1. Introduction
Diabetes mellitus (DM) is currently affecting 537 million people worldwide, and its already high prevalence, nearly 10%, is continuously increasing, especially in developing countries. Despite their different pathogenic mechanisms, both type 1 and type 2 DM show similar complications, among which diabetic foot syndrome is one of the most severe complications of diabetes and the most common cause of hospitalization in diabetic patients. Patients with diabetic foot syndrome have a risk of developing diabetic foot ulcers (DFU) up to 25%, and too often, DFU entails limb amputation. Further, diabetic foot complications are associated with a higher mortality rate than diabetes alone. Thus, diabetic foot syndrome is associated with very high public health and economic burdens, a very relevant impact on the quality of life for patients and their families, and can represent a major burden for healthcare professionals and institutions [
1].
On average, the prevalence of DFU in people with diabetes reaches 6.3% worldwide; in Italy, it is in the range of 5.4–6.2% and raises to 20% in patients over 75 years [
2]. According to the International Working Group on the Diabetic Foot (IWGDF) [
3], therapeutic footwear is effective for primary and secondary ulcer prevention, even though its efficacy is often limited by poor adherence [
4], despite valuable randomized controlled trials proving the effectiveness of offloading and ulcer healing techniques [
5].
A 2020 systematic review [
6] synthesized the following concepts: neuropathic DFU, which occurs mostly at the plantar forefoot and is very often associated with areas of peak plantar pressure; limited joint mobility, which likely contributes to the observed forefoot peak pressures. Furthermore, plantar pressure patterns are used to guide footwear and insole selections, adaptation and manufacturing, and to assess their effectiveness. Lowering plantar pressures represents a key factor for wound healing and ulcer prevention, where footwear and insoles are essential treatments for offloading pressures, with the desired reduction of dynamic in-shoe plantar pressure being >30% of the baseline or <200 kPa at the forefoot [
6].
Despite, cautiously, the recommended thresholds in [
6] should be intended as referred to specific measurement instrumentation and setup [
7], the key-message of redistributing plantar pressure has a general validity.
The standard of care (SoC) for managing diabetic foot plantar pressures is represented by customized insoles [
8,
9], which exploit soft, accommodating material in the shape of open and closed cell foams, for example, polyurethane foam, to absorb compressive stress. These insoles, which are usually covered with a weight-bearing surface congruent with the patient’s foot, have been reported as mechanically effective, even in the presence of active ulcers [
8]. The SoC insoles’ manufacturing process often requires hand craftsmanship, which may entail variability in the insole’s design and therapeutic impact [
9]. Those fabrication processes, which in some cases have been judged as inefficient and outdated, also heavily rely on clinical expertise and manual post-manufacturing adjustments (i.e., the creation of a manufactured depression, insertion of low-density foam disks, material removal from the base of the insole) to accommodate various patient-specific conditions and to ensure a proper fit to the patient’s foot and shoes [
10]. Further, they likely require more than one visit at the outpatients’ service, eventual additional visits at the manufacturer, and quite long delivery times.
Advances in 3D-printing, materials science and software seem promising for significantly improving the offloading performance of the insoles and to optimize the whole fabrication process. While, in fact, the SoC insoles for the diabetic foot, though customized to the patient-specific geometry and conditions, rely on generic material properties, the 3D-printing approach is based on the concept of personalized materials to also address patient-specific stiffness and structural behavior [
10,
11]. Of course, biocompatible materials are mandatory for manufacturing 3D-printed insoles, which are intended to come in touch with human skin. As such, among the several printing techniques and materials, a suitable solution appears in the use of the fused deposition molding (FDM) technique to print polylactic acid (PLA) filaments. PLA is, in fact, a biodegradable thermoplastic polyester derived from natural resources that complies with the required biocompatibility and can be printed at a low temperature. A wide discussion on 3D-printing techniques and materials for constructing plastic materials can be found in Nguyen et al. [
12], where infill printing and mechanical properties of PLA-printed models were investigated in detail, with a focus on investment casting. Another relevant, constructive issue to take into consideration when using the FMD technique and PLA material is the warpage of the model, which may occur in large-size models, as in the case of insoles. Huynh et al. [
13] addressed this issue and showed that warpage can be reduced after consideration of the thermal effects and adhesion force.
Evidence from robust clinical studies is still lacking in the literature; however, pioneering papers confirm the mandatory safety and the potential effectiveness of personalized materials and graded stiffness and of the overall innovative approach, either specifically for diabetic foot management or with reference to other foot pathologies, such as a flat foot or the high-arched foot [
8,
9,
10,
11,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28]. A more detailed digression of the relevant literature is reported in the Discussion section.
Despite technological advances, the prescription of 3D-printed insoles for diabetic foot syndrome is not yet reimbursed by the Italian public healthcare service. However, a decree by the Italian Minister of Health has been recently (April 2023) approved by the State-Regions Conference, which establishes that the insoles for diabetic feet fabricated by using 3D-printing technologies, will also be reimbursable by the Public Healthcare Service, similarly to the SoC insoles. The updated document is expected to come officially into force in April 2024 [
29].
Within the Italian project DIAPASON, regarding an innovative, multidimensional model of care for diabetic foot complications in very old people with diabetes and neuropathy, an ongoing pilot study in territorial healthcare facilities is exploring the feasibility and the potentiality of a novel insole fabrication process. Briefly, the process, which is based on a patented process for 3D personalized insoles modeling and manufacturing (Patents EP3916346A4, IT201900006076A1, IT201800010667A1) [
11], has been integrated with measurements from a consolidated in-shoe pressure assessment [
7], and from the patient’s functional and behavioral assessment. In agreement with the diabetic foot-related issues listed in this introduction, the focus of the DIAPASON investigation was on: the appropriateness and safety of 3D-printed insoles for diabetic foot management; the feasibility of a novel 3D-printing-based workflow in an ambulatory setting; its portability in primary care settings, long-term care facilities or other community settings; the possible clinical relevance of the custom 3D-printed insoles; and the overall impact of the entire manufacturing process and its refundability by the Italian National Healthcare Service. Prior to the pilot implementation, which involved 20 outpatients aged 75 and over and one younger patient who volunteered as the first on-the-field tester, the present paper reports on the single case study of this younger patient as a vehicle to explore the core topics of the DIAPASON investigation.
Outline of the Paper
The present paper is articulated according to the conventional paper sections, namely the Introduction, Materials and Methods, Results, Discussion and Conclusions.
The Materials and Methods section addresses three main topics, namely: the context, scope and main objectives of the DIAPASON Project, i.e., the project within which the case study has been designed and executed; the fabrication process of the 3D-printed insoles, based on the 3D-printing workflow of a patented process integrated with new elements purposely developed within the DIAPASON Project; and relevant information on the case study.
The Results section is articulated in three paragraphs that report on the main outcomes of the instrumental and functional assessment of the case study’s volunteers on Visit 1, whose suitable subset was sent to the 3D-insoles manufacturer; the fabrication process of the custom 3D insoles for the volunteers; the testing of the custom 3D insoles at Visit 2; and the data analysis and interpretation of the case study’s main outcomes.
The Discussion section is articulated in the following three paragraphs: a brief digression on the relevant recent literature on the use of 3D-printed insoles for diabetic foot management and for other foot pathologies; the interpretation and potential impact of the case study’s outcomes, dealing with the outcomes impact within the DIAPASON project and in the clinics, the portability of the reported solution, and its possible refundability within the Italian National Health Service; and a brief discussion of the main limitations of the study.
Finally, the Conclusions section summarizes the impact of the presented case study, in general, and in the peculiar Italian scenario.
2. Materials and Methods
2.1. The DIAPASON Project
The project proposal won an internal application for a small grant from the Italian National Institute of Health (ISS) as a two-year experimental pilot project, running from July 2021 to July 2023. The full name of the project is “DIAPASON: DIAbetic PAtients Safe ambulatiON. Enhancing resilience of very old patients with Diabetes and Neuropathy to maintain safe ambulation: an innovative multidimensional care model integrating new orthotics technology and new metabolic biomarkers”. The research topic of the new metabolic biomarkers is outside the scope of this paper and will no longer be discussed. To address the topic of the novel care model exploiting new orthotics technology, ISS has been working in collaboration with the territorial primary care premises of the ASL ROMA2 Lazio Region healthcare service and with the Medere s.r.l. Company (Rome, Italy)—the owner of the patented process. The following key topics of the project were addressed:
Integration of the ISS instrumental assessment protocol [
7] with the existing diabetic foot care processes (foot screening, footwear prescription, manufacturing, testing, approval and reimbursement). The protocol, as detailed in [
7], is based on the in-shoe pressure assessment measurement based on the Pedar-X system (novel
GmbH, Munich, Germany) and on the ad hoc risk thresholds, which are slightly more restrictive than the 200 kPa threshold [
6,
7]. Three additional functional tests have been integrated into the DIAPASON protocol namely the HHD (Hand-Held Dynamometry), the HRT (Heel Raise Test) and the TUG (Timed-Up-and-Go test) [
30,
31,
32];
Use, feasibility and optimization of the patented process to deliver 3D-printed personalized insoles; the insoles were meant to be inserted in proper, pre-selected home shoes to implement foot care prevention at home;
Assessment of the appropriateness of the 3D-printed personalized insoles, especially in terms of safety and effectiveness;
Information, education and enhanced motivation of patients and caregivers to reach higher adherence to the foot care interventions.
The DIAPASON project could be implemented on the basis of a scientific agreement already in force between ISS and ASL ROMA2 and of the ethical approval provided by the ASL ROMA2 Committee in 2019 and renewed in 2022 (ASL ROMA2 Resolution, number 1948 (20 September 2019); ASL ROMA2 Resolution, number 1570 (18 October 2022)). All study documents and actions were prepared and conducted in compliance with the Helsinki Declaration and with the GDPR (General Data Protection Regulation). For the DIAPASON pilot study (which is still ongoing and will be reported in future publications), twenty people of 75 years or more with type 2 diabetes and neuropathy were enrolled among those referring to the outpatients’ Diabetic Foot Service of the ASL ROMA2 territorial facilities. Due to their foot complications (either in primary or secondary prevention), they were already in charge of the Diabetic Foot Service; thus, they were informed of the study (together with their relatives or caregivers) when they came to the service for their periodic examination. For those who agreed to participate, the clinical history was reviewed and completed so as to assess the presence of possible serious exclusion reasons, among which, scheduled hospitalization for any reason, locomotion prevented also at home, hostile approach towards using the prescribed footwear, home-shoes and the 3D-printed insoles. Written informed consent was gathered from those who agreed to participate. They did not receive any compensation. Two of them refused to participate since they were not available to wear prescribed footwear and insoles. Two of them abandoned the study for other complications requiring long hospitalizations. Those patients were highly representative of the people leaving with diabetes in the ASL ROMA2 healthcare district since up to 20% of people with type 2 diabetes and more than 50% of people with type 2 diabetes and neuropathy in the district have age and main clinical features comparable with the enrolled patients. The sample size was established on the basis of previous knowledge about ulcer rates and injuries from falls (20% and 45%, respectively, in the population from which we extracted our sample over a 12-month period), and also taking into consideration the project scheduling (12 months of intervention and follow-up) and resources (human resources and a budget for assessment and for 3D insoles fabrication). The sample size was adequate to detect an improvement in the two mentioned clinical outcomes of ≥6% (G*Power 3.1.9.7, α = 0.05, 1 − β = 0.8).
One younger patient was also enrolled to volunteer as the first ambulatory tester, whose only difference from the DIAPASON sample consisted of her age (50 years old). She received the information on the study and signed the informed consent. While the characterization of the DIAPASON sample has been detailed here to introduce the aim and the clinical scenario that also originated the case study, the outcomes associated with the 20 patients will not be further mentioned in the present paper, which only focuses on the outcomes from the younger volunteer.
2.2. The 3D Insoles Fabrication Process
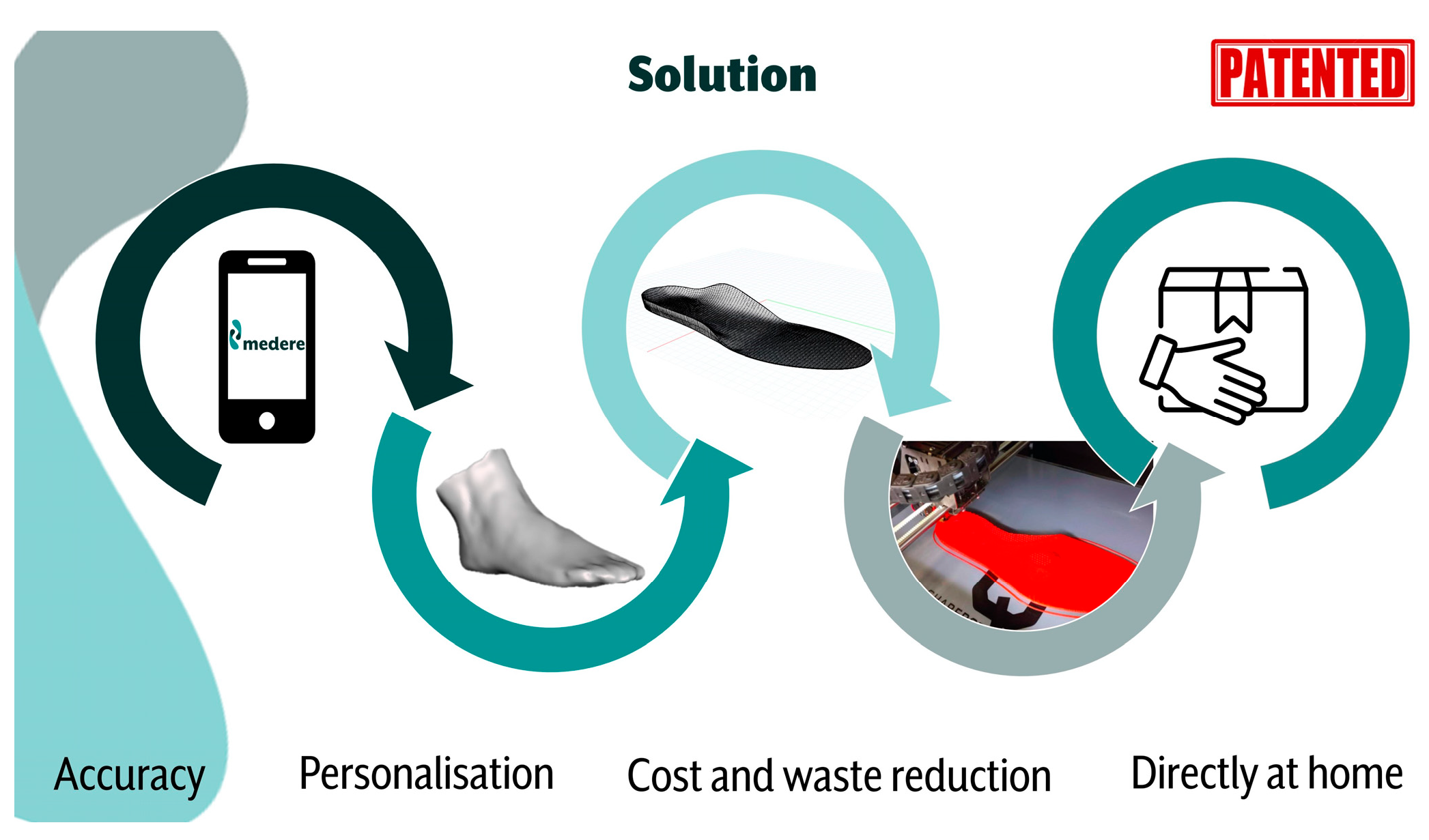
The Medere patented process (
Figure 1) allows the collection of the patient’s anatomical data by means of a proprietary App (Medere) available for both IOS and Android mobile operating systems. At regimen, healthcare professionals can be easily trained to use the App. During the DIAPASON project, to ensure procedure repeatability, the PI (Principal Investigator) of the DIAPASON project acted as the measurements manager and collected the data in person. The anatomical data acquisition includes the use of the smartphone to 3D scan the foot and to acquire a sequence of foot images under loaded and unloaded predefined conditions (unilateral half-loaded internal sagittal view; unilateral half-loaded internal sagittal view with maximum dorsi-flexion of the 1st metatarso-phalangeal joint; bilateral frontal view with joined heels; bilateral rear view with parallel feet). The App also asks for basic anthropometrics (height and body mass) and a picture and some details of the shoes where the insoles should be inserted; in the DIAPASON project, this section was integrated with additional accurate measurements of the home-shoes previously selected to host the 3D insoles and of their commercial insoles to replace. Further, additional information was also delivered to Medere to optimize the modeling process, among which:
plantar pressure measurements: at least 12 at regimen footprints for each foot were extracted from the three repetitions of the TUG and averaged to calculate the map of the peak pressures and the map of the pressure impulses (dedicated novel software packages by novelGmbH, Munich, Germany; OriginPro 2022, OriginLab Corp, Northampton, MA, USA);
anthropometric asymmetry, if present (especially in case of clinically relevant asymmetry in the lower limb length);
semi-quantitative (normal; reduced; increased) joint mobility and muscle performance, based on video recordings during HHD, unloaded HRT and loaded HRT, and on force measurements by the wireless Biometrics Myometer (Biometrics Ltd., Nine Mile Point Industrial Estate, Ynysddu, UK) during MTT; video semi-quantitative analysis was done by using Kinovea 0.8.15 tools (Joan Charmant developer;
https://www.kinovea.org/) (2 July 2023)
history of falls and ulcers;
behavioral information about daily activity and habits.
Following all those data acquisitions, Medere then proceeded to model the insole according to the patented workflow, as summarized in the following steps:
The video and image datasets are used to accurately reconstruct the anatomy of the feet. The second stage involves the automatic creation of the 3D model of the foot using a photogrammetric algorithm based on the Structure From Motion (SFM) technique. This computer vision-based approach, validated as reliable and accurate in numerous fields (e.g., geosciences, cultural heritage, digital object reconstruction), significantly reduces both the modeling time and material waste. The algorithm works via SIFT (scale-invariant characteristic transform) and SURF (accelerated robust transform) to accurately identify foot geometries and their relative orientation in space. A point cloud is generated and finally converted into a mesh object. Dedicated filtering procedures are implemented to reduce inaccuracies when detected.
The creation of the 3D model of the footbed is then performed using the computer-aided drafting (CAD) modeling technique. CAD modeling is the gold standard tool for prototype creation and optimization. This technique provides a high level of customization since, following the guidelines of a clinician, it is possible to modify the personalized footplate model to achieve the desired result. The method validated and patented by Medere consists of the creation of a starting model based on the patient’s shoe geometry to create the best-fitting outline. A few transversal lines are then added following each foot geometry and modified to create the final plantar surface of the custom-made insoles. A more detailed description can be found in the patents [
33,
34,
35]. The final model is then divided into regions to be printed with different density and mechanical properties.
In the final stage of the process, insoles are produced using an additive manufacturing process (3D printing) based on the fused material deposition approach (FDM). FDM is a method that allows users to make almost any type of design while optimizing material waste with respect to standard production methods. The inner part of the insoles has an internal structure (infill) that can be adjusted and modified. Changing the geometric characteristics of the filler has a direct impact on the properties of the insoles and the mechanical behavior of the final object. Different shapes and densities of internal structures are used to maximize the required mechanical response (e.g., shock absorption and the required level of elasticity).
The plantar surface of the insoles is covered with antibacterial ethylene vinyl acetate (EVA) sheet with a Shore A 35 to maximize smoothness and reduce the friction of the foot.
Within the DIAPASON project, the model was further refined before printing on the basis of the additional information (pressure maps, destination home shoes, patient’s clinical history and behavioural habits, and any other relevant information) (
Figure 2).
Once manufactured, the 3D insoles were shipped to the ASL ROMA2 ambulatory, where the patient underwent the same instrumental assessment protocol as for the SoC insoles testing. Approval of the 3D insoles was thus based on a visual examination by the reference diabetologist and by the expert health professional who managed the patient’s whole screening and assessment on instrumental testing outcomes (pressure patterns within the acceptable ranges, acceptable stability and balance during the TUG test), and on feedback from the patient. In case of criticalities, similar to the SoC procedure, the 3D insoles were sent back to the manufacturer for remodeling and reprinting.
2.3. The Case Study
The woman who first volunteered for the feasibility study was younger with respect to the 20 enrolled patients of the DIAPASON Project and had the following clinical, biological and behavioral features collected during the anamnesis and the podiatric screening:
The patient was a woman;
50 years old, 1.62 m, 85 kg;
Mild obesity (BMI: 32.4 kg·m−2);
Type 2 diabetes mellitus (first diagnosed in 2019);
Neuropathy: Vibration Perception Threshold (VPT) > 25 V; Michigan Neuropathy Score Index (MNSI) = 6 (normal reference < 1);
No peripheral arterial disease (ankle–brachial index (ABI) > 0.90); with
Normal vascular stiffness and peripheral pulses;
Diabetic foot disease in primary prevention (no history of DFU);
Bilateral flatfoot, hallux valgus and overlapping toes;
Self-reported imbalance and postural instability but no history of falls;
Left ankle osteotomy for Achilles tendon pain in 2018; the same problem is currently suspected for the right ankle;
Acquired hypothyroidism since 2019 (treated with radiometabolic therapy for thyrotoxicosis);
No history of smoking;
No regular sports activity practiced;
Needs to walk and stand for daily activities (housework, job, family).
3. The Case Study Results
3.1. Instrumental Assessment and Data Collection at Visit 1
On Visit 1, after the clinical anamnesis and the podiatry screening (the most relevant data are summarized in
Section 2.3), the woman underwent the following protocol:
HHD at each foot, under a maximum push-and-pull task against the resistance of the trained healthcare professional, three repetitions each, with the patient supine and the feet perpendicular to the ground (the force data were acquired by using the Biometrics Myometer and a video recording in the sagittal view,
Figure 3). The maximum right push-and-pull reached 8.6 kg (10.1% of body mass) and 6.4 kg, respectively; the maximum left push-and-pull reached 7.2 kg (8.5% of body mass) and 5.6 kg, respectively;
Barefoot standing for 10 s (the pressure data were from Pedar-X; wide insoles’ size VW; the video recordings (webcams) were taken in the rear and sagittal views,
Figure 4a); “barefoot” means wearing special socks purposely hand-made to host the Pedar insoles and to keep them solid with the foot, and to fix three markers roughly on the fifth metatarsal head, the lateral malleolus and along the ideal line joining the lateral malleolus with the head of the fibula. During barefoot standing, the rearfoot resulted more loaded than the forefoot, and the right more than the left, with a maximum average pressure of 245 kPa;
The HRT (barefoot condition) of both feet, simultaneously while sitting (unloaded conditions), included 10 consistent repetitions (the pressure data were from Pedar-X; the video recordings (webcams) were taken in the rear and sagittal views,
Figure 4b). The task lasted 15 s and showed the simultaneous raising of the heels, with greater force on the left and at the central repetitions;
The HRT (barefoot condition) of both feet, simultaneously while standing (loaded conditions), included 10 consistent repetitions (the pressure data were from Pedar-X; the video recordings (webcams) were taken in the rear and sagittal views,
Figure 4c). The task lasted 26 s (73% longer than the HRT while sitting) and showed asynchronous and variable raising patterns of the two heels, with comparable force, higher at the central repetitions;
The TUG test (barefoot condition), comprising three repetitions (the pressure data were from Pedar-X; the video recordings (webcams) were taken in the rear and sagittal views,
Figure 5). The total task lasted 44.4 s, and the average duration of each repetition was 14.1 s; maximum peak pressures of >200 kPa were found: at the heel (638 kPa) and hallux (238 kPa) on the left foot; at the heel (465 kPa), midfoot (280 kPa), forefoot (275 kPa) and hallux (285 kPa) on the right foot;
Video recordings, alternatively, of the right and the left foot while sitting with the foot perpendicular to the ground and the hallux dorsiflexed were recorded. (the video acquired through the Medere app on an iPhone 8,
Figure 6a,b);
The sequence of pictures of the barefoot feet is described in paragraph 2.2 (the pictures were acquired through the Medere app on an iPhone 8,
Figure 6c–h).
The whole ambulatory visit lasted roughly one hour, during which one expert health professional and one engineer worked to collect all the needed data and information.
At the end of the instrumental assessment, the diabetologist was involved in preliminary data analyses and, together with the health professional, authorized and started the consolidated prescription procedure (SoC shoes and insoles) and identified the off-the-shelf home shoe to host the 3D insoles (MAC2 Fanny home shoe, (Optima Molliter s.rl., Civitanova Marche, Italy), an MD-certified shoe for the prevention of diabetic foot complications). The selection was conducted among a certain number of MD-certified home shoes made available at the healthcare premises for the DIAPASON feasibility study and whose commercial standard insoles had been previously scanned in the sizes from EU 36 to EU 45. The final decision was taken after the volunteer’s agreement.
The data collected through the Medere app were immediately sent to Medere; some additional processing time was required to prepare the synthesis of the additional data and information.
3.2. 3D Insoles Fabrication Process
The 3D-insoles manufacturer received the data collected through the Medere app and the following additional information to model the insoles:
A woman, 50 years, 1.62 m, 85 kg;
Type 2 DM with neuropathy;
Bilateral flatfoot, hallux valgus and overlapping toes;
Achilles tendon pain at right (solved at left after ankle osteotomy);
Active at home (walking and standing);
Self-reported unbalance and postural instability;
Ankle joint mobility was slightly reduced when unloaded and compromised when loaded;
Ankle dorsi-flexion was slightly weaker than plantarflexion; the left leg was slightly weaker than the right;
Barefoot pronation and hallux scarcely loaded during gait;
Very high plantar pressure (peaks and impulses) at the hindfoot, more on the right foot; abnormally high pressures on the left foot; unexpected offload of the forefoot (
Figure 7, containing peak pressure maps and pressure impulse maps);
The selected home shoe was a Molliter Fanny, EU size 38 (the technical details of the shoe and insole were already available in the Medere DIAPASON database).
Based on the received data, Medere modeled the insoles based on the patented process (as previously described) and integrated the received additional information.
The model was created to maximize the contact between the plantar surface of the insole and the foot of the patient, with the aim of distributing the pressure evenly across the foot and increasing the level of stability. The arch support was modeled to reduce barefoot pronation.
The foot pressure map was aligned with the CAD model of the insoles to identify the area of interest (e.g., peak pressure regions) and to subdivide the insoles into different parts. Using a parametrization method based on the anthropometric and pressure data, the density of the different parts of the insoles was calculated. The initial step was to calculate the density of the main part and then the density of the parts where the pressure needed to be reduced.
The model was imported into a slicing software (Simplify3D V4.1.2—Simplify3D, LLC.). The parts were aligned and positioned on the printing bed surface, and the previously calculated parameters for the density were assigned to each part. For this case study, the regions of max pressure were identified in correspondence with the heels only. Therefore, the model was divided into two parts, which were printed with the following parameters:
A flexible filament with Shore A 82 was used for the printing process (Filaflex82A-RECREUS INDUSTRIES S.L., Elda (Alicante) Spain). The insoles were then covered with EVA Shore A 35 sheet.
The 3D insoles were then shipped to the project PI, who preliminarily checked them for congruency and alerted the ASL ROMA2 ambulatory of Visit 2 for the volunteer.
3.3. 3D-Insoles Testing at Visit 2
The whole 3D fabrication process took one week (standard fabrication time, no priority was asked to the Manufacturer). Three weeks were however needed to have the SoC footwear ready and to allow the volunteer to go to the shoemaker to gather them. During visit 2 both products were tested, to optimise the number of visits.
The instrumental assessment protocol at visit 2 was much simpler than visit 1, and only consisted of the following steps (testing of the SoC footwear, very similar to this, is not reported since it falls outside the scope of this paper):
With the home shoes and their commercial insoles: standing for 10 s and three repetitions of the TUG test (the pressure data were from Pedar-X; wide insoles size VW; the video recordings (webcams) were taken in the rear and sagittal views,
Figure 8a). The TUG test lasted 41.7 s in total (barefoot total duration: 44.4 s); the mean TUG duration was 13.2 s (barefoot mean TUG duration: 14.1 s);
With the home shoes and the 3D-printed personalized insoles: standing for 10 s and three repetitions of the TUG test (the pressure data were from Pedar-X; wide insoles size VW; the video recordings (webcams) were taken in the rear and sagittal views,
Figure 8b). The TUG test lasted 38.9 s in total, and the mean TUG duration was 12.5 s.
The pressure data were processed to investigate the appropriateness of the 3D insoles with respect to the risk thresholds and gait balance (only data from at-regimen footprints) and their eventual advantage with respect to the commercial accommodative insoles of the home shoes. Briefly: the peak pressures were greatly reduced by the home shoes and their commercial insoles with respect to the barefoot conditions, with the maximum peak at the right hindfoot being reduced by roughly 45% (
Figure 8a); the 3D-printed personalized insoles, together with the home shoes, performed even better, with the maximum peak pressure at the right hindfoot—the only remaining area with pressures above the 200 kPa threshold—reduced by >50% (
Figure 8b). The overall assessment, including visual examinations from the clinician and health professional, feedback from the volunteer, comparisons with Visit 1’s barefoot assessment and a review of the video recordings, fetched a positive evaluation of the 3D insoles.
4. Discussion
4.1. 3D-Printed Insoles: Feedback from the Recent Literature
Advances in 3D technology may represent a promising, significant improvement in the optimization of the therapeutic performance of plantar orthoses.
In the specific field of diabetic foot care, robust clinical studies are still needed to provide evidence either of a comparable clinical efficacy of 3D-printed insoles, with respect to custom conventional insoles (SoC solutions), or even of their superiority. However, several preliminary studies have reported on the potentialities of 3D-printed insoles for diabetic foot management or with respect to other relevant foot and musculoskeletal pathologies.
Zuniga et al. [
15] reported a proof-of-concept of 3D-printed insoles for patients with diabetes and tested it on one volunteer only. Their developed 3D-printed insoles used two polymers, thermoplastic polyether-polyurethane and thermoplastic polyurethane polyester-based polymer, and they assessed their performance through measurements of plantar pressure distribution during walking. The two 3D-printed insoles performed as well as a standard insole, with no significant difference in the average peak pressures. They concluded that 3D-printed insoles have the potential for diabetic foot management and that the digital manufacturing workflow of customized insoles can be helpfully implemented in middle-income countries.
Chhikara et al. [
16] recently conducted a valuable review on the effectiveness of 3D-printed orthoses for diabetic foot management. They reported on the following human subject studies: Telfer et al. [
17] compared standard milled insoles with 3D-printed insoles in 20 patients with type 2 diabetes and proved that the former performed better than the latter in improving plantar offloading. Anggoro et al. [
18] conducted a preliminary study on the expectations and satisfaction of two patients with a long history of diabetes after having used the 3D-printed insoles for 6 weeks and obtained high satisfaction and expectation scores and overall satisfactory performance and good comfort. Tang et al. [
19] conducted an exploratory study on one healthy volunteer, and the supplied 3D-printed insoles showed an effective reduction in the peak plantar pressures (>33%) compared to SoC insoles. Hudak et al. [
10] enrolled one patient with diabetes to compare an SoC insole, a hybrid 3D-printed insole with a bi-laminate foam top, and a fully 3D-printed insole: the latter showed improved durability, reduced shear stiffness and lower plantar pressures. The authors of review [
16] concluded that: the available literature on development of 3D-printed orthosis for patients with Diabetes is still limited; more generally, the 3D-printed orthoses demonstrated equivalent performance in clinical aspect; 3D-printing process may bring to biomechanical changes in the foot, however further validation is required to confirm that these changes can indeed be associated with clinically relevant outcomes; additive manufacturing applied to the diabetic foot management may benefit of the integration with Finite Elements Analysis (FEA), biomechanical measurements and modelling; there is still a lack of orthoses for post-ulcer diabetic foot and 3D-printed insoles impact as an intervention against the foot ulcer progression is yet to be tested [
20]; patients with partial foot amputations can also be managed using 3D-printed partial foot orthosis [
21,
22].
Shaikh et al. [
23] conducted quite an extensive, experimental study involving 200 patients suffering from various foot-related problems and joint pain; 18 of them (38–69 years old) suffered from diabetic foot complications. Their 3D-printed insoles were designed using plantar pressure systems and a clinical practitioner’s assessment and, for patients with diabetes, also providing additional podiatry elements. In particular, diabetic 3D-insole fabrication exploited the slicing options, which allowed for variable density printing and the possibility to add elements for corn pressure relief, metatarsal bar and pads since the insole design phase. The insoles were tested under walking and other relevant motion tasks, with only two dropouts (with active ulcers and obesity) among patients with diabetes. The authors found that the custom 3D-printed insoles provided biomechanical correction whenever required, contributed to alleviating pain and relief from high peak pressures, and showed the potential of being long-lasting (still well-performing after 21 months for the patients who participated in the follow-up).
Daryabor et al. [
24] recently published a systematic review aimed at evaluating custom 3D-printed insoles for flat feet. As the main outcome of their narrative analysis, based on 10 studies, including 225 subjects with flexible flatfeet, the evidence from the literature was found to be weak; however, it emerged that using custom 3D-printed insoles may positively affect pain and foot function, with no significant change in the vertical loading rate during walking or running. However, the authors reported insufficient evidence to conclude the comparison between 3D-printed insoles and other types of insoles.
Xu et al. [
25] compared custom 3D-printed insoles with traditional prefabricated rehabilitation insoles in 80 patients with bilateral symptomatic flatfoot. After 8 weeks, their RCT showed that the 3D-printed insoles reduced the pressure on the metatarsals and redistributed it over the midfoot significantly more than the prefabricated insoles.
Jandova et al. [
26] showed that, in both flatfoot and high-arched feet, 3D-printed insoles perform as comparably well as traditional, customized insoles. The study relied on 51 adults, and comparisons were conducted on the basis of plantar pressure distribution. The authors concluded that in the case of a high-arched foot, where peak pressures are higher and more difficult to compensate for, 3D-printed insoles might reach even better results than traditional customized insoles.
Jin et al. [
27] tested a customized 3D-printed heel support insole on a sample of 30 healthy male participants. The authors found that the biomechanical properties of the customized 3D-printed heel support may be better than those of the traditional heel support insole, especially when there is a need for an additional increase in heel height. Their volunteers did not decrease midfoot motion function while using the insoles.
Prakotmongkol et al. [
28] compared custom 3D-printed insoles with regard to custom conventional insoles for flatfeet, focusing on foot and ankle function, navicular height, patient satisfaction and insole durability. Their RCT (60 patients in total) lasted for three months and revealed that the scores of foot and ankle functions and insole use significantly improved at three months in both the intervention and the control group; deformation of insoles was found in both groups with no significant difference between them, and durability and patient-reported satisfaction were significantly higher for the intervention group.
4.2. Interpretation and Potential Impact of the Case Study Outcomes
When dealing with patients, the adoption of this new technology mandatorily requires an investigation of the feasibility of the whole fabrication process, thus also including those phases of the workflow which mostly impact the patient, namely the acquisition of the input data, the identification of the most suitable shoes to use and/or adapt the final product testing.
The hereby reported case study showed that an integrated process to obtain 3D-printed personalized insoles for patients with diabetic foot disease may be feasible, safe and effective in delivering an appropriate offloading device while also optimizing the number and duration of the patient’s visits. Specifically, the case study was conducted by rigorously applying the procedure designed and approved within an Italian project, the DIAPASON project, where the SoC process for diabetic ulcer prevention in the territorial healthcare facilities of the ASL ROMA2 Regional Health Agency had been integrated (i) with an instrumental assessment of the in-shoe plantar pressure profile at the SoC footwear prescription and testing phases and (ii) with functional tests (HHD, HRT and TUG) at the prescription phase only. Additional data collection was required, specifically for the 3D fabrication process, consisting of a 3D scan of the foot and a sequence of foot images under pre-established loading conditions, all acquired by means of a dedicated app. Gathering a few models and sizes of home shoes marketed as MD-certified for the diabetic foot and scanning their commercial insoles (to be replaced by the 3D-printed personalized insoles), represented the remaining preliminary actions needed to complete the input dataset of the 3D workflow. Key findings from the case study may thus be summarized as follows: the 3D insoles fabrication process was feasible and required only two visits by the patient to the diabetic foot outpatient service (ambulatory setting): Visit 1 lasted about one hour and it involved one health professional and one technology expert to gather and process data, and one diabetologist to approve the data synthesis and home shoes selection, while Visit 2 lasted <20 min and involved the same personnel as Visit 1. The experimental setup for quantitative and semi-quantitative data collection was safe and adequate for the purpose, with no risks of adverse events for the volunteer.
In agreement with [
10], the proposed integrated fabrication process resulted in effectively optimizing the resources, delivery times and burden on the patient. According to the Italian SoC process, in fact, at least two additional visits are needed at the manufacturer’s premises: the first for the imprint collection and the shoe selection, and the second to test the insole fit with the patient’s foot and the selected shoe before the delivery for clinical testing.
Results of the case-study were well in agreement with Hudak et al [
10], who described a comparable approach to deliver 3D-fabricated personalized insoles for the diabetic foot. Differently from the foot data acquisition method used in the hereby described case-study (foot 3D scan and images), they started their fabrication workflow by scanning the foam crush box impression of the patient’s foot. However, to define the 3D model of the foot, they similarly collected the patient-specific plantar pressure distribution using the same in-shoe Pedar-X device. They defined the offloading regions using the 200 kPa threshold value [
6], and manually identified anatomical landmarks of the foot (heel and first and fifth metatarsal heads) to facilitate proper insole positioning and sizing. Finally, they modelled the insole to match the geometry of the patient’s scanned foot, and defined the offloading segments on the basis of the plantar pressure map. The reported results of the technical assessment showed a matched or improved durability, a reduced shear stiffness, and a reduction in plantar pressure of their 3D insoles compared to SoC insoles. Further, the Authors stressed the advantage of the new process when compared with the SoC one, where: manually performed modifications are usually done to the plaster model; layers of foam of different compositions are usually glued together; the pressure-relieving region is based on clinical judgments and obtained by adding material to the positive plantar model, or using disks of low-density foam, or by removing material from the base of the insole in the desired region; the insole is finally manually shaped to ensure proper fit to the patient’s foot and shoes; last but not least, the entire fabrication process typically requires patient visits over multiple days and may last for weeks.
Despite the fact that therapeutic personalized insoles based on 3D technology are not yet refundable in Italy, a regulatory official act already exists [
29], which is expected to enter into force by April 2024. The current approved budget for SoC personalized insoles reimbursement may be adequate for the 3D-printed personalized insoles. Of course, manufacturers should reorganize their workflow. In the case of a remote process, which is more convenient for the patient, it will be necessary that the clinical foot service reorganizes itself so as to acquire all the needed measurements and scans and to have a set of shoes and home shoes available to allow for on-site identification as the best solution for the specific patient. The reported case study, and the pilot study, which is ongoing within the DIAPASON project on very old and fragile patients, may thus result as valuable for the spread of 3D-printing techniques in the Italian scenario of diabetic foot management, and similar feasibility and experimental studies are needed and welcome.
The case study also allowed us to explore the portability of the proposed novel care model in other healthcare structures or even in long-term care facilities or community settings, so as to reach those fragile and disadvantaged patients who have serious limitations to reach dedicated labs, shoemaker settings or outpatient’s clinics. The assessment phases of the whole process showed the potential to be moved from the ambulatory to other local settings; however, this means that the portable equipment and the expert personnel shall move twice for each patient to reach the local setting. Reasonably, the process might become feasible and sustainable if groups of patients are scheduled for assessment on the same day.
4.3. Limitations of the Study
The outcomes of the reported case study, though encouraging, remain valid within the specific, adopted 3D fabrication process, including materials, algorithms and modeling features. The case study relied on a patented process integrated with a consolidated assessment protocol; however, to generalize the feasibility and effectiveness of 3D-printing technology with respect to diabetic foot management, many experimental studies are still needed. Insole effectiveness was tested with respect to plantar pressure distribution and the quantified outcomes of the functional tests addressing balance and force, as well as on visual inspection from an expert podiatrist and diabetologist and on the patient’s feedback; however, shear stress measurements would have completed the overall insole assessment. Further, the study did not contain information on the durability and follow-up outcomes; the patient is, however, followed, and the eventual relevant outcomes will be documented in future works.
Transferability of the results to other patients might represent another limitation. The volunteer in this case study was quite young (50 years old); however, all tasks and measurements had already been validated on cohorts of very old and fragile patients, and they had been found safe and feasible, even in the case of partially impaired patients, where the use of walking aids and the assistance from healthcare personnel was included (and properly accounted for) in the functional assessment.
5. Conclusions
Despite the limited evidence from the literature, custom 3D-printed insoles seem to have the potential for diabetic foot management, their effectiveness appearing as at least comparable or even greater than custom conventional insoles, with an expected longer durability. Further validation is, however, still required to confirm that these high-level performances can indeed be translated into clinically relevant outcomes.
The hereby reported case study proved the feasibility and safety of an integrated workflow, including in-shoe pressure measurements and the outcomes from functional tests to obtain 3D-printed insoles for patients with diabetic foot disease. The insoles were found effective in delivering the appropriate offloading while also optimizing the number and duration of the patient’s visits: specifically, only two visits at the diabetic foot outpatient service were needed, with no additional visits at the manufacturer’s premises (as it happens in the case of SoC insoles manufacturing). The workflow, successfully tested during the case study, can thus be used to implement a pilot study—the ongoing DIAPASON project—at the territorial healthcare facilities of the Italian ASL ROMA2 Regional Health Agency, dealing with very old patients with type 2 diabetes and foot complications. The workflow also has the potential to be used in long-term care facilities or community settings, with an expected relevant impact on diabetic foot management, the burden of care for fragile or disadvantaged patients and healthcare resources. The essential requirement is, of course, that the portable equipment and expert, trained personnel are available to reach local settings twice for each patient. Italian legislation has already officially approved the reimbursement of 3D-printed insoles for diabetic foot management; the law is expected to enter into force within the spring of 2024.