction work to fruition with sufficient production to allow numerous pharmaceutical companies around the world as well as the Connaught Laboratories in Toronto to make insulin available for the treatment of humans on a larger scale than ever before. Adding to an already tumultuous partnership among the four principles, Banting immediately sent a telegram to Dr. Elliot Joslin, then hosting Charles Best in a meeting in Boston, that Banting would share his portion of the Nobel Prize with Best. Macleod responded in kind and announced that he would share his portion of the prize with Collip. It would take many years before the animosities of the four Canadian investigators would be resolved, and now, some 90+ years after this Nobel Prize was awarded, there still remain many who would have favored other insulin investigators for the pioneering work that they did to make it all happen, such as Zuelzer, Gley, Scott, Kleiner, Paulescu, etc.
Figure 8 shows now world-famous photographs of before and after pictures of several patients representing the face of pediatric diabetes and the miracle of insulin in bringing them back to life as photographed by Dr. Ralph Major in Kansas City and Dr. H. Rawle Geyelin in New York City in
Figure 9.
In the years that followed, further improvements in removing impurities made the multiple insulin injections easier to tolerate for patients. Research competition between Nordisk and Novo as well as Lilly and others helped develop protamine zinc long-acting insulin (PZI) in or around 1936 and then what became known as NPH, neutral protamine Hagedorn as an intermediate-acting insulin in or around 1946. PZI and NPH were used in addition to regular insulin based on the patient’s urine glucose readings. In 1954, different formulations of zinc were used to change the time course of insulin into an alternative intermediate-acting insulin called lente insulin, combining proportions of a shorter acting semilente insulin with the longer-acting ultralente insulin (
Figure 10 and
Figure 11). In or around 1973, more purified preparations, especially those purely extracted from pig pancreas, became available to help with those allergic still to the impurities of insulin.
In the 1970s, scientific progress helped researchers create semi-synthetic insulin starting from animal-source insulin and modifying some of the amino acids in the insulin molecule itself for the first “human” insulins available. In the 1980s, bioengineering had harnessed the knowledge of genetics using modified E.coli or yeast cells to become “factories” to produce insulin, and thus bioengineered human insulin became available from the major manufacturers: insulin analogs (
Figure 12). With each progressive step, impurities were reduced, and some of the allergic problems, as well as most—but not all—of the lipoatrophy and lipohypertrophy, also believed to be related to such impurities, diminished.
In 1985, insulin pens (
Figure 13) became available, first in Denmark, then in the rest of Europe, and afterward in America and around the world. Such pens used smaller needles, were therefore less painful than syringes, easier to use, and easier to carry around as multiple injection therapy was becoming more popular and attempts at improving overall glycemic control in conjunction with self-monitoring of blood glucose became more available.
During these years, research studies such as the KROC study pointed to the long-term complications of diabetes as likely being directly related to short- and long-term glycemic control so that efforts were directed more at lowering glycemic targets safely. Insulin pumps were introduced but were initially cumbersome and very expensive. The multicentered Diabetes Control and Complications Trial (DCCT) (
Figure 14) began in 1982, recruited its first patients in 1983, and reported its first final formal study results—a year earlier than originally anticipated because the results were so dramatically conclusive—at the American Diabetes Association annual scientific meetings in Las Vegas in 1993 to an enormous audience [
15]. The small cohort of adolescents recruited for the DCCT was more difficult to manage, as expected, than the large adult study cohort but showed the same patterns of metabolic memory as did the adult cohort [
16,
17]. DCCT follow-up studies, EDIC, continue to highlight the importance of “glucose memory” and its role in all these known long-term complications associated with type 1 diabetes even 20–30 years after initiation of improved glycemia as part of the original DCCT study cohort [
18].
2. Insulin Analogs: Fast-Acting Lispro, Aspart, and Glulisine
During the 1980s and 1990s, insulin analogs became available as molecular scientists deciphered the actual alpha and beta chain molecular structure of insulin and its connecting peptide from the proinsulin molecule. Thereafter, bioengineering maneuvers allowed pharmacologists to create the genetic sequencing to produce human insulin by inserting these newly identified sequences into genetically modified bacteria or yeast. Rapid-acting insulin analogs began to replace bioengineered human regular insulin to provide faster initial absorption subcutaneously, more physiologic postprandial glycemic coverage, shorter overall duration of insulin effect, and improved flexibility of treatment at the expense of more daily injections of prandial insulin. The potential for reducing oxidative stress with reductions in postprandial glycemic excursions has also been documented with all three such analogs [
19]. Lilly produced the first available such rapid-acting insulin analog, lispro, (Humalog
®) (
Figure 15) in 1996 [
20], followed thereafter by aspart insulin (Novolog
® or Novorapid
®) by Novo Nordisk in 1999 and glulisine (Apidra
®) by Sanofi-Aventis. All three were available in pen or syringe and vial format coupled with improvements in pen mechanics and use.
Lispro insulin (see
Figure 16,
Figure 17 and
Figure 18) is biosynthetic human insulin produced by modified E.coli with lysine and proline amino acids 28 and 29 switched in their positions on the beta chain of the insulin molecule. With this modification, the regular insulin was less likely to form hexamers (see
Figure 17) as its three-dimensional configuration was altered and thus absorption characteristics were changed, and speedier uptake occurred as there were more dimers and monomers being picked up in the subcutaneous space [
21]. A shorter total duration of activity was also noticed so that there was significantly less nocturnal and late-onset hypoglycemia in the following 3–12 h time periods [
22]. Because of the earlier fast-acting insulin uptake, better postprandial glycemia was noted [
23], and patients reported 60% improved ease of use, 58% improved meal timing and preparation, and 30% improvement in daily activities [
24], and significant treatment satisfaction [
25].
Prepubertal lispro studies in which we participated [
26] using home blood glucose monitoring profiles during routine activities in comparison with regular insulin coupled with then available basal NPH dosage showed that pre- and post-meal lispro insulin could be utilized with good therapeutic results, although pre-meal dosage was slightly better than post-meal dosage and both lispro dose strategies were equal or better than regular insulin for prandial dosing so that the proof-of-concept for this new form of insulin was verified per protocol intent.
In the peripubertal period as well as in the pubertal and post-pubertal age groups, the same results were published in multidose insulin protocols with an emphasis in many such studies on the decrease in nocturnal hypoglycemia (
Figure S1), presumably reflecting the shorter “tail-effect” of the faster analog compared to regular insulin [
27]. Life-table analysis in the same studies confirmed this relative decrease of overnight hypoglycemia in the adolescents participating (
Figure S2). In a separate study published the same year where twice-a-day basal NPH was provided in addition to prandial lispro insulin and efforts were made to optimize the NPH dosage according to blood glucose self-report, comparisons of glycosylated hemoglobin results and frequency of even mild hypoglycemia showed improvement (
Figure S3). This was particularly significant since the DCCT studies showed the importance of such lowering of GHb results but at the expense of more frequent hypoglycemia; the lispro studies showed that the overall glycemic exposure (i.e., GHb) could be lowered in a safer fashion with the rapid-acting analogs as prandial performers [
28], and this was true at all GHb levels achieved.
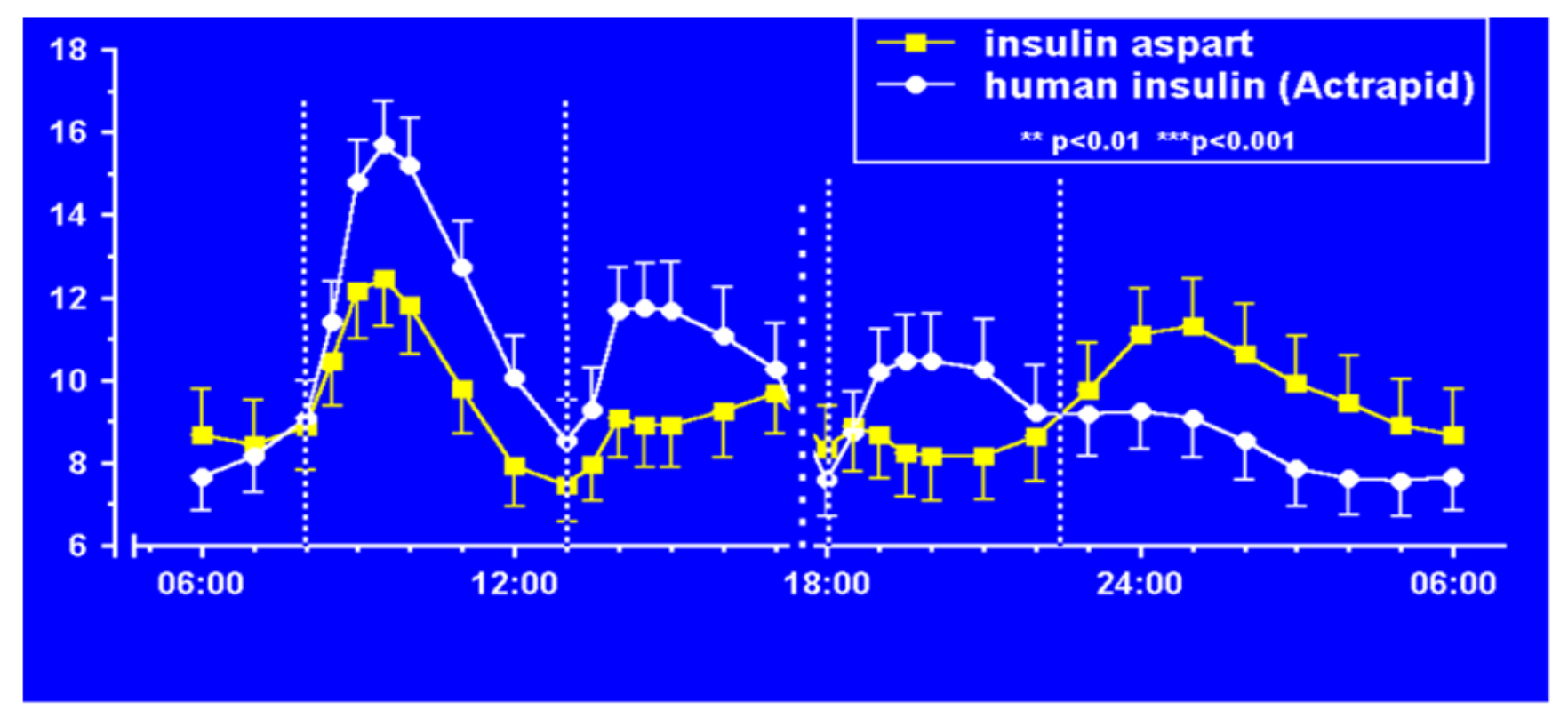
Aspart insulin (Novolog
® or Novorapid
®), a modification of the B-chain 28th amino acid with aspartic acid (
Figure 19), was introduced a few years later by Novo Nordisk also in pen and vial and syringe format (
Figure 20). In their studies (
Figure 21), improvements in postprandial glycemic excursions were documented for all three meals in young patients compared to regular insulin used prandially. In addition, the same improvement in avoiding nocturnal hypoglycemia was documented as with the lispro studies [
29]. Fructosamine and glycemia area under the curve both showed very statistically significant improvements in these somewhat shorter studies. These studies were designed to not only show equivalent efficacy with animal source and semisynthetic human regular insulin but succeeded in showing better results postprandially (i.e., lower glucose levels) at the same time that they decreased late-onset hypoglycemia after the meals and in the middle-of-the-night [
30].
The third rapid-acting insulin available was created by Sanofi-Aventis, glulisine insulin (Apidra
®) (
Figure 22, pen format) and involves substation of lysine for asparagine in the B3 position and lysine replaced by glutamic acid in the B29 position (
Figure 23) to modify the action of the insulin molecule in the subcutaneous space. It has similar beneficial characteristics, acts in an improved manner as a prandial insulin, and decreases postprandial hypoglycemia and nocturnal hypoglycemia hours after the meals in a similar fashion clinically to lispro and aspart insulins [
31].
In practical terms, all three of the rapid-acting insulins cost about the same but are more expensive than the previous animal source as well as biosynthetic human regular insulin preparations. All three can be used in infants, children, adolescents, adults, and the elderly without any difficulties understanding that they would ideally be provided about 15 min ahead of food to try to match glycemic excursions of most foods and snacks and would need to be coupled with some type of basal/background insulin for between-meal glycemic control. All three fast-acting insulin analogs have the same potential benefits and have minimal differences in terms of clinical performance, lessening of nocturnal and postprandial hypoglycemia, improvement in coverage for postprandial glycemic excursions, and equal or improved A1c results. Because of their fast uptake, in the very young child who does not always eat a full meal or in those where this is not possible psychologically, postprandial dosing still works quite well as a “back-up” plan [
32].
With insulin pumps, the three fast-acting insulin analogs, lispro, aspart, and glulisine, are all available, and all work quite well. As with an MDI regimen, with CSII [
33] use, there is less daytime as well as nocturnal hypoglycemia occurring and specifically far less nocturnal severe episodes of hypoglycemia-related loss of consciousness or seizures, the most severe types of hypoglycemia. The actual bolus effect seems to diminish by about 30% per hour after the bolus is administered, so after the first hour, about 70% of the insulin action occurs; after the second hour, about 40% occurs; after the third hour, this tapers to about 10% with very little, if any, effect on the blood glucose readings by about the fourth hour after bolus pump administration.
3. Insulin Analogs: Basal Slow-Acting Glargine and Detemir
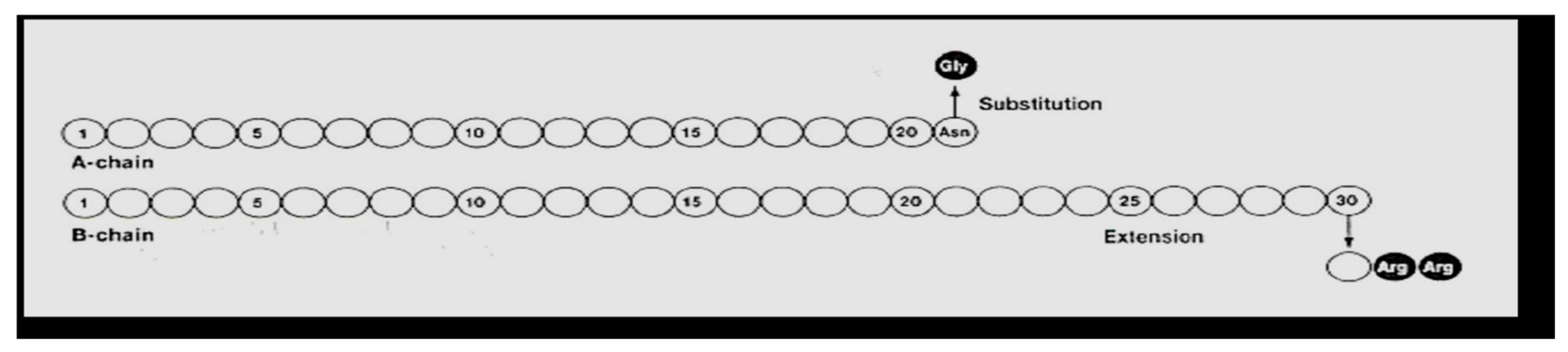
Hoechst began clinical trials in 1998 with what was then called HOE 901, later glargine insulin. Glargine (Lantus
®) (
Figure 24 and
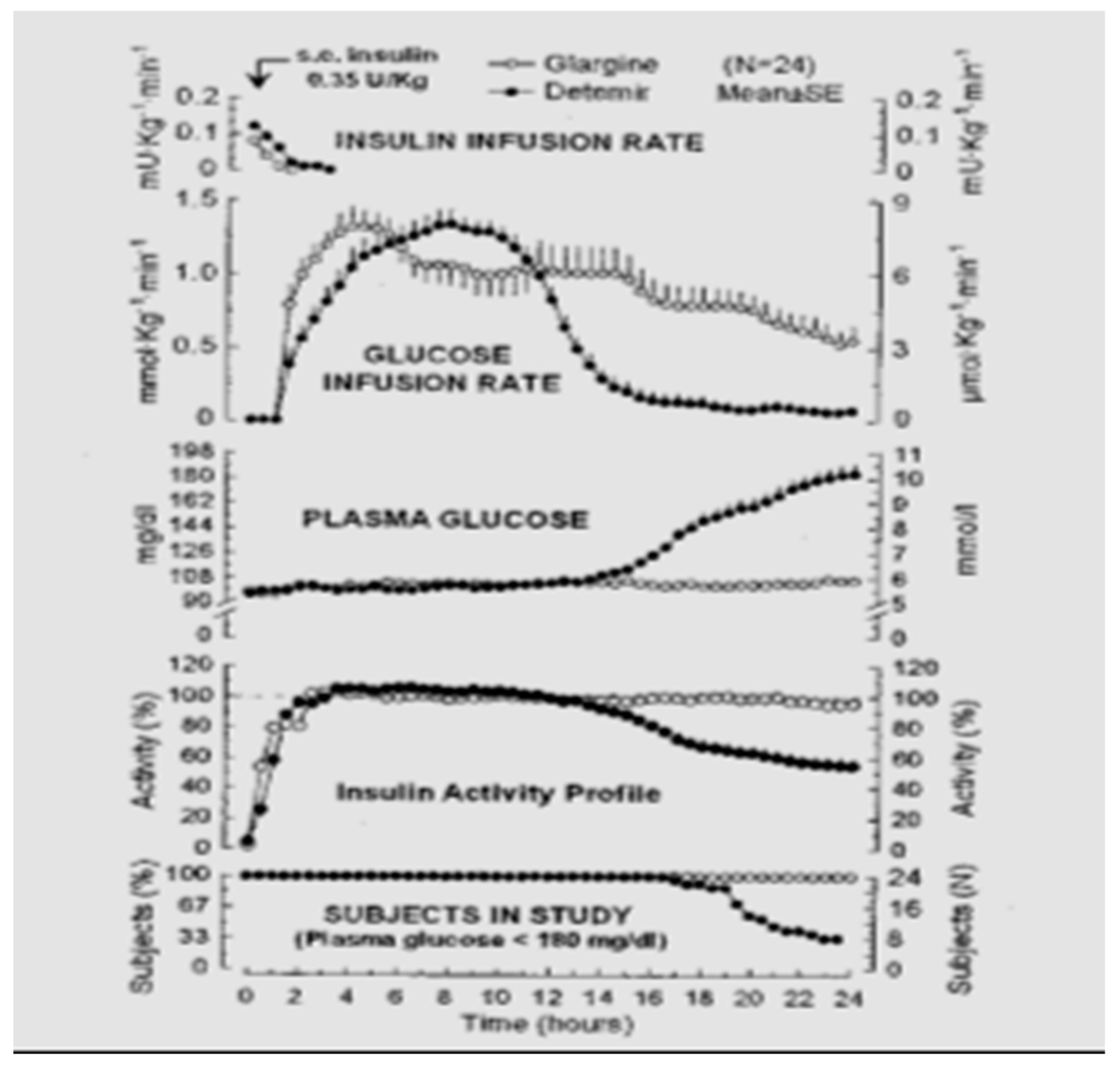
Figure 25) was genetically modified human insulin with an A-21 glycine substitution for the A-chain end terminal amino acid ASN in addition to an arginine-arginine addition to the end of the B-chain of the insulin molecule. Glargine had a flatter action curve compared to NPH, lente, or ultralente insulin at the same time; it was “relatively peakless”, and this was especially important for their success in documenting less nocturnal hypoglycemia [
34]. Several different zinc formulations of glargine were studied, and all showed the same therapeutic benefits with lowered fasting glucose levels; at the same time, there were fewer nocturnal hypoglycemic episodes [
35]. The comparison data (see
Figure 26) were demonstrated [
36] using glucose levels achieved as well as glucose infusion rates and the expected earlier NPH peak effect compared to the lower, relatively peakless, and more prolonged glargine effects. In some patients with glargine, there is a mild peak effect between 12 and 16 h after injection, while in many patients using relatively small doses of glargine, the initial 24+ hour duration of glargine waned somewhat. This was especially seen in the younger patients using smaller overall dosage (compared to the adults or more overweight patients) so that while it is typical to start with a bedtime-only glargine dose, depending upon actual blood glucose profile data analysis, it is not uncommon for pediatric, adolescent and young adult patients to require glargine twice-a-day with a ratio of about 70–80% glargine at bedtime and 20–30% glargine with breakfast in the morning. Some even have a reverse need for the larger morning glargine dose and the smaller bedtime dose of glargine. Others have a different time action course with the mild peak occurring post-breakfast or prelaunch so that a suppertime rather than bedtime glargine dose works better. These decisions can be recognized with frequent blood glucose monitoring and data analysis [
37].
Novo Nordisk (see
Figure 27) entered the insulin market with their basal insulin, detemir, in 2005. Detemir (Levemir
®) substituted a threonine for the terminal B29 lysine amino acid and attached a C14 fatty acid chain (myristic acid) to the threonine (
Figure 28). Through this process, the detemir insulin molecule binds to albumin in tissue and blood and is therefore very slowly released to the general circulation [
38]. Detemir became the second basal bioengineered insulin to be made available with similar favorable clinical results [
39] plus it had “less binding” to the IGF-1 receptors [
40], and so there was potentially less mitogenicity associated with detemir compared to glargine (
Figure 29); clinically, there has not been any long-term studies to answer this question scientifically. There were some initial worries that glargine had some association with cancer risk increases, but these turned out to be statically incorrect, and further longer studies have not drawn these same conclusions. Detemir also had a relatively flat effect on glucose levels, but its duration was a bit shorter than glargine, and this difference was magnified a bit in the younger patients using smaller doses than the more overweight adult population studied. For most practical purposes, while detemir can be started as a single night-time basal insulin, it is most frequently utilized as a twice-a-day, pre-breakfast plus bedtime overlapping basal insulin, with the same caveats of utilizing blood glucose monitoring profiles to identify those who need higher or lower doses either in the evening or morning, more equal doses for the twice-a-day detemir treatment or even reversed distribution as mentioned with glargine insulin provision. Detemir, like glargine, is associated with improved fasting glucose levels (i.e., lower); at the same time, there is less nocturnal and between-meal hypoglycemia compared to NPH or other intermediate or longer-lasting insulin products [
41].
At our center, NEDEC, we utilize all three rapid-acting analogs as long as they have equal availability and insurance company coverage. Insulin decisions are individualized in terms of doses required for the three major meals of the day, and when there is little activity or large snacking, then sometimes snacks are also covered in prandial fashion using a model of insulin–carb ratios created for that person as well as correction factors in a style similar to what we teach for insulin pump treatment. None of this is rigid and dogmatic, and all is aimed to provide maximum flexibility for day-to-day variability, especially with changes in toddlers, children, and adolescents as well as young adult lifestyles. We teach the importance of waiting about 15 min prior to food intake to allow the subcutaneous rapid-acting insulins to begin to reach peak insulin efficacy about the same time as the food intake is reaching peak glycemic load. In this fashion, we believe that we maximize opportunities for improved insulin coverage of food peaks and allow the rapid-acting insulin analogs to begin to decrease their effects and concentrations in the body at about the same time that the food effects are waning. In comparison with the Hvidore study reports of A1c in a multicenter analysis [
42], NEDEC A1c results are in the “excellent, green” range without excessive or severe hypoglycemia reported either during the daytime hours or overnight time period (
Figure 30). Nevertheless, despite maximizing educational and re-educational efforts [
43], this remains a challenge to optimize such insulin–food timing for many of our patients and their families [
44]. In keeping with the message of the DCCT, we would summarize the NEDEC Type 1 Diabetes Treatment Goals as aiming for blood glucose as close as possible to those without diabetes, but without excessive or severe episodes of hypoglycemia. The education approach is a psychosocially oriented approach wherein the entire team of health care professionals, physicians, nurses, nurse-educators, nutrition specialists, exercise specialists, psychologists, and social workers work with the same philosophy and with the same goals individualized for the patient and his or her family to optimize such treatment. This usually involves a multi-dose basal-bolus model with one of the three fast-acting analogs given at times when food is eaten coupled with one of the two longer-lasting basal analogs (see below). The NEDEC philosophy was spelled out in some detail in several summary clinical articles [
45]. When the transition from beef and pork insulin to human insulin took place, it was an easy change because this meant fewer instances of localized lipoatrophy and lipohypertrophy were seen. The rare case of allergic reactions locally or systemically to beef or pork insulin products also became even rarer. With the advent of the synthetic insulin analogs, both the fast-acting and the slower basal insulin analogs, the transition continued since not only did such local problems became even more rare, but the improved physiological balance of insulin to food helped decrease hypoglycemia while at the same time made the quality-of-life improvements because the timing of insulin administration and the use of pens over syringes were easier for our patients and their families. This all happened at the expense of more daily injections and was seen in many other centers as well. Insulin pump use also increased from about 10% of the NEDEC patient population in the 1990s to now about 60% using pumps, with about 45% using combinations of insulin pumps and continuous glucose sensors. An example of the improvements in A1c outcomes [
46] is presented from the Barbara Davis Diabetes Center at the University of Colorado in Denver when one of the insulins being used was changed to a faster-acting analog prandial lispro (
Figure 31).
When eating away from home at school or at a restaurant, the fast-acting analogs could be better timed with the food consumed. If food flexibility was desirable, decisions to cover extra snacks could easily be accommodated with these fast-acting analogs (
Figure 32). For the preschoolers whose parents could not always guarantee that a certain amount of food would actually be eaten, fast-acting insulins could be provided after the meal rather than before with almost equal coverage of the food. When changes in activities or exercise occurred without advance warning, adaptations with food and insulin could also be realized easier than with the earlier insulin preparations using the basal-bolus model. What might be called the “Professor Harry Dorchy” model of flexibility and adaptation [
47,
48] was possible with the prior insulin preparations but more easily facilitated with the analogs and their physiological adaptations and time-course of action based on frequent blood glucose monitoring and analysis of such monitoring results. Detailed logbooks help in this learning process, as do newer computer downloading of meters, which make it easier to graph patterns, identify trends, and allow proactive as well as reactive responses. Insulin–carbohydrate ratios for foods and glycemic index of foods both are more ideally treated at NEDEC with insulin pumps because square waves and dual waves, as well as temporary basal adjustments, make such adaptations easy to be taught and accommodated. Similar concepts are also taught with multi-dose insulin regimens (MDI), which makes the transition from MDI to CSII much easier to accomplish since the same terminology is utilized by the educational team.
4. NEDEC Transition from Previous Insulin Regimens to Basal-Bolus MDI with Analogs
Coupled with the fast-acting meal-time and snack-time analogs are either of the two readily available longer-lasting “basal” insulin analogs that replaced NPH, lente, and ultralente insulin: glargine and detemir insulin. With type 2 diabetes patients, bedtime glargine or detemir can often be utilized just once a day, but with smaller doses required for children and also in growing adolescents, usually twice-a-day glargine and twice-a-day detemir seem to be needed to provide smoother basal insulin coverage. This, too, is not done dogmatically but rather started with a single night-time basal insulin dose of glargine or detemir once a day. Thereafter, blood glucose levels throughout the day and the night guide the treatment team, patient, and family as to the necessity of once- or twice-a-day basal insulin analog provision. The smaller the dose of insulin, usually the need for two smaller overlapping basal insulin analog doses each day. Occasionally, a reverse pattern is required wherein only morning glargine or detemir seems to work better than bedtime or twice-a-day basal insulin, and this too is decided based upon actual BG results and pattern analysis of food and activity.
At NEDEC, when the new basal analogs became available, most of our patients were using MDI with overlapping three or four doses of NPH; in an attempt to provide optimal basal insulin at the same time, there were lower doses at any moment, thus decreasing hypoglycemia risks between meals and especially overnight. This proved to be very successful and assisted the safe achievement of DCCT-level GHb results; at the same time, there were minimal severe episodes of hypoglycemia taking place. When the new analogs showed improvement in this same fashion, but with even fewer injections needed and even lowered nocturnal hypoglycemia, the patients were slowly but nearly universally changed to either detemir or glargine basal insulin preparations. The more common use of analogs has been documented in many studies for these similar reasons [
49].
The NEDEC changeover protocol that was successfully developed was as follows:
Add up the total cloudy insulin doses for the entire day (i.e., all three or four NPH doses being used);
Start with eliminating all the NPH doses the next day but providing pre- and postprandial blood glucose testing and coverage with either lispro, aspart, or glulisine prandially;
Give the first basal insulin dose as glargine or detemir at 80% of the total basal insulin dose previously used and give it at bedtime;
Keep this dose steady for 2–3 days with some 3–4 am blood glucose checks to prove that there is no nocturnal hypoglycemia occurring;
Titrate the prandial bolus insulin analog dose according to pre and postprandial paired BG readings;
Titrate the bedtime glargine or detemir dose based upon the next morning’s pre- and post-breakfast as well as prelaunch BG readings aiming for a target of fasting BG of 100 mg/dL (~5.5 mmol);
With prebreakfast hypoglycemia, decrease the bedtime glargine or detemir;
With prebreakfast hyperglycemia, increase the bedtime glargine or detemir;
With the expectation that only 10–20% of pediatric and adolescent patients provided bedtime glargine or detemir alone will have sufficient basal insulin effect to last a full 24 h, expect that some morning glargine or detemir will be added based on late afternoon–evening hyperglycemia;
When the morning basal insulin is added, expect the evening glargine or detemir dose to be decreased by approximately an equal amount;
Understand that sometimes the reverse pattern occurs and more morning glargine or detemir is needed than evening basal analog;
Similarly, in some individuals, the morning and bedtime long-lasting analog doses are equal, but this is a smaller portion of patients;
Once the overall pattern of basal insulins is established, go back and fine-tune to adjust the prandial fast-acting analogs by looking at pre and postprandial glycemic excursions with reminders about appropriate pre-meal timing of analog doses to actual timing of when food is ingested (usually about 15 min);
Sometimes there is also a need to change the bedtime basal insulin to suppertime when there is a more prolonged peak from the glargine or detemir;
If there is a large morning or afternoon snack, these may also need prandial bolus insulin analog coverage according to activity and BG results;
Many no longer need mid-morning, mid-afternoon, or even bedtime snacks because of the relatively peakless nature of the basal insulin compared to the stronger NPH peaks used previously, and so calories can be cut back, and these snacks eliminated.
A major benefit of the NEDEC approach [
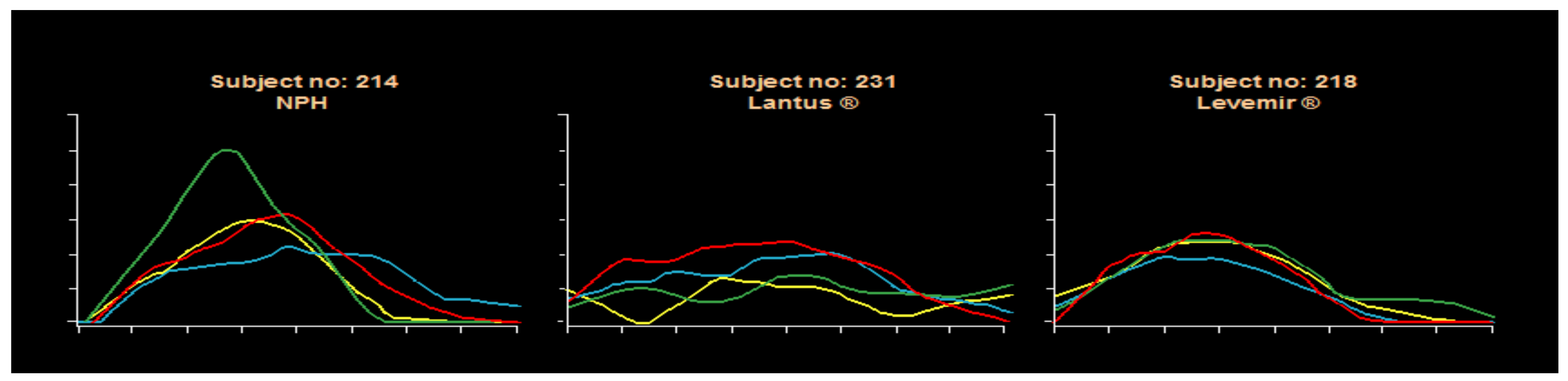
50] with analog MDI insulins is a reduction in frequency and severity of hypoglycemia at the same time that overall improvement in glycemic control. This produced better GHb results and less glycemic variability using a flexible carbohydrate counting approach coupled with frequent monitoring of blood glucose levels for analysis either in a color-coded logbook or by downloading blood glucose meters for computer graphic review. The analogs offer more predictable effects than the beef-pork, purified animal insulins, or semisynthetic human insulin preparations. Pen use has increased for ease of provision since there is a separate decision needed for prandial bolus dosing with this MDI approach. Pens are also available for both glargine and detemir basal insulins. Syringes can be used in those who prefer them or for cost considerations, with vials of analog insulin also available per individual circumstances. The MDI basal-bolus insulin approach is easier to teach than with premixed insulins, allows for dose adjustments for activity and food changes as well. Usually, glycemic excursions are decreased, and carbohydrate counting benefits become more apparent when using this educational approach to insulin delivery coupled with more daily pre and postprandial BG checking. Some detemir studies have suggested that detemir was somewhat more predictable than glargine, but these have not been conclusive. Within-subject variability remains rather large from day-to-day with old as well as new insulin systems because of vagaries of measurement, dose administration, subcutaneous uptake, differences in glycemic food excursions and food variability, carbohydrate counting expertise, and perhaps also with inconsistencies of activity and stress effects on day to day management of type 1 diabetes inherent in the human condition: gym or no gym, after-school sports or homework, video gaming stress, family dynamics, and friend-to-friend relationships, exam-taking, sleeping late, etc. A unique Novo Nordisk clamp study using NPH, glargine, and detemir documented the day-to-day variabilities with these three subjects’ clamp results shown as an example in
Figure 33.
As Sperling elegantly has written [
51], the difficulties of type 1 diabetes remain with the inherent variability of food ingested, changes in activity from day-to-day, vagaries of insulin absorption and effect metabolically, counter-regulatory responses, and the ongoing potential dangers and fears of hypoglycemia; what he has called the Scylla and Charybdis of blood glucose control in children but this is somewhat lessened with the newest analogs compared to earlier preparations.
7. Blood Glucose Monitoring with AI, Artificial Pancreas Hybrid Closed Loops, and Closed Loops: Decision Support Systems for Insulin Adjustment
Cell phones, computers, blue tooth, wi-fi, and the internet have all contributed to amazing interactivity [
53], and this type of technology has been increasingly incorporated into diabetes technology management in an effort to assist day to day management hurdles. Continuous glucose monitoring systems have begun replacing self-blood glucose measurement, allowing better analysis of glucose data information as well as more identification of patterns for both hyperglycemia and hypoglycemia, particularly overnight and post-meal glycemic excursions [
54]. Insulin data can also be recorded with these same systems with more technological advancements and data processing capabilities not only with pumps but also with insulin pens facilitating such processing capacity. Medical integration with other technologies such as smart watches and GPS connectivity allow more and more of such data to become available for health care providers as well as families and patients themselves. Enormous amounts of such data require sophisticated data processing, which frequently increases the psychological burden of analyzing the data, the time needed by the health care team for analysis, and, of course, also home processing between follow-up visitation [
55]. Clearly, there is also stress and anxiety in the use of such equipment that also needs to be recognized and addressed.
Availability of such new technologies is expensive, and systems for incorporation for reimbursement also need to be developed as well as acknowledging that this does not automatically improve glycemic control without support services vis-à-vis education, strategies for safe use, and efforts to promote continued and ongoing usage as well as integration so that anxiety and monitoring stresses are also reduced at the same time overall glycemic improvement occurs. This has been demonstrated [
56] in younger children (and their parents), school-agers, and adolescents as well as young and older adults, and even in type 2 diabetes patients, it is now being evaluated for such technological advanced usage.
However, there remains a data paradox that having more information and more sophisticated methods of delivering insulin, as well as ways to counter-balance food and activity as well as stress effects on glucose levels per se, sometimes also correlates with worse health outcomes, and this is thought to reflect the increased financial and emotional burden of such systems. Time spent during health care visits also may become more face-time with computers than actual health care professional: patient/family time during visits as well as between visits, especially in the beginning phase of such treatment processes—and even with ongoing support and encouragement. Health care professionals have to learn to use such tools in somewhat different ways compared to more traditional approaches and, of course, in areas where there are limited or only medium resources available, such tools remain impractical under such circumstances (see section below). Nevertheless, with more availability of cellular technologies, even in LRC or MRC areas of the world, electronic reminders and ways to communicate are being facilitated increasingly. Reminders to health care providers also can be incorporated into such systems. Focused patient reports and ways to highlight important aspects of such results can also facilitate improving care.
Such decisions involve food and snack choices, carbohydrate counting, and ways to more optimally balance insulin delivery to improve glucose time in range at the same time as decreasing hypoglycemia and hyperglycemia occurrences. Initial efforts in recent years have focused on reducing hypoglycemic events, especially severe hypoglycemia associated with loss of consciousness and/or seizures, while attempting to improve time in range instead of merely shifting the glycemic results into hyperglycemic ranges with raised A1c results and known long-term complications risks. This has been demonstrable with numerous insulin delivery systems and different types of pumps and sensor systems [
57]. The person with diabetes (PWD) needs to cope and make decisions about insulin when food changes, amounts of food or types of food change, the timing of eating changes, and also when activity or illness, as well as emotional stresses of normal life, occur and this can actually be documented to involve 1–2 h every single day. As stated by Dr. Nimri: “Both the complexity and intensity of diabetes management justify an overwhelming need for further decision-support that is becoming available”. With the recent COVID-19 worldwide pandemic and the associated decreases in face-to-face office and clinic consultations, such digital diabetes data applications and reviews have been taken place in many parts of the world where teleconferencing and connectivity are possible. Dr. Nimri has further documented more than 1000 diabetes-related applications currently available on the Apple App Store and Google Play to assist health care providers as well as PWD and their families. These help to collect data, summarize and highlight important aspects of such data, and/or remind/encourage healthier choices with increasingly available digitized systems beginning to use medical-based artificial intelligence to help with actual decision making. Automatic downloading has been shown to facilitate such systems actually being used when time can be saved in the process. Not only pump and sensor systems but also blood glucose meters can be connected in such systems for automatic or semi-automatic analysis. Insulin dose titration algorithms can also be recommended in many of these systems with the goal of more consistent application, better pattern recognition, and treatment recommendations to consider. Medtronic Carelink, Abbott Libre, Tandem t:connect, and Dexcom Clarity are some of the available systems involving pumps and sensors. Blood glucose meters by Johnson and Johnson, Ascencia, Abbott and Roche, and others also have similar systems, and independent downloading systems such as Glooko and Tidepool have also become available for sharing such data.
8. Data Analysis
One of the more important graphs for home review as well as in-office/clinic review is the standard or modal day pattern. This can be obtained from the usual blood glucose stand-alone systems downloaded to most of the home, office/clinic/hospital computerized programs currently available and can generate many types of graphic displays, pie charts, and numerical summaries depending on individual preferences and circumstances. This allows home data analysis of blood glucose information on a daily, weekly, or monthly basis and helps facilitate potential changes in food, activity, and/or insulin choices accordingly. Not all PWD will have access to such electronics, and not all PWD will have the mathematical skills to use these on their own. Appropriate educational and psychosocial support should be an integrated part of the health care team’s responsibilities and ongoing discussions to help meet the needs of the PWD and his or her family members [
58].
Examples of such data analysis are presented in the next figure, which shows overall patterns as well as color-coded day-by-day comparative analysis.
For this author, one of the more potent graphs for home review as well as in-office review is the standard or modal day pattern to see if there are outliers. If these can be explained and learned, then it should be possible to utilize such information to be proactive and therefore prevent future occurrences more and more often. In
Figure 34, for instance, there are several days of post-dinner hyperglycemia that would suggest either poor carbohydrate potion control not being covered by sufficient prandial insulin, lack of use of the extended bolus function for high-fat foods at this meal, or just not timing the insulin bolus correctly without allowing sufficient time prior to insulin administration to adequately cover the meal quantities. Because of the 24 h time period for these several days, the “correct” answer in this instance was not utilizing the square wave bolus function for high fat-carbohydrate foods on those three days; just making this correction “solved” the problem on future evenings quite readily.
Alternative programs (
Figure 35) have alternative graphic displays, and some are easier to use for individual patients so that whatever works is the program that should be utilized. Some will identify averages but not individual day-by-day readings of either home blood glucose readings or CGMS data. Newer pump statistical downloads allow identification of hypoglycemic episodes, meal-time analysis vs. overnight analysis, as well as more detailed statistics including averages, percentage hyperglycemia vs. hypoglycemia, total average insulin dose, basal–bolus ratios, and differences between sensor glycemia and blood glucose capillary data.
A key issue for the health care team is to have a unified approach to recommendations for how often downloading at home should occur, whether or not it should take place only at the office visits or between visits with this author recommending optimal downloading twice a week so that specific aberrations or variance can be identified and remembered in an effort to become more proactive and less reactive. It remains extremely important to have nurses, dieticians, social workers/psychologists, students, and physicians making consistent recommendations and approaches to minimize confusion for the PWD or family members. This also facilitates improved compliance while still emphasizing individual needs and differences. Both reactivity to high and low values as well as being more preventive (proactivity) are very important to learn and practice so that improved glycemic control, lowered glycemic variability, and ultimately better quality of life, and fewer short- and long-term complications may occur. Some patients can learn this quite effectively, and others who have math or language difficulties may take longer to learn to respond, if at all. Sometimes other members of the family must be called to assist under such circumstances. Practice in the office and practice at home between visits need to be encouraged, and assistance with computer problems from meters, pumps, and CGMS manufacturers also may be required to help with home systems.
In the past few years, the CGMS have improved dramatically [
59] so that less effort is needed for daily calibration, and more and more of the newest CGMS will be factory calibrated in the near future. Three major sensors now are available DexCom
®, Enlite
® and Libre
®. Most provide round-the-clock every 5 min automatic subcutaneous glucose data on a continuous basis, and most sensors last from 4 to 14 days depending upon the particular system. The DexCom
® sensors transmit their information to their own receivers as well as download it to computers and phones. More recently, they also transmit such information to several insulin pump systems, with the pumps beginning to also have the capability of “responding” to such data automatically. The PWD or family members can still manually respond and make basal and/or bolus dose adjustments as well. Improvements of systems using these DexCom CGMS using better and more sophisticated artificial intelligence algorithms have worked well for reducing hypoglycemic events, reducing the severity of hypoglycemia, and even reducing hyperglycemia as well as glycemic variability [
60]. This is true for Omnipod
®, Roche
®, Animas
®, and T:Slim pump systems working with DexCom sensors [
61].
With the initial Medtronic pump systems coupled with Enlite
® sensors, there was some more variability, and the DexCom sensors seemed to be more reliable and more accurate; over recent years, the Enlite sensors have improved dramatically so that there is virtually no difference in accuracy or specificity any longer with virtually identically MARD values of 0.4 [
62]. The biggest advantage currently of the Medtronic pumps is not only that the Enlite sensors are more accurate and reliable but also that the Enlite sensors send information to mobile phones and home computers as well as directly to Medtronic insulin pumps acting as information receivers. The screens of the Medtronic 530G insulin pump (
Figure 36) and more recently the 630, 640, and 670G Medtronic pumps, in color (
Figure 37), now show every 5 min actual blood glucose readings from the sensor, graphic analysis (24 h, 12 h, 6 h, and 3 h updated curves) looking at trend analysis and arrows that highlight upward or downward trends for easier identification. Information about the connectivity of the sensors as well as battery power and insulin reserve is also provided on most pump screens.
Other systems are available for those who prefer raw mathematical data identifying amount of time in range, above or below individually identified target glycemic goals and pie-charts for graphic representation looking at overall time periods as well as specifically identified hours of the day or night.
Figure 38 shows one such pie chart analysis.
As older PWD and family members get more comfortable with analysis of their own pump and CGMS data, not only the modal day and summary data are important but better problem solving can take place when reviewing the individual daily summary downloads. These provide specific information to verify the accuracy or inaccuracy of the sensor graphs compared to capillary blood glucose calibration information since the graphic display overlays this quite easily for review. Insulin delivery and use (or lack of use of square and dual waves), as well as manual or automatic low glucose suspension features of the pumps, is also demonstrable. Carbohydrate counting errors, missed information, exercise, and any other events so code also can help assess glycemic responses. These daily response graphs are most dramatic when people believe that the sensors are inaccurate, yet their own data can help convince them under what circumstances this is valid or misconstrued information.
Figure 39 shows such a daily summary download chart showing such features.
A demonstration of the DexCom G4 sending information to the DexCom receiver and the newer DexCom G4 sending information to a mobile phone app are presented in
Figure 40 demonstration of two weeks of DexCom sensor data and the superb response of the PWD showing remarkable improvement in glycemic variability on the second week of DexCom sensor use (
Figure 41).
Figure 42 demonstrates the Abbott FreeStyle Libre Pro
® results pages which is a stand-alone sensor that can be used with multidose insulin regimens so does not require an insulin pump and shows time in range, averages, percentage time above target and below target, as well as low glucose events and their duration. Estimated glycohemoglobin results are also provided mathematically. Data scanning increased throughout the day, and routine fingerstick BGs were eliminated by 91% of the participants since no calibration was required (
Figure 43,
Figure 44 and
Figure 45).
The latest advance in the march towards the artificial pancreas was approved by the US FDA in September 2016 and is called the “hybrid” artificial pancreas as presented by Medtronic utilizing the Medtronic 670G pump and, more recently, upgrades to 780G system. It involves a color screen, is waterproof, and includes the LGS (low glucose suspension) automatic features of earlier models. In addition, it keeps the Guardian
® CGMS integrated with bidirectional communication to the newest pumps with a “hybrid closed loop” (HCL) [
63] that automatically adjusts basal rates trending upwards if there is a pattern of increasing glycemia and, in addition, automatically also adjusts basal rates trending downward—before LGS initiates, to attempt to prevent more severe hypoglycemia. Two levels of personalization with this 670G “hybrid” artificial pancreas: suspend before low option and auto mode option, which adjusted basal insulin delivery every 5 min based on defined sugar levels to maintain improved target range glycemia day and night without operator activity [
64], as shown in
Figure 46.
The 780G CSII systems with Guardian Connect
® assisted hybrid closed-loop (AHCL) pediatric and adolescent studies were presented at the 2021 Annual (virtual) Meeting of ISPAD [
65]. Such systems not only improve time in range but continue to minimize hypoglycemic events. Such systems allow connection to mobile cellular telephones, including access to programed alerts for patients and their family members to take action. Cloud data analysis for health care professionals is also readily available. Such shared data are available with DexCom systems as well, and efforts to make more consistent downloaded glucose data available have been forthcoming from many groups working around the world in close collaboration with diabetes teams and diabetes supply manufacturers.
In an e-poster presented at the 2021 Annual (virtual) Meeting of ISPAD [
66], improvements in A1c as well as time in the range were confirmed with such hybrid closed-loop systems while maintaining minimal overall hypoglycemia and completely avoiding all severe daytime and nocturnal hypoglycemic events (
Figure 47,
Figure 48 and
Figure 49).
The JDRF has set its “Road to Fully Automated Closed-Loop Pumps”, as presented by Kowalski [
67] in
Figure 50.
We have moved through phases 1–3 and are now “officially” in phase 4 with the introduction of the newest hybrid closed-loop systems. We started back in the 1970s with proposed models of what the artificial mechanical pancreas, such as that shown in
Figure 50 from Dr. Soeldner, the first “wearable” artificial pancreas that allowed blood glucose monitoring and insulin delivery.
Figure 51 and a picture of the Ames Biostator as it was used in hospitals almost fifty years ago is shown in
Figure 52. The first Ames Glucometer ® (the “brick”)
Figure 53 that brought bedside, in clinic and at home self blood glucose monitoring its early start.
The latest development over the past few years has also been the introduction of parents working with computers, opening up pumps, and advancing CGMS software to allow communication with each other, with their children and spouses, and also to talk to mobile phones in a more facilitated fashion. This has not happened with official government or industry sanction, and it has opened up some intriguing ownership, legal, and medial quandaries about who owns the software rights, computer maneuvering rights, and data when someone has “purchased” an insulin pump, blood glucose meter, or continuous glucose monitor. Nevertheless, the work has continued around the world, with the internet serving as a vehicle for communications, sharing, and improvement in software and hardware. Open Source, OpenAp, and NightScout (
Figure 54 and
Figure 55) are some of the groups operating in this environment to bring improvements such as advanced waterproofing, better communication possibilities, better alarm notification, and improved quality of life for all utilizing these new developments in artificial intelligence, insulin pumps, monitoring, and access.
12. Ultra-Slow-Acting Insulins
The rationale for the “ultra-slow-acting insulins” is the same rationale as was proposed for the development of PZI, then CZI, then glargine and detemir insulins: smoother, longer duration, and perhaps needing fewer than daily injections to provide basal insulin coupled with less insulin exposure and therefore less hypoglycemia, particularly nocturnal hypoglycemia. They would be utilized in conjunction with prandial analogs designed to cover the immediate effects of food intake in a more physiologic fashion mimicking the normal pancreatic insulin delivery.
Novo Nordisk’s degludec (Ideg or Tresiba
®) has completed proof of concepts studies as well as initial safety and efficacy trials [
82] with approval by some authorities already obtained in 2013 and with two pen dose formulations available (
Figure 61). The half-life for degludec is about 25 h. Degludec consists of multiple hexamers with very slow release to monomers from the subcutaneous depot through adding a fatty acid side chain to the terminal aminoacid of the B chain of the human insulin molecule (
Figure 62). The result [
83] is insulin with twice the duration of activity of glargine or detemir and a flatter, smoother profile of effect as well—both desirable properties for basal insulin [
84]. It can be given either in the morning or the evening and may not need a daily dose because of its long half-life in the circulation but would still require prandial insulins for meal and snack coverage. It is not yet approved for use in those under 18 years of age, but clinical studies are in progress to address safety and efficacy issues [
85,
86]. Postprandial as well as nocturnal hypoglycemia is the same or less than other products in testing to date [
87].
Lilly has developed a pegylated lispro insulin analog called peglispro (LY2605541) [
88] with an even longer half-life of 48–72 h. Peglispro begins with the lispro analog that is then modified with a 28 KDa polyethylene glycol (
Figure 63) to slow absorption as well as slow renal clearance. As with Ideg
®, peglispro has a more consistent, flatter, and longer duration of activity and a decrease in overall hypoglycemia and especially a decrease in nocturnal hypoglycemia as a result. Clinical trial studies are ongoing [
89,
90].
Longer-lasting glargine analogs also have been studied and are coming to market with flatter curves of action and smaller or no peak effects with the hopes of decreasing hypoglycemia further and making basal insulin availability more standardized from day to day and therefore helping decrease glycemic variability.
Uniform multidisciplinary team care combined with more available monitoring, more adaptable food and snack choices, and more physiological insulin delivery using newer analogs, or insulin pumps combined with SBGM or automated or semi-automated more detailed BG testing have shown significant promise of overcoming the differences in different centers of specialty excellence around the world. The key improvement occurred when teams in both Sweden and the UK identified their discordant messages and actually worked very specifically to present a more unified approach for motivational teaching and training utilizing psychosocial approaches, learning theory, and group dynamics to form the basis of such programs—and produced significant improvements in A1c outcomes while reducing hypoglycemic episodes, glycosuria, postprandial hyperglycemia, etc. [
91].