Real-World Life Analysis of a Continuous Glucose Monitoring and Smart Insulin Pen System in Type 1 Diabetes: A Cohort Study
Abstract
:1. Background
1.1. Aim
1.1.1. Primary Objective
1.1.2. Secondary Objectives
2. Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Sample and Criteria
2.4. Endpoints
2.5. Statistical Analysis
2.5.1. General Methodology
2.5.2. Data Analysis
2.5.3. Derived Variables
2.5.4. Handling of Missing Data and Outliers and Validation Requirements
3. Results
3.1. Baseline Characteristics
3.2. Time Study
3.3. Sensor Use
3.4. Glycemic Outcomes with Medtronic Smart MDI
3.5. Comparison Between Standard MDI and Medtronic Smart MDI
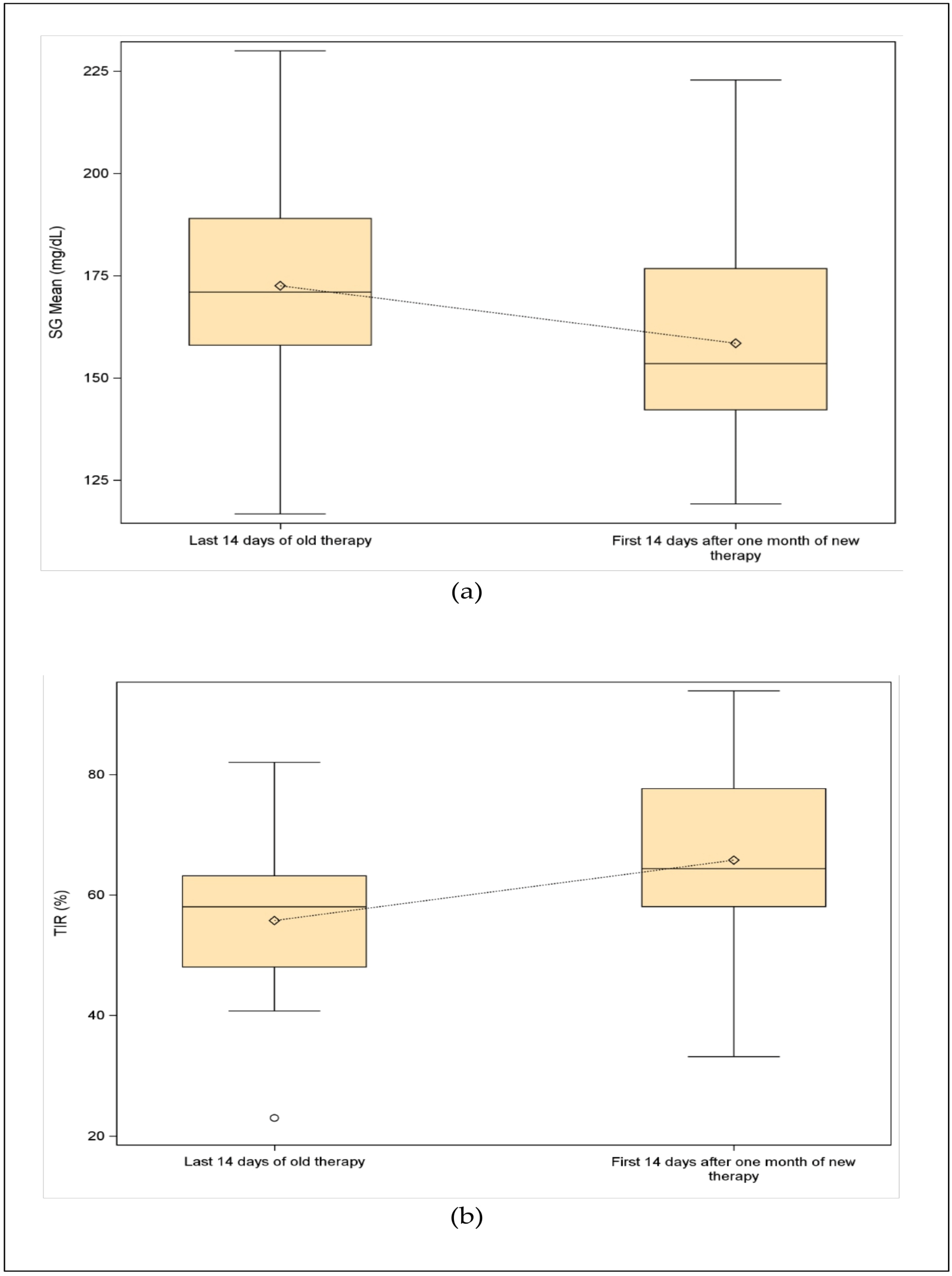
3.6. Boxplot Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Magliano, D.J.; Boyko, E.J. Committee IDFDAtes. IDF diabetes atlas. In IDF Diabetes Atlas; International Diabetes Feeration©: Brussels, Belgium, 2021; Volume 2021. [Google Scholar]
- Wong, N.D.; Sattar, N. Cardiovascular risk in diabetes mellitus: Epidemiology, assessment and prevention. Nat. Rev. Cardiol. 2023, 20, 685–695. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 25 November 2024).
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2021. Results. Institute for Health Metrics and Evaluation. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 1 November 2024).
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Diseases and Injuries Collaborators. Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Jacobsen, L.M.; Sherr, J.L.; Considine, E.; Chen, A.; Peeling, S.M.; Hulsmans, M.; Charleer, S.; Urazbayeva, M.; Tosur, M.; Alamarie, S.; et al. Utility and precision evidence of technology in the treatment of type 1 diabetes: A systematic review. Commun. Med. 2023, 3, 132. [Google Scholar] [CrossRef]
- Mallik, R.; Kar, P.; Mulder, H.; Krook, A. The future is here: An overview of technology in diabetes. Diabetologia 2024, 67, 2019–2026. [Google Scholar] [CrossRef]
- Handelsman, Y.; Hellman, R.; Lajara, R.; Roberts, V.L.; Rodbard, D.; Stec, C.; Unger, J. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons with Diabetes Mellitus. Endocr. Pract. 2021, 27, 505–537. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 7. Diabetes Technology: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S126–S144. [Google Scholar] [CrossRef]
- Jayedi, A.; Zargar, M.S.; Emadi, A.; Aune, D. Walking speed and the risk of type 2 diabetes: A systematic review and meta-analysis. Br. J. Sports Med. 2024, 58, 334–342. [Google Scholar] [CrossRef]
- Cao, L.; An, Y.; Liu, H.; Jiang, J.; Liu, W.; Zhou, Y.; Shi, M.; Dai, W.; Lv, Y.; Zhao, Y.; et al. Global epidemiology of type 2 diabetes in patients with NAFLD or MAFLD: A systematic review and meta-analysis. BMC Med. 2024, 22, 101. [Google Scholar] [CrossRef]
- Gregory, G.A.; Robinson, T.I.G.; Linklater, S.E.; Wang, F.; Colagiuri, S.; de Beaufort, C.; Donaghue, K.C.; International Diabetes Federation Diabetes Atlas Type 1 Diabetes in Adults Special Interest Group; Magliano, D.J.; Maniam, J.; et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: A modelling study. Lancet Diabetes Endocrinol. 2022, 10, 741–760. [Google Scholar] [CrossRef] [PubMed]
- Anandhakrishnan, A.; Hussain, S. Automating insulin delivery through pump and continuous glucose monitoring connectivity: Maximizing opportunities to improve outcomes. Diabetes Obes. Metab. 2024, 26, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Farhat, I.; Drishti, S.; Bochner, R.; Bargman, R. Do hybrid closed loop insulin pump systems improve glycemic control and reduce hospitalizations in poorly controlled type 1 diabetes? J. Pediatr. Endocrinol. Metab. 2024, 37, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Cangelosi, G.; Scuri, S.; Pantanetti, P.; Lavorgna, F.; Faldetta, F.; De Carolis, C.; Rocchi, R.; Debernardi, G.; Florescu, A.; et al. Diabetes and technology: A pilot study on the management of patients with insulin pumps during the COVID-19 pandemic. Diabetes Res. Clin. Pract. 2020, 169, 108481. [Google Scholar] [CrossRef]
- Petrovski, G.; Al Khalaf, F.; Campbell, J.; Umer, F.; Almajaly, D.; Hamdan, M.; Hussain, K. One-year experience of hybrid closed-loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: Drivers to successful outcomes. Acta Diabetol. 2021, 58, 207–213. [Google Scholar] [CrossRef]
- McAuley, S.A.; Lee, M.H.; Paldus, B.; Vogrin, S.; de Bock, M.I.; Abraham, M.B.; Bach, L.A.; Burt, M.G.; Cohen, N.D.; Colman, P.G.; et al. Australian JDRF Closed-Loop Research Group. Six Months of Hybrid Closed-Loop Versus Manual Insulin Delivery with Fingerprick Blood Glucose Monitoring in Adults With Type 1 Diabetes: A Randomized, Controlled Trial. Diabetes Care 2020, 43, 3024–3033. [Google Scholar] [CrossRef]
- Cobry, E.C.; Kanapka, L.G.; Cengiz, E.; Carria, L.; Ekhlaspour, L.; Buckingham, B.A.; Hood, K.; Hsu, L.J.; Messer, L.; iDCL Trial Research Group; et al. Health-Related Quality of Life and Treatment Satisfaction in Parents and Children with Type 1 Diabetes Using Closed-Loop Control. Diabetes Technol. Ther. 2021, 23, 401–409. [Google Scholar] [CrossRef]
- Benioudakis, E.; Karlafti, E.; Kalaitzaki, A.; Kaiafa, G.; Savopoulos, C.; Didangelos, T. Technological Developments and Quality of Life in Type 1 Diabetes Mellitus Patients: A Review of the Modern Insulin Analogues, Continuous Glucose Monitoring and Insulin Pump Therapy. Curr. Diabetes Rev. 2022, 18, e031121197657. [Google Scholar] [CrossRef]
- National Health Service (NHS) Digital. National Diabetes Audit 2021–22, Type 1 Diabetes—Overview. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-audit-type-1-diabetes/nda-type-1-2021-22-overview (accessed on 24 November 2024).
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef]
- Tejera-Pérez, C.; Chico, A.; Azriel-Mira, S.; Lardiés-Sánchez, B.; Gomez-Peralta, F. on behalf of the Área de Diabetes-SEEN. Connected Insulin Pens and Caps: An Expert’s Recommendation from the Area of Diabetes of the Spanish Endocrinology and Nutrition Society (SEEN). Diabetes Ther. 2023, 14, 1077–1091. [Google Scholar] [CrossRef]
- Nimri, R.; Nir, J.; Phillip, M. Insulin Pump Therapy. Am. J. Ther. 2020, 27, e30–e41. [Google Scholar] [CrossRef] [PubMed]
- Cernea, S.; Raz, I. Insulin Therapy: Future Perspectives. Am. J. Ther. 2020, 27, e121–e132. [Google Scholar] [CrossRef] [PubMed]
- Nevo-Shenker, M.; Phillip, M.; Nimri, R.; Shalitin, S. Type 1 diabetes mellitus management in young children: Implementation of current technologies. Pediatr. Res. 2020, 87, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Schoelwer, M.J.; DeBoer, M.D.; Breton, M.D. Use of diabetes technology in children. Diabetologia 2024, 67, 2075–2084. [Google Scholar] [CrossRef]
- Ng, S.M.; Wright, N.P.; Yardley, D.; Campbell, F.; Randell, T.; Trevelyan, N.; Ghatak, A.; Hindmarsh, P.C. Long-term assessment of the NHS hybrid closed-loop real-world study on glycaemic outcomes, time-in-range, and quality of life in children and young people with type 1 diabetes. BMC Med. 2024, 22, 175. [Google Scholar] [CrossRef]
- Lingen, K.; Pikounis, T.; Bellini, N.; Isaacs, D. Advantages and disadvantages of connected insulin pens in diabetes management. Endocr. Connect. 2023, 12, e230108. [Google Scholar] [CrossRef]
- Gildon, B.W. InPen Smart Insulin Pen System: Product Review and User Experience. Diabetes Spectr. 2018, 31, 354–358. [Google Scholar] [CrossRef]
- Yoo, J.H.; Kim, J.H. Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control. Diabetes Metab. J. 2023, 47, 27–41. [Google Scholar] [CrossRef]
- Ospelt, E.; Noor, N.; Sanchez, J.; Nelson, G.; Rioles, N.; Malik, F.S.; Basina, M.; Indyk, J.; Vendrame, F.; Schmitt, J.; et al. Facilitators and Barriers to Smart Insulin Pen Use: A Mixed-Method Study of Multidisciplinary Stakeholders from Diabetes Teams in the United States. Clin. Diabetes 2022, 41, 56–67. [Google Scholar] [CrossRef]
- Jendle, J.; Ericsson, Å.; Gundgaard, J.; Møller, J.B.; Valentine, W.J.; Hunt, B. Smart Insulin Pens are Associated with Improved Clinical Outcomes at Lower Cost Versus Standard-of-Care Treatment of Type 1 Diabetes in Sweden: A Cost-Effectiveness Analysis. Diabetes Ther. 2021, 12, 373–388. [Google Scholar] [CrossRef]
- Jendle, J.; Pöhlmann, J.; de Portu, S.; Smith-Palmer, J.; Roze, S. Cost-Effectiveness Analysis of the MiniMed 670G Hybrid Closed-Loop System Versus Continuous Subcutaneous Insulin Infusion for Treatment of Type 1 Diabetes. Diabetes Technol. Ther. 2019, 21, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Strengthening the Reporting of Observational Studies in Epidemiology, Strobe. Available online: https://www.strobe-statement.org/ (accessed on 29 November 2024).
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Medtronic. Care Link Report. Available online: https://www.medtronicdiabetes.com/customer-support/carelink-software-support/carelink-reports (accessed on 30 November 2024).
- Vigersky, R.A.; McMahon, C. The Relationship of Hemoglobin A1C to Time-in-Range in Patients with Diabetes. Diabetes Technol. Ther. 2019, 21, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Puhr, S.; Calhoun, P.; Welsh, J.B.; Walker, T.C. The Effect of Reduced Self-Monitored Blood Glucose Testing After Adoption of Continuous Glucose Monitoring on Hemoglobin A1c and Time in Range. Diabetes Technol. Ther. 2018, 20, 557–560. [Google Scholar] [CrossRef]
- Alazmi, A.A.; Brema, I.; Alzahrani, S.H.; Almehthel, M.S. The Relationship Between Hemoglobin A1c, Time in Range, and Glycemic Management Indicator in Patients with Type 1 and Type 2 Diabetes in a Tertiary Care Hospital in Saudi Arabia. Cureus 2024, 16, e63947. [Google Scholar] [CrossRef]
- Hellman, J.; Hartvig, N.V.; Kaas, A.; Møller, J.B.; Sørensen, M.R.; Jendle, J. Associations of bolus insulin injection frequency and smart pen engagement with glycaemic control in people living with type 1 diabetes. Diabetes Obes. Metab. 2024, 26, 301–310. [Google Scholar] [CrossRef]
- Elian, V.; Popovici, V.; Ozon, E.A.; Musuc, A.M.; Fița, A.C.; Rusu, E.; Radulian, G.; Lupuliasa, D. Current Technologies for Managing Type 1 Diabetes Mellitus and Their Impact on Quality of Life-A Narrative Review. Life 2023, 13, 1663. [Google Scholar] [CrossRef]
- Cleves-Valencia, J.J.; Roncancio-Moreno, M.; De Luca Picione, R. Beyond Therapeutic Adherence: Alternative Pathways for Understanding Medical Treatment in Type 1 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2024, 21, 320. [Google Scholar] [CrossRef]
- Lai, Y.R.; Chiu, W.C.; Huang, C.C.; Tsai, N.W.; Wang, H.C.; Lin, W.C.; Cheng, B.-C.; Su, Y.-J.; Su, C.-M.; Hsiao, S.-Y.; et al. HbA1C Variability Is Strongly Associated with the Severity of Peripheral Neuropathy in Patients With Type 2 Diabetes. Front. Neurosci. 2019, 13, 90. [Google Scholar] [CrossRef]
- Edqvist, J.; Rawshani, A.; Rawshani, A.; Adiels, M.; Franzén, S.; Bjorck, L.; Svensson, A.-M.; Lind, M.; Sattar, N.; Rosengren, A. Trajectories in HbA1c and other risk factors among adults with type 1 diabetes by age at onset. BMJ Open Diabetes Res. Care 2021, 9, e002187. [Google Scholar] [CrossRef]
- Beck, R.W.; Bergenstal, R.M.; Riddlesworth, T.D.; Kollman, C.; Li, Z.; Brown, A.S.; Close, K.L. Validation of Time in Range as an Outcome Measure for Diabetes Clinical Trials. Diabetes Care 2019, 42, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Urakami, T.; Terada, H.; Tanabe, S.; Mine, Y.; Aoki, M.; Aoki, R.; Suzuki, J.; Morioka, I. Clinical significance of coefficient of variation in continuous glucose monitoring for glycemic management in children and adolescents with type 1 diabetes. J. Diabetes Investig. 2024, 15, 1669–1674. [Google Scholar] [CrossRef] [PubMed]
- Schiaffini, R.; Lumaca, A.; Martino, M.; Rapini, N.; Deodati, A.; Amodeo, M.E.; Ciampalini, P.; Matteoli, M.C.; Pampanini, V.; Cianfarani, S. Time In Tight Range in children and adolescents with type 1 diabetes: A cross-sectional observational single centre study evaluating efficacy of new advanced technologies. Diabetes Metab. Res. Rev. 2024, 40, e3826. [Google Scholar] [CrossRef] [PubMed]
- Eviz, E.; Killi, N.E.; Karakus, K.E.; Can, E.; Gokce, T.; Yesiltepe Mutlu, G.; Hatun, S. Assessing the feasibility of time in tight range (TITR) targets with advanced hybrid closed loop (AHCL) use in children and adolescents: A single-centre real-world study. Diabet. Med. 2024, 41, e15333. [Google Scholar] [CrossRef] [PubMed]
- Bahillo-Curieses, P.; Fernández Velasco, P.; Pérez-López, P.; Vidueira Martínez, A.M.; Nieto de la Marca, M.O.; Díaz-Soto, G. Utility of time in tight range (TITR) in evaluating metabolic control in pediatric and adult patients with type 1 diabetes in treatment with advanced hybrid closed-loop systems. Endocrine 2024, 86, 539–545. [Google Scholar] [CrossRef]
- MacLeod, J.; Vigersky, R.A. A Review of Precision Insulin Management with Smart Insulin Pens: Opening Up the Digital Door to People on Insulin Injection Therapy. J. Diabetes Sci. Technol. 2023, 17, 283–289. [Google Scholar] [CrossRef]
- MacLeod, J.; Im, G.H.; Smith, M.; Vigersky, R.A. Shining the Spotlight on Multiple Daily Insulin Therapy: Real-World Evidence of the InPen Smart Insulin Pen. Diabetes Technol. Ther. 2024, 26, 33–39. [Google Scholar] [CrossRef]
- Danne, T.P.A.; Joubert, M.; Hartvig, N.V.; Kaas, A.; Knudsen, N.N.; Mader, J.K. Association Between Treatment Adherence and Continuous Glucose Monitoring Outcomes in People with Diabetes Using Smart Insulin Pens in a Real-World Setting. Diabetes Care 2024, 47, 995–1003. [Google Scholar] [CrossRef]
- Ekberg, N.R.; Hartvig, N.V.; Kaas, A.; Møller, J.B.; Adolfsson, P. Smart Pen Exposes Missed Basal Insulin Injections and Reveals the Impact on Glycemic Control in Adults with Type 1 Diabetes. J. Diabetes Sci. Technol. 2024, 18, 66–73. [Google Scholar] [CrossRef]
- Scuri, S.; Tesauro, M.; Petrelli, F.; Argento, N.; Damasco, G.; Cangelosi, G.; Nguyen, C.T.T.; Savva, D.; Grappasonni, I. Use of an Online Platform to Evaluate the Impact of Social Distancing Measures on Psycho-Physical Well-Being in the COVID-19 Era. Int. J. Environ. Res. Public Health 2022, 19, 6805. [Google Scholar] [CrossRef]
- Adolfsson, P.; Hartvig, N.V.; Kaas, A.; Møller, J.B.; Hellman, J. Increased Time in Range and Fewer Missed Bolus Injections After Introduction of a Smart Connected Insulin Pen. Diabetes Technol. Ther. 2020, 22, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Cangelosi, G.; Mancin, S.; Morales Palomares, S.; Pantanetti, P.; Quinzi, E.; Debernardi, G.; Petrelli, F. Impact of School Nurse on Managing Pediatric Type 1 Diabetes with Technological Devices Support: A Systematic Review. Diseases 2024, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Galindo, R.J.; Ramos, C.; Cardona, S.; Vellanki, P.; Davis, G.M.; Oladejo, O.; Albury, B.; Dhruv, N.; Peng, L.; Umpierrez, G.E. Efficacy of a Smart Insulin Pen Cap for the Management of Patients with Uncontrolled Type 2 Diabetes: A Randomized Cross-Over Trial. J. Diabetes Sci. Technol. 2023, 17, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sguanci, M.; Mancin, S.; Gazzelloni, A.; Diamanti, O.; Ferrara, G.; Morales Palomares, S.; Parozzi, M.; Petrelli, F.; Cangelosi, G. The Internet of Things in the Nutritional Management of Patients with Chronic Neurological Cognitive Impairment: A Scoping Review. Healthcare 2024, 13, 23. [Google Scholar] [CrossRef]
- Cangelosi, G.; Mancin, S.; Pantanetti, P.; Nguyen, C.T.T.; Morales Palomares, S.; Biondini, F.; Sguanci, M.; Petrelli, F. Lifestyle Medicine Case Manager Nurses for Type Two Diabetes Patients: An Overview of a Job Description Framework—A Narrative Review. Diabetology 2024, 5, 375–388. [Google Scholar] [CrossRef]
- Cranston, I.; Jamdade, V.; Liao, B.; Newson, R.S. Clinical, Economic, and Patient-Reported Benefits of Connected Insulin Pen Systems: A Systematic Literature Review. Adv. Ther. 2023, 40, 2015–2037. [Google Scholar] [CrossRef]
- Akturk, H.K.; Bindal, A. Advances in diabetes technology within the digital diabetes ecosystem. J. Manag. Care Spec. Pharm 2024, 30 (Suppl. S10-b), S7–S20. [Google Scholar] [CrossRef]
- Tian, T.; Aaron, R.E.; Du Nova, A.Y.; Jendle, J.H.; Kerr, D.; Cengiz, E.; Drincic, A.; Pickup, J.C.; Chen, K.Y.; Schwartz, N.; et al. Diabetes Technology Meeting 2023. J. Diabetes Sci. Technol. 2024, 18, 1208–1244. [Google Scholar] [CrossRef]


| Variable | Derivation for a Given Period |
|---|---|
| Sensor usage (%) | [Number of CGM measurements/(Number of minutes in the period of interest/5)] × 100 |
| SG mean, SD, and CV | Mean, SD, and CV of CGM measurements |
| TIR metrics | (Number of CGM measurements in the range of interest/Number of CGM measurements) × 100 |
| Measure | Summary Statistic | Total (n = 21) |
|---|---|---|
| Age (years) | Available Measures (%) | 21 (100.0%) |
| Mean ± SD | 51.5 ± 16.1 | |
| Median (IQR) | 53.0 (40.0–63.0) | |
| Min–Max | 17.0–76.0 | |
| Female | % (n/Available Measures) | 38.1% (8/21) |
| BMI | Available Measures (%) | 20 (95.2%) |
| Mean ± SD | 24.7 ± 4.1 | |
| Median (IQR) | 24.7 (23.0–28.6) | |
| Min–Max | 14.6–31.1 | |
| Duration of T1D (years) | Available Measures (%) | 20 (95.2%) |
| Mean ± SD | 21.9 ± 12.2 | |
| Median (IQR) | 21.5 (14.5–28.5) | |
| Min–Max | 4.0–52.0 | |
| Smoke | ||
| No | % (n/Available Measures) | 55.0% (11/20) |
| Yes | % (n/Available Measures) | 25.0% (5/20) |
| Former smoker | % (n/Available Measures) | 20.0% (4/20) |
| Previous therapy | ||
| SMBG | % (n/Available Measures) | 15.0% (3/20) |
| Other CGM | % (n/Available Measures) | 65.0% (13/20) |
| MEDTRONIC GC | % (n/Available Measures) | 20.0% (4/20) |
| Patient | Previous Therapy | Last 14 Days of Old Therapy | First 14 Days After One Month of Medtronic Smart MDI | First 30 Days of Medtronic Smart MDI | First 90 Days of Medtronic Smart MDI |
|---|---|---|---|---|---|
| 1 | SMBG | 95.44 | 93.68 | 94.93 | |
| 2 | None | 89.66 | 60.72 | 80.83 | |
| 3 | Other CGM | 89.0 | 97.89 | 95.58 | 90.99 |
| 4 | SMBG | 92.81 | 96.25 | 96.98 | |
| 5 | Other CGM | 74.0 | 94.00 | 93.31 | 94.22 |
| 6 | Other CGM | 77.0 | 98.66 | 94.21 | 96.47 |
| 7 | Other CGM | 89.0 | 55.13 | 93.24 | 54.00 |
| 8 | Other CGM | 100.0 | 99.43 | 95.89 | 97.25 |
| 9 | SMBG | 97.20 | 95.88 | 96.94 | |
| 10 | MEDTRONIC GC | 92.8 | 95.98 | 94.22 | 94.59 |
| 11 | MEDTRONIC GC | 77.0 | 59.25 | 85.84 | 80.76 |
| 12 | Other CGM | 79.0 | 97.25 | 96.60 | 97.94 |
| 13 | Other CGM | 77.0 | 98.29 | 80.37 | 90.40 |
| 14 | MEDTRONIC GC | 95.2 | 95.19 | 94.46 | 95.61 |
| 15 | Other CGM | 94.0 | 99.68 | 99.63 | |
| 16 | Other CGM | 96.0 | 97.87 | 93.18 | 96.05 |
| 17 | Other CGM | 96.0 | 98.74 | 96.53 | 97.60 |
| 18 | Other CGM | 99.0 | 93.63 | 91.93 | 81.97 |
| 19 | MEDTRONIC GC | 94.5 | 84.40 | 84.86 | 86.92 |
| 20 | Other CGM | 100.0 | 72.00 | 95.50 | 88.99 |
| 21 | Other CGM | 98.0 | 99.98 | 96.40 | 98.01 |
| Measure | Summary Statistic | First 14 Days After One Month of Medtronic Smart MDI (n = 21) | First 30 Days of Medtronic Smart MDI (n = 21) | First 90 Days of Medtronic Smart MDI (n = 20) |
|---|---|---|---|---|
| SG mean (mg/dL) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 157.8 ± 26.5 | 161.6 ± 27.4 | 156.6 ± 18.5 | |
| Median (IQR) | 153.5 (142.2–176.7) | 156.5 (141.5–171.1) | 158.2 (140.4–171.3) | |
| Min–Max | 119.2–222.9 | 123.1–248.3 | 123.9–191.3 | |
| SD (mg/dL) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 52.2 ± 13.0 | 55.6 ± 10.7 | 55.0 ± 10.0 | |
| Median (IQR) | 52.5 (45.2–59.5) | 54.7 (48.9–60.4) | 54.0 (49.0–62.0) | |
| Min–Max | 27.4–75.0 | 39.3–76.6 | 38.2–75.6 | |
| CV (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 33.0 ± 5.6 | 34.6 ± 5.1 | 35.2 ± 5.3 | |
| Median (IQR) | 33.6 (28.1–38.0) | 33.3 (30.4–38.7) | 35.5 (29.7–39.5) | |
| Min–Max | 22.9–41.3 | 27.4–45.3 | 27.4–42.6 | |
| TBR2 (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 0.5 ± 0.9 | 0.5 ± 1.1 | 0.6 ± 0.9 | |
| Median (IQR) | 0.0 (0.0–0.4) | 0.1 (0.1–0.4) | 0.1 (0.1–0.5) | |
| Min–Max | 0.0–3.3 | 0.0–4.7 | 0.0–3.0 | |
| TBR1 (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 2.2 ± 2.5 | 2.1 ± 2.3 | 2.4 ± 2.2 | |
| Median (IQR) | 0.8 (0.6–3.2) | 1.4 (0.5–2.9) | 1.4 (0.7–3.6) | |
| Min–Max | 0.0–9.5 | 0.0–8.6 | 0.0–8.0 | |
| TBR (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 2.7 ± 3.3 | 2.7 ± 3.2 | 3.0 ± 3.0 | |
| Median (IQR) | 0.8 (0.6–4.1) | 1.4 (0.6–3.4) | 1.5 (0.9–4.2) | |
| Min–Max | 0.0–11.7 | 0.0–13.3 | 0.0–10.8 | |
| TIR (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 66.5 ± 16.3 | 63.8 ± 16.1 | 66.3 ± 12.5 | |
| Median (IQR) | 64.4 (58.0–77.6) | 64.9 (57.8–72.5) | 64.2 (57.8–76.8) | |
| Min–Max | 33.1–97.9 | 18.6–87.3 | 43.0–89.5 | |
| TAR1 (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 22.9 ± 10.5 | 24.4 ± 9.0 | 23.6 ± 9.0 | |
| Median (IQR) | 26.2 (16.2–29.1) | 24.0 (17.9–30.1) | 22.1 (16.8–29.5) | |
| Min–Max | 1.5–42.3 | 10.6–43.6 | 8.9–44.1 | |
| TAR2 (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 7.9 ± 9.0 | 9.1 ± 10.8 | 7.2 ± 4.8 | |
| Median (IQR) | 4.2 (2.2–12.0) | 6.5 (3.3–9.9) | 6.9 (2.4–10.9) | |
| Min–Max | 0.0–38.6 | 0.8–49.9 | 1.1–16.8 | |
| TAR (%) | Available Measures (%) | 21 (100.0%) | 21 (100.0%) | 20 (100.0%) |
| Mean ± SD | 30.8 ± 16.9 | 33.5 ± 16.8 | 30.7 ± 13.0 | |
| Median (IQR) | 34.2 (19.8–41.3) | 33.4 (22.7–40.2) | 32.7 (20.3–40.0) | |
| Min–Max | 1.5–66.0 | 12.3–81.0 | 10.0–56.9 |
| Measure | Summary Statistics | Last 14 Days of Old Therapy (n = 17) | First 14 Days After One Month of New Therapy (n = 17) | p-Value |
|---|---|---|---|---|
| SG mean (mg/dL) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.035 |
| Mean ± SD | 172.5 ± 25.3 | 158.5 ± 26.5 | ||
| Median (IQR) | 171.0 (158.0–189.0) | 153.5 (142.2–176.7) | ||
| Min–Max | 116.7–230.0 | 119.2–222.9 | ||
| SD (mg/dL) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.159 |
| Mean ± SD | 48.3 ± 12.5 | 52.9 ± 12.8 | ||
| Median (IQR) | 47.0 (41.1–54.0) | 52.5 (45.2–59.5) | ||
| Min–Max | 30.0–72.0 | 30.0–75.0 | ||
| CV (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.009 |
| Mean ± SD | 28.1 ± 6.3 | 33.3 ± 5.4 | ||
| Median (IQR) | 28.1 (25.1–31.7) | 33.6 (28.5–38.0) | ||
| Min–Max | 16.3–41.6 | 24.4–41.3 | ||
| TBR2 (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.320 |
| Mean ± SD | 1.0 ± 1.5 | 0.6 ± 1.0 | ||
| Median (IQR) | 0.0 (0.0–2.0) | 0.0 (0.0–0.9) | ||
| Min–Max | 0.0–5.0 | 0.0–3.3 | ||
| TBR1 (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.747 |
| Mean ± SD | 3.1 ± 3.5 | 2.3 ± 2.6 | ||
| Median (IQR) | 1.0 (1.0–6.0) | 0.8 (0.6–3.2) | ||
| Min–Max | 0.0–10.0 | 0.2–9.5 | ||
| TBR (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.378 |
| Mean ± SD | 4.1 ± 4.8 | 2.8 ± 3.5 | ||
| Median (IQR) | 1.0 (1.0–8.0) | 0.8 (0.6–4.1) | ||
| Min–Max | 0.0–12.1 | 0.3–11.7 | ||
| TIR (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.005 |
| Mean ± SD | 55.7 ± 13.7 | 65.8 ± 15.6 | ||
| Median (IQR) | 58.0 (48.0–63.2) | 64.4 (58.0–77.6) | ||
| Min–Max | 23.0–82.0 | 33.1–93.9 | ||
| TAR1 (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.040 |
| Mean ± SD | 27.6 ± 9.0 | 23.3 ± 9.4 | ||
| Median (IQR) | 28.0 (25.0–33.0) | 26.2 (16.8–29.1) | ||
| Min–Max | 5.3–40.0 | 5.4–42.3 | ||
| TAR2 (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.023 |
| Mean ± SD | 12.6 ± 10.3 | 8.1 ± 9.8 | ||
| Median (IQR) | 12.0 (5.0–14.0) | 3.1 (2.2–12.0) | ||
| Min–Max | 0.5–43.0 | 0.0–38.6 | ||
| TAR (%) | Available Measures (%) | 17 (100.0%) | 17 (100.0%) | 0.015 |
| Mean ± SD | 40.3 ± 15.0 | 31.4 ± 16.3 | ||
| Median (IQR) | 39.0 (35.0–46.0) | 34.2 (19.8–41.3) | ||
| Min–Max | 5.9–76.0 | 5.4–66.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantanetti, P.; Cangelosi, G.; Morales Palomares, S.; Ferrara, G.; Biondini, F.; Mancin, S.; Caggianelli, G.; Parozzi, M.; Sguanci, M.; Petrelli, F. Real-World Life Analysis of a Continuous Glucose Monitoring and Smart Insulin Pen System in Type 1 Diabetes: A Cohort Study. Diabetology 2025, 6, 7. https://doi.org/10.3390/diabetology6010007
Pantanetti P, Cangelosi G, Morales Palomares S, Ferrara G, Biondini F, Mancin S, Caggianelli G, Parozzi M, Sguanci M, Petrelli F. Real-World Life Analysis of a Continuous Glucose Monitoring and Smart Insulin Pen System in Type 1 Diabetes: A Cohort Study. Diabetology. 2025; 6(1):7. https://doi.org/10.3390/diabetology6010007
Chicago/Turabian StylePantanetti, Paola, Giovanni Cangelosi, Sara Morales Palomares, Gaetano Ferrara, Federico Biondini, Stefano Mancin, Gabriele Caggianelli, Mauro Parozzi, Marco Sguanci, and Fabio Petrelli. 2025. "Real-World Life Analysis of a Continuous Glucose Monitoring and Smart Insulin Pen System in Type 1 Diabetes: A Cohort Study" Diabetology 6, no. 1: 7. https://doi.org/10.3390/diabetology6010007
APA StylePantanetti, P., Cangelosi, G., Morales Palomares, S., Ferrara, G., Biondini, F., Mancin, S., Caggianelli, G., Parozzi, M., Sguanci, M., & Petrelli, F. (2025). Real-World Life Analysis of a Continuous Glucose Monitoring and Smart Insulin Pen System in Type 1 Diabetes: A Cohort Study. Diabetology, 6(1), 7. https://doi.org/10.3390/diabetology6010007






