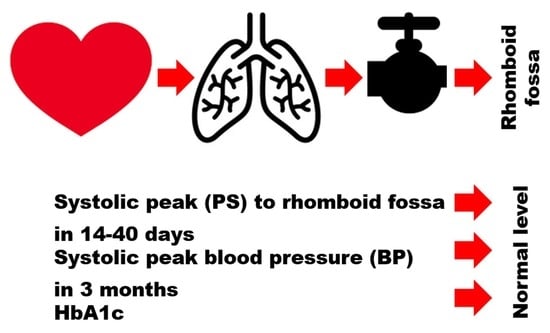
Hypothetical Reason for the Restoration of HbA1c Level for Pre-Diabetic Patients through the Recovery of Arterial Blood Flow Access to Rhomboid Fossa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Selection
2.3. Therapy
2.4. Statistical Analysis
3. Results
4. Discussion
- Abdominal obesity;
- Elevated blood triglyceride levels;
- Low blood high-density lipoprotein (HDL) cholesterol;
- HBP;
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacCracken, J.; Hoel, D. From ants to analogues. Puzzles and promises in diabetes management. Postgrad. Med. 1997, 101, 138–150. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. 1), S62–S67. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef] [PubMed]
- WHO. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia—Report of a WHO/IDF Consultation; WHO Document Production Services: Geneva, Switzerland, 2006; p. 50. [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin. Diabetes 2015, 33, 97–111. [Google Scholar] [CrossRef]
- Bower, J.K.; Appel, L.J.; Matsushita, K.; Young, J.H.; Alonso, A.; Brancati, F.L.; Selvin, E. Glycated hemoglobin and risk of hypertension in the atherosclerosis risk in communities study. Diabetes Care 2012, 35, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Selvin, E.; Steffes, M.W.; Zhu, H.; Matsushita, K.; Wagenknecht, L.; Pankow, J.; Coresh, J.; Brancati, F.L. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N. Engl. J. Med. 2010, 362, 800–811. [Google Scholar] [CrossRef]
- Adams, R.J.; Appleton, S.L.; Hill, C.L.; Wilson, D.H.; Taylor, A.W.; Chittleborough, C.R.; Gill, T.K.; Ruffin, R.E. Independent association of HbA(1c) and incident cardiovascular disease in people without diabetes. Obesity (Silver Spring) 2009, 17, 559–563. [Google Scholar] [CrossRef]
- Song, J.; Wei, N.; Zhao, Y.; Jiang, Y.; Wu, X.; Gao, H. Elevated glycosylated hemoglobin levels and their interactive effects on hypertension risk in nondiabetic Chinese population: A cross-sectional survey. BMC Cardiovasc Disord 2020, 20, 218. [Google Scholar] [CrossRef]
- Zhukov, K.V.; Vetcher, A.A.; Gasparuan, B.A.; Shishonin, A.Y. Alteration of Relative Rates of Biodegradation and Regeneration of Cervical Spine Cartilage through the Restoration of Arterial Blood Flow Access to Rhomboid Fossa: A Hypothesis. Polymers 2021, 13, 4248. [Google Scholar] [CrossRef]
- Mahmoud, M.; Kokozidou, M.; Auffarth, A.; Schulze-Tanzil, G. The Relationship between Diabetes Mellitus Type II and Intervertebral Disc Degeneration in Diabetic Rodent Models: A Systematic and Comprehensive Review. Cells 2020, 9, 2208. [Google Scholar] [CrossRef]
- Vetcher, A.A.; Zhukov, K.V.; Gasparuan, B.A.; Shishonin, A.Y. The cerebellum role in arterial hypertension. Med. Hypotheses 2022, 162, 110835. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W.H. Freeman: New York, NY, USA, 2008. [Google Scholar]
- Levit, S.; Giveon, S.; Philippov, Y.I.; Panchev Domuschiev, I.; Zivony, A. Type 2 diabetes therapeutic strategies: Why don’t we see the ELEPHANT in the room? Diabetes Mellit. 2016, 19, 341–349. [Google Scholar] [CrossRef]
- Shishonin, A. Method for Treating Cervical Osteochondrosis. Patent RF RU 2,243,758C2, 6 February 2003. [Google Scholar]
- Vetcher, A.A.; Zhukov, K.V.; Gasparuan, B.A.; Shishonin, A.Y. The cervical blood flow parameters with the best correlation from arterial blood pressure in hypertension cases. Int. J. Recent Sci. Res. 2021, 12, 42957–42958. [Google Scholar]
- Gloy, V.; McLennan, S.; Rinderknecht, M.; Ley, B.; Meier, B.; Driessen, S.; Gervasoni, P.; Hirschel, B.; Benkert, P.; Gilles, I.; et al. Uncertainties about the need for ethics approval in Switzerland: A mixed-methods study. Swiss Med. Wkly. 2020, 150, w20318. [Google Scholar] [CrossRef]
- Talantov, P.; Niyazov, R.; Viryasova, G.; Dranitsyna, M.; Yasny, I. Unapproved clinical trials in Russia: Exception or norm? BMC Med. Ethics 2021, 22, 46. [Google Scholar] [CrossRef]
- Beltrame, R.T.; Littig, L.B.; Covre, C.; Martins, A.B.; Quirino, C.R.; Costa, R.L.D. Automatic and manual Doppler velocimetry measurements of the uterine artery in pregnant ewes. Anim. Reprod. Sci. 2017, 181, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Curtelin, D.; Morales-Alamo, D.; Torres-Peralta, R.; Rasmussen, P.; Martin-Rincon, M.; Perez-Valera, M.; Siebenmann, C.; Perez-Suarez, I.; Cherouveim, E.; Sheel, A.W.; et al. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb Blood Flow Metab. 2018, 38, 136–150. [Google Scholar] [CrossRef] [PubMed]
- He, Z.B.; Lv, Y.K.; Li, H.; Yao, Q.; Wang, K.M.; Song, X.G.; Wu, Z.J.; Qin, X. Atlantoaxial Misalignment Causes High Blood Pressure in Rats: A Novel Hypertension Model. Biomed. Res. Int. 2017, 2017, 5986957. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Hyperchloremic normal gap metabolic acidosis. Minerva Endocrinol. 2019, 44, 363–377. [Google Scholar] [CrossRef]
- Regolisti, G.; Fani, F.; Antoniotti, R.; Castellano, G.; Cremaschi, E.; Greco, P.; Parenti, E.; Morabito, S.; Sabatino, A.; Fiaccadori, E. Metabolic acidosis. G. Ital. Nefrol. 2016, 33, 2. [Google Scholar]
- Sajan, A.; Horowitz, J.; Murakami, N.; McFarlane, I.M. Recurrent Anion Gap Metabolic Acidosis. Am. J. Med. Case Rep. 2019, 7, 200–202. [Google Scholar] [CrossRef] [PubMed]


| Parameter | M | F |
|---|---|---|
| Sample size | 19 | 29 |
| Age, years | 63.1± 11.7 | 65.5 ± 12.2 |
| BP before treatment PAB, torr | 159.5 ± 18.3 | 163.5 ± 17.9 |
| BP after treatment PAA, torr | 132.3 ± 19.2 | 131.7 ± 16.6 |
| Changes in BP, torr | −27.2 ± 10.1 | −31.7 ± 11.3 |
| Positive changes in BP | 19 | 29 |
| Critical values for α = 0.005 | 32 | 100 |
| PS before treatment PAB, cm/s | 22.5 ± 8.1 | 21.9 ± 9.3 |
| PS after treatment PAA, cm/s | 41.7 ± 6.7 | 43.2 ± 7.4 |
| Changes in PS, cm/s | 19.2 ± 7.7 | 21.3 ± 7.1 |
| Positive changes in PS | 19 | 29 |
| Critical values for α = 0.005 | 32 | 100 |
| HbA1c before treatment, % | 6.03 ± 0.34 | 6.11 ± 0.45 |
| HbA1c after treatment, % | 5.7 ± 0.63 | 5.73 ± 0.51 |
| Changes in HbA1c, % | −0.33 ± 0.58 | −0.38 ± 0.42 |
| Positive changes in HbA1c | 17 | 24 |
| Negative changes in HbA1c (rank) | 0 | 1 (1) |
| Actual sample size | 17 | 25 |
| Critical values for α = 0.005 | 23 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vetcher, A.A.; Zhukov, K.V.; Gasparyan, B.A.; Shishonin, A.Y. Hypothetical Reason for the Restoration of HbA1c Level for Pre-Diabetic Patients through the Recovery of Arterial Blood Flow Access to Rhomboid Fossa. Diabetology 2022, 3, 470-476. https://doi.org/10.3390/diabetology3030035
Vetcher AA, Zhukov KV, Gasparyan BA, Shishonin AY. Hypothetical Reason for the Restoration of HbA1c Level for Pre-Diabetic Patients through the Recovery of Arterial Blood Flow Access to Rhomboid Fossa. Diabetology. 2022; 3(3):470-476. https://doi.org/10.3390/diabetology3030035
Chicago/Turabian StyleVetcher, Alexandre A., Kirill V. Zhukov, Bagrat A. Gasparyan, and Alexander Y. Shishonin. 2022. "Hypothetical Reason for the Restoration of HbA1c Level for Pre-Diabetic Patients through the Recovery of Arterial Blood Flow Access to Rhomboid Fossa" Diabetology 3, no. 3: 470-476. https://doi.org/10.3390/diabetology3030035
APA StyleVetcher, A. A., Zhukov, K. V., Gasparyan, B. A., & Shishonin, A. Y. (2022). Hypothetical Reason for the Restoration of HbA1c Level for Pre-Diabetic Patients through the Recovery of Arterial Blood Flow Access to Rhomboid Fossa. Diabetology, 3(3), 470-476. https://doi.org/10.3390/diabetology3030035