Abstract
Background: Microvascular disease (MVD) describes systemic changes in small vessels (~100 µm diameter or smaller) that impair tissue oxygenation and perfusion. MVD has been demonstrated to play an independent role in the risk of limb loss. Despite this relevance, MVD is not regularly assessed clinically because tools used to evaluate and quantify the severity of MVD of the foot remain limited. We sought to evaluate if the Semmes-Weinstein 10-g Monofilament (SWM) can be used to stratify clinical MVD severity. Methods: We evaluated a racially diverse cohort of 124 patients (with 248 limbs). SWM testing was performed on the plantar aspect of the feet at 1st, 3rd, and 5th metatarsophalangeal joints. Clinical MVD was stratified in an ascending order of severity into: no diabetes; type 2 diabetes (DM); diabetes+ neuropathy (DM+N); diabetes + neuropathy + retinopathy (DM+N+R). Logistic regression models were used to examine the association between a patient’s clinical MVD severity and an abnormal SWM test. Results: Sixty-four patients (51.6%) tested had an abnormal sensation. The odds of an abnormal SWM test were significantly higher for patients with DM+N and DM+N+R compared to those with no DM respectively. (DM vs. No DM: OR: 3.58, [0.98–13.09], p = 0.05; DM+N vs. No DM: OR: 30.46, [10.33–105.17], p < 0.001; DM+N+R vs. No DM: OR: 43.00, [9.89–309.17], p < 0.001). Furthermore, we categorized SWM based on the degree of sensation loss and found that the proportion of people with a higher degree of sensation loss increased across the clinical MVD severity spectrum. Conclusions: Abnormal SWM sensation strongly correlates with the severity of clinical MVD. This suggests that a simple, non-invasive, 1-min SWM test that can be done in the clinic is a promising tool in assessing MVD in the feet, which is particularly significant considering MVD involvement in limb loss.
1. Introduction
Despite attention to and advances in clinical care to prevent lower extremity wounds and limb amputations, there remains an unacceptably high incidence of lower limb amputations in the United States [1,2,3]. These lower limb amputations are largely attributed to peripheral arterial disease (PAD) and diabetes [1,3,4,5]. However, in addition to these diseases, recent evidence showed that systemic changes in small vessels also contributes to limb amputation [6,7,8]. These systemic changes in small vessels are often referred to as microvascular disease (MVD) [9,10].
MVD, also known as small-vessel disease, describes a range of pathological conditions that affect the arterioles, venules, and/or capillaries—blood vessels with diameters of 100 µm or less—leading to impaired tissue oxygenation and perfusion [11]. MVD is common in patients with type 2 diabetes (DM), but is not exclusive to them as data suggests that MVD can also occur independent of DM and its severity [7,12,13].
MVD plays an independent role in the risk of limb loss, increasing risk by 3.4-fold [6,7]. MVD also synergistically acts with large vessel PAD to increase the risk of limb loss by 22.7-fold [6]. In addition, MVD has been shown to independently predict diabetic foot ulcers [14]. Despite this clinical significance, MVD of the limb is not regularly evaluated for or quantified clinically and is often not accounted for in clinical limb salvage algorithms [15].
Limb salvage interventions are largely driven by PAD status as generally defined by ankle/brachial and toe/brachial pressure indices (ABI and TBI) [3,16,17,18], but these measurements have limitations in defining tissue perfusion (especially in patients with DM) and do not capture MVD status [11,19]. Although there are no well-defined treatments for MVD in the lower extremities, methods to quantify MVD could help studies better understand its relevance and promote targeted therapies.
The 10 g Semmes Weinstein Monofilament (SWM) is a clinical instrument that has been validated to test sensory nerve function (light touch sensation) in patients with suspected neuropathy [20]. Recently this tool has been shown to significantly and independently predict future foot ulceration and or amputation [21]. SWM is designed to minimize variability in applied force between users, unlike other tests such as the vibratory tuning fork. It is usually applied to test sites until it buckles. The buckling force of the monofilament, which is the force felt by the patient when monofilament bends is set at 10 g [21].
Clinically, retinopathy and neuropathy are considered systemic markers of MVD [22,23,24], however, these markers act as surrogate endpoints for MVD severity and do not directly provide information on the state of the microvasculature of the feet [15]. In this observational study, our aim was to evaluate the utility of a non-invasive point of care tool called the Semmes-Weinstein 10-g Monofilament (SWM) in assessing sensation loss in the feet. We sought to determine whether a patient’s clinical MVD severity would correlate with an abnormal sensation test result using the SWM.
2. Methods
2.1. Study Population
One hundred and thirty-nine patients with 263 limbs were prospectively recruited from a single institution—Banner University Medical Center in Tucson, Arizona, USA from June 2017 to December 2022. Eligibility criteria included adult patients (>18 years old) admitted to the in-patient services of podiatry, vascular surgery, internal medicine and those seen in podiatry and vascular surgery outpatient clinics. By design, this included patients with and without type 2 DM. We further excluded patients with unilateral amputation of their lower limb including a transmetatarsal amputation (n = 15) as a SWM test would be impossible in this population. All patients included in the study had SWM measurements of both feet. A research assistant completed a questionnaire with the patient requesting patient demographics and clinical information. All clinical diagnoses were confirmed upon EMR review. Informed consent was obtained from the patients and approved by the University of Arizona Institutional Review Board (IRB) on 7 August 2016; IRB approval number: 1606633762. The deidentified data utilized in the present study will be made available upon reasonable request to the corresponding author.
2.2. Semmes Weinstein 10 g Monofilament Testing
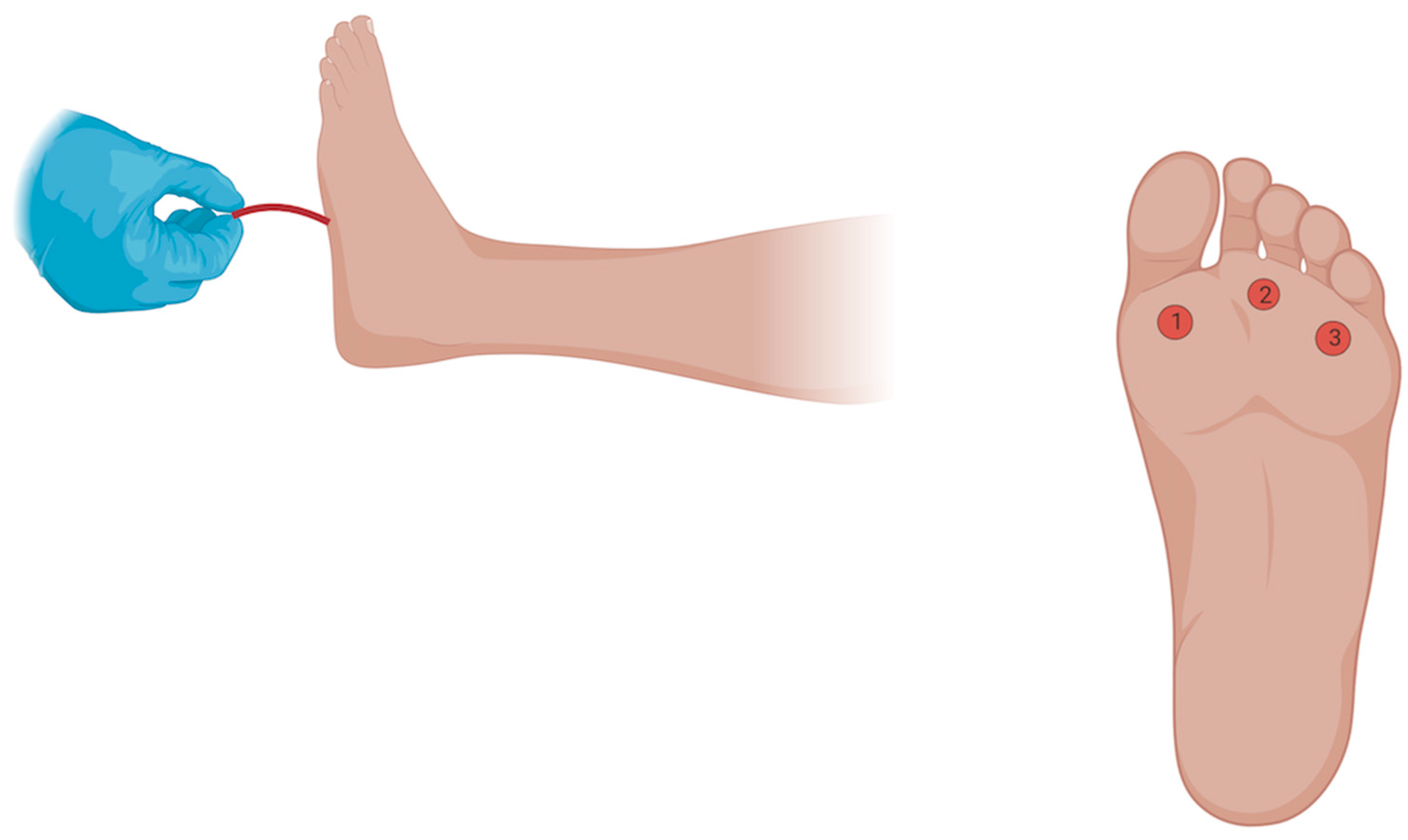
The Semmes Weinstein 10 g monofilament consists of a plastic or paper handle connected to a nylon monofilament which measures sensation by buckling at 10 g of force [25]. For our study, we used the Baseline ADA Monofilament Sensory Test™ obtained from Fabrication Enterprises Inc. (Elmsford, NY, USA). Patients were placed in a supine position with the feet exposed. Following this, patients were asked to close their eyes and verbally respond with “yes” whenever they felt a sensation. We avoided prompting the patients and applied the stimuli in irregular time intervals to minimize bias and tested the plantar aspect of the 1st, 3rd and 5th metatarsophalangeal joints (MPJ), until the monofilament buckled. If a sensation was missed on the first attempt in any location, a second trial was performed at the same location for confirmation of sensation loss (Figure 1).
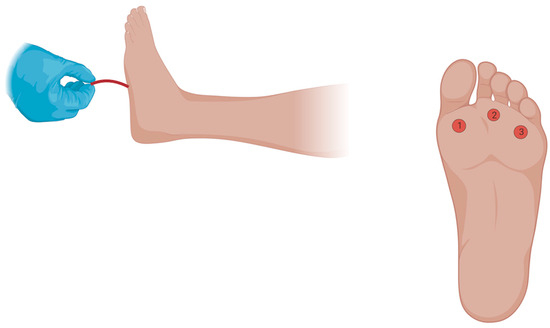
Figure 1.
Testing Sensation with the 10 g Semmes Weinstein Monofilament. The Semmes Weinstein 10 g monofilament consists of a plastic or paper handle connected to a nylon monofilament which measures sensation by buckling at 10 g of force. Patients were placed in a supine position with the feet exposed. They were asked to close their eyes and verbally respond with “yes” whenever they felt a sensation on the plantar aspect of either the 1st, 3rd and 5th MPJ. The stimuli were applied in irregular time intervals to reduce potential subject bias. For each location, a checkmark or an X was written on the sheet based on whether the patient did or did not feel the sensation. If the patient did not feel the sensation on the first attempt, a second attempt was performed at the same location to confirm sensation loss. A normal (Category 0) result occurs when the patient feels the sensation at all three locations, while an abnormal result occurs when the patient does not feel one (Category 1), two (Category 2), or all three locations (Category 3). Figure was created using Biorender.com, https://www.biorender.com/, accessed on 20 September 2024.
2.3. Independent Variable and Outcomes of Interest
The independent variable of interest was clinical MVD. For this study, we used the existing clinical diagnoses of DM, neuropathy, and retinopathy to represent MVD severity. These diagnoses aligned as a spectrum, as all patients with neuropathy had DM, and all patients with retinopathy had neuropathy (and DM) [11]. Thus, the clinical MVD spectrum on an order of increasing severity was 1. No DM. 2. DM 3. DM + Neuropathy (DM+N) 4. DM+N+Retinopathy (DM+N+R) [11]. All diagnoses (including Type 2 DM, neuropathy and retinopathy) were self-reported by patients and were further confirmed upon electronic medical records (EMR) review. Type 2 DM was defined as a self-reported diagnosis confirmed by an EMR entry documenting Type 2 DM. Neuropathy and retinopathy were defined based on self-reported diagnosis which was also confirmed with EMR data. PAD was defined using the Ankle-Brachial Index (ABI) and/or Toe-brachial Index (TBI) of less than 0.9 and 0.7 respectively. We excluded nephropathy from the clinical spectrum of MVD because it occurred independently of the other forms of MVD in contrast to the stepwise severity of those analyzed.
The primary outcome measure was an abnormal SWM test. For a foot to be categorized as a normal, the patient must feel sensations in all 3 areas tested (1st, 3rd and 5th MPJ). An abnormal SWM test was defined as a missed sensation of the SWM on at least one foot. SWM scores further stratified into 3 categories based on the increasing degree of sensation loss. Category 0 had feet with no sensation loss (0/3), category 1 had feet with one or two sites of sensation loss (1/3 or 2/3) and category 2 had the most severe form of sensation loss with feet having complete sensation loss in all three sites tested (3/3). For each patient, out of both feet, we used the foot with the highest SWM category for our analysis.
2.4. Statistical Analyses
Baseline demographics and comorbidities were summarized using descriptive statistics. Categorical data were reported as counts and percentages while continuous data were reported as medians and interquartile ranges. We tested for differences in MVD groups using Fisher’s exact test for categorical variables and Kruskal Wallis test for age. To examine the association between clinical MVD severity and an abnormal SWM test, we used logistic regression models. Model 1 was unadjusted, model 2 was adjusted for age and sex while model 3 was adjusted for covariates: age, sex, smoking status and end stage renal disease (ESRD). SWM scores were further stratified into 3 categories based on the increasing degree of sensation loss: 0/3 (no sensation loss, category 1), 1/3 or 2/3 (partial sensation loss, category 2), and 3/3 (complete sensation loss, category 3). Proportional odds model was conducted to determine the odds ratio for being in a higher degree of sensation loss for each level of clinical MVD status (DM only, DM+N, DM+N+R) versus No DM. We tested the assumption of proportional odds and found no violation (p = 0.34). The statistical values were presented as odds ratio with a corresponding 95% confidence interval (95% CI). Diagnostic testing accuracy of the SWM against clinical MVD was performed [Supplemental Table S1]. Statistical significance was defined as a p-value of <0.05. All analyses were completed using R, version 4.2.3 [26].
3. Results
3.1. Baseline Characteristics
A total of 124 patients (248 limbs) were included in our final analysis. The median age of the patients was 63.5 years. Of the patients included in the study, 56.5% were male, 58.9% had DM, 53.2% had a known public insurance status, 41.1% with a history of smoking, 13.7% had ESRD and 16.9% had PAD. (Table 1).

Table 1.
Baseline Characteristics of Patients.
3.2. Primary Outcome: Presence of Abnormal Monofilament Sensation
An abnormal SWM test was defined as the inability to feel the sensation in any or all the three areas tested (plantar aspect of the 1st, 3rd and 5th MPJ) in at least one foot. The odds of an abnormal SWM test were significantly higher for patients with DM with neuropathy and DM with neuropathy and retinopathy, compared to those with no DM respectively. (DM vs. No DM: OR: 3.58, 95% CI: 0.98–13.09, p = 0.05; DM+Nvs. No DM: OR: 30.46, 95% CI: 10.33–105.17, p < 0.001; DM +N+R vs. No DM: OR: 43.00, 95% CI: 9.89–309.17, p < 0.001). (Table 2). After adjusting for age, sex, smoking and ESRD, the trend persisted with the odds of an abnormal SWM becoming greater across the clinical spectrum of MVD. (Table 2).

Table 2.
Relationship between clinical MVD severity and an abnormal SWM test.
3.3. Secondary Outcome: Observed Proportion of Sensation Loss According to the Severity of Clinical Microvascular Disease
SWM testing was further stratified into 3 categories based on the increasing degree of sensation loss. Category 0 defined a foot with sensation intact on all sites (no sensation loss), category 1 defined a foot with sensation loss in one or two sites (partial sensation loss) and category 2 had the most severe form of sensation loss with zero sensation in all three sites tested (complete sensation loss). For each patient, out of both feet, we used the foot with the highest SWM category for our analysis.
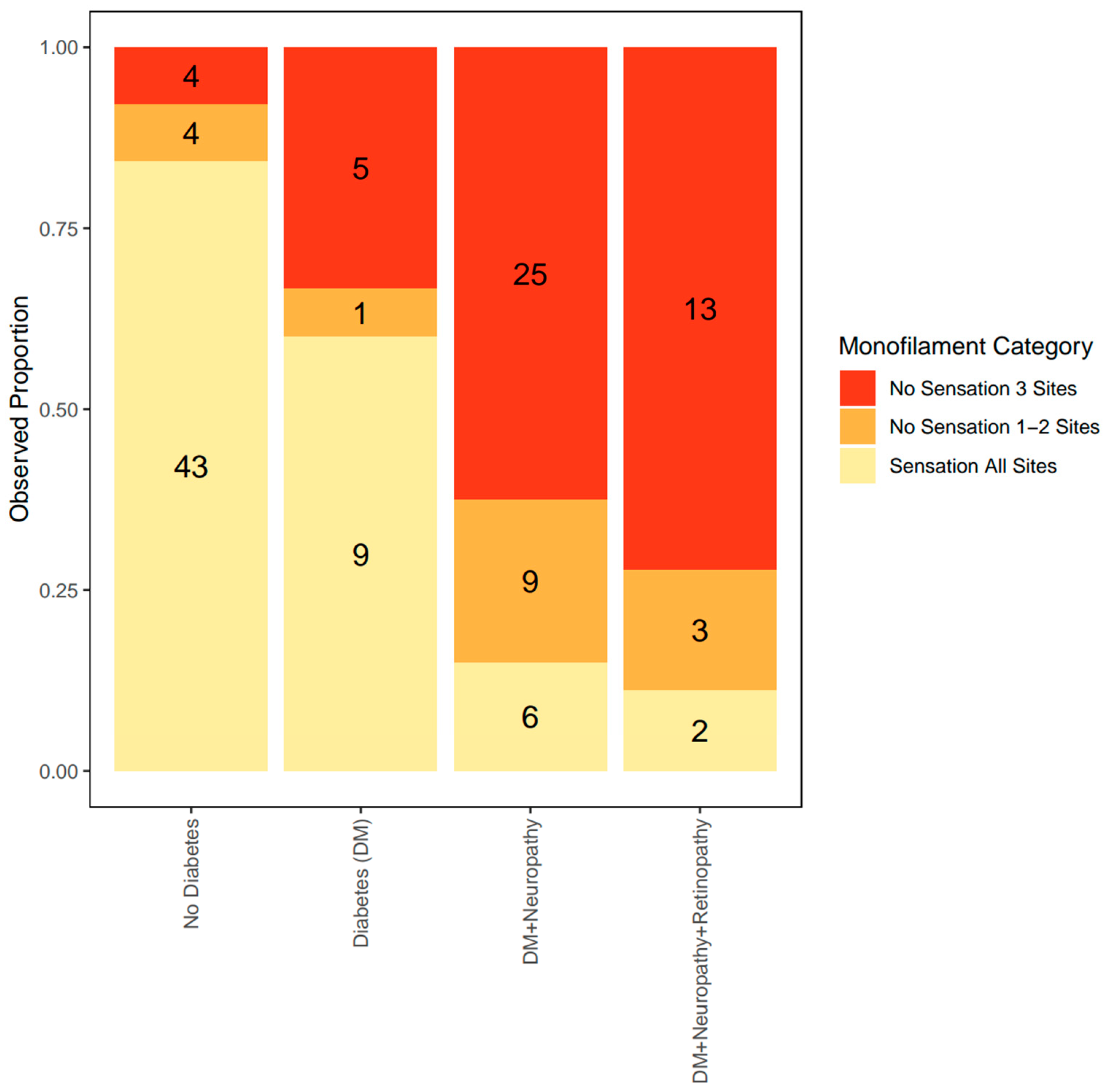
The group with no DM had the lowest proportion of patients with complete sensation loss [category 2] (7.8%). The group with DM had 33.3% of patients with complete sensation loss; DM+N group had 62.5% of patients with complete sensation loss, while the DM+N+R group had the highest proportion of patients with complete sensation loss (72.2%) (Figure 2).
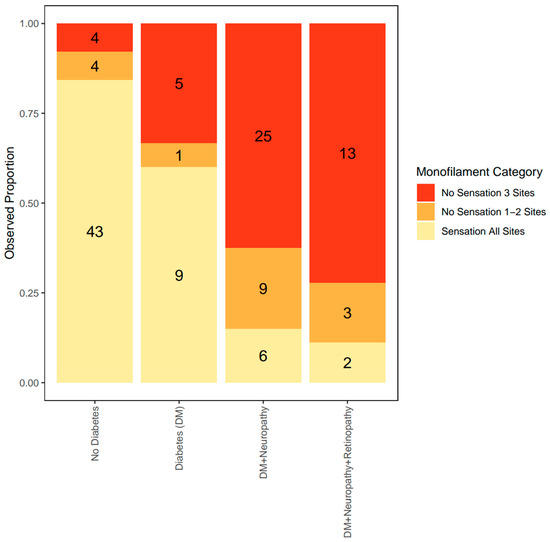
Figure 2.
Observed proportion of each level of monofilament category according to the severity of clinical MVD. The observed proportion of sensation loss was compared across the spectrum of clinical MVD. The proportion of patients with sensation loss (shown in red and orange) increases with clinical MVD severity.
Using the proportional odds model, when a patient has DM, the odds of having complete sensation loss were 4.37 times higher compared to when the patient has no DM (p < 0.05). In DM+N, the odds of having complete SWM sensation loss were 23.46 times higher compared to when the patient has no DM (p < 0.05). For patients with DM+N+R, the odds of having complete monofilament sensation loss were 35.34 times higher compared to when the patients who have no DM (Table 3).

Table 3.
Odds of having no SWM sensation based on the spectrum of clinical MVD.
4. Discussion
MVD has been demonstrated to play an independent role in the risk of limb loss [6,7]. As a result of this, there is a clinical need to diagnose and quantify MVD to better understand limb loss risk and this patient population. Our study evaluated the utility of a non-invasive point of care tool called the Semmes Weinstein 10-g Monofilament (SWM) in predicting a patient’s clinical MVD severity. This study found an association between clinically defined MVD and SWM testing of the feet. The data showed that the odds of an abnormal SWM test were significantly higher for patients with DM+N, and DM+N+R, compared to those without DM. This remained true after adjusting for patient-level factors including age, sex, ESRD and smoking. Furthermore, after the SWM test was stratified based on the degree of sensation loss, our data showed that the number of patients with a higher degree of sensation loss proportionally increased as the severity of MVD worsened.
In our study, the baseline demographic characteristics across the clinical MVD spectrum showed that male patients had more severe MVD compared to females. Contemporary work reports mixed results regarding sex differences for MVD [27,28]. This could be related to potential sex differences in MVD pathophysiology, risk factor exposure and susceptibility [28,29]. This study also found that a greater proportion of patients with severe clinical MVD were publicly insured. This could indicate that individuals with public insurance might have limited access to preventive healthcare or early interventions leading to advanced disease at the time of diagnosis [30]. Furthermore, our data demonstrated that the median age of patients reduced as clinical MVD severity worsened which was unexpected [31]. We suspect this finding could be related severity of diabetes seen in Native Americans and Latinos in this population, which can affect these populations at younger ages. Existing literature suggest that these populations especially Native Americans experience significantly higher incidence of diabetic microvascular complications at younger ages compared to other groups [32]. In our study, 72.7% of the patients with severe clinical MVD were Hispanic and Native Americans. We also observed that the mean age of those with severe clinical MVD was notably lower which is consistent with literature showing that Native Americans and Hispanic patients with severe MVD tend to be younger than those from other groups. This suggests that the progression of disease likely began earlier, with social determinants of health playing a significant role in both care and disease progression. Further studies are needed to elucidate these associations which are beyond the scope of our paper. It would be interesting to examine how the duration of diabetes impacts the progression to microvascular disease especially among Hispanic and Native Americans.
These data were adjusted for age, sex, ESRD and smoking because literature has shown that these factors independently contribute to MVD [31,33]. Aging is associated with a decline in the function of the microvasculature as a result of reduced angiogenic capacity and impaired vasodilatory function in the microcirculation [31]. Nicotine is a predictor of microvascular dysfunction [34] and biological sex has also been shown to affect the microvascular function of patients with DM [33]. ESRD was also adjusted for because it can result from nephropathy, a type of clinical MVD [35]. In our study, we excluded nephropathy from the clinical spectrum of MVD because it occurred independently of the other forms of MVD in contrast to the step-wise severity of those analyzed, which us done in other studies [11]. PAD can also coexist and may be a potential confounder for MVD. PAD status was unable to be defined in 60% of our cohort as they had no clinical record of ankle brachial index (ABI) or toe brachial index (TBI); thus, we were unable to adjust for PAD in our regression models with that substantial amount of missing data. However, our sub-analysis showed that the odds ratio of an abnormal SWM testing increased with the severity of MVD (DM+N) compared to no DM for both patients with and without PAD, indicating these results can likely be extended to those patients with and without PAD [Supplemental Table S2].
MVD is a common complication of DM; however, it can also occur independent of DM [6,7]. The data from our study supports this as 15.8% of patients with no DM had an abnormal SWM test. Routine screening for MVD is currently recommended in patients with DM only [23,36], thus many patients with MVD unrelated to DM could be potentially missed. Even among the patients with DM, our data shows that 45.1% of these patients had an abnormal SWM test without any clinical MVD equivalent (neuropathy and retinopathy). Both findings underscore the potential relevance of the SWM test in improving clinical evaluation of MVD.
We observed that among patients with severe MVD (DM+N+R), there were 13% who had intact sensation in all sites when tested with the SWM. This indicates there may be a disconnect between clinical MVD status and SWM-defined sensation. One explanation for this finding could be that MVD can occur in one vascular bed independent of another vascular bed [37]. The exact organ-specific pathophysiology in MVD is not fully understood but some hypothesis suggest that because the microcirculatory networks differ between organs due to variations in vascular architecture, hemodynamics and cell type, there can be a spectrum of clinical presentation of MVD [38]. Another possible explanation for why some of the patients who reported a diagnosis of neuropathy or both neuropathy and retinopathy had intact SWM sensation could be because the SWM may only capture sensation loss that occurs in large fiber neuropathy [39]. Overall, our findings are interesting because they raise the possibility that some patients who would be presumed to have no MVD, may actually have MVD and others with clinical signs of MVD may remain with less or insignificant MVD. Future histopathological studies should help clarify discrepancies between SWM and clinical MVD status.
Our study should be interpreted in the context of some limitations. (1.) Our study contains a small sample size could increase the possibility of type I or type II errors and resulted in wide confidence intervals for the stratified analyses. (2.) Nephropathy was excluded as a surrogate clinical marker of MVD in our cohort because we did not have the urine protein-to-creatinine ratio for these patients, which is used to test for nephropathy, and many patients with ESRD were unable to specify the precise etiology of their renal disease limiting our ability to reliably use nephropathy as a surrogate marker for MVD. (3.) PAD as measured by ABI and/or TBI was not available in about 60% of our cohort, thus we were unable to adjust for PAD in our regression models given the small number of patients with available data, however our sensitivity analysis to observe for the effect of PAD showed that odds of abnormal SMW testing increased across MVD spectrum regardless of PAD status. (4.) The current gold standard for assessing MVD is biopsy and histological analysis. We were unable to directly assess the microvasculature with microscopic evaluation, as this was considered medically invasive for the patients who had no clinical indications for this procedure. As a result, we utilized surrogate clinical markers of MVD (neuropathy and retinopathy) to define MVD of the feet [11].
Future research should focus on grading the duration of diabetes and the clinical control of diabetes using markers such as HbA1c to determine whether variations in these factors correlate with sensation in the SWM test. Additionally, testing patients before undergoing surgical procedures- such as wound debridement or surgical amputation- could provide valuable opportunities to obtain tissue sample for histopathological diagnosis of microvascular disease. These biopsy results could then be correlated with results obtained from non-invasive tools to help refine and validate methods for MVD. Furthermore, while our study was cross-sectional and limited in evaluating changes in microvascular disease progression over time, prospectively designed longitudinal studies will be crucial in assessing how MVD evolves in individuals and if the non-invasive testing tools such as SWM test can detect disease progression in individual patients.
5. Conclusions
This observational study shows that an abnormal SWM sensation strongly correlates with the severity of clinical MVD. This raises the possibility that a simple, non-invasive, 1-min SWM test that can be done in the clinic is a promising tool in assessing MVD in the feet, which is particularly relevant clinically considering MVD involvement in limb loss.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diabetology6040024/s1, Table S1: Diagnostic testing accuracy of the monofilament against clinical MVD; Table S2: Odds of Abnormal Monofilament by PAD status.
Author Contributions
Conceptualization, T.-W.T. and C.C.W.; Data curation, I.K.B.-R., S.R.F., M.T. and C.C.W.; Formal analysis, I.K.B.-R., S.M.K., P.G.-F. and C.C.W.; Funding acquisition, C.C.W.; Investigation, I.K.B.-R., S.R.F., S.M.K., M.T., J.C.A., P.G.-F., B.A., T.-W.T. and C.C.W.; Methodology, I.K.B.-R. and C.C.W.; Project administration, I.K.B.-R., S.R.F., J.C.A. and C.C.W.; Resources, B.A., T.-W.T. and C.C.W.; Supervision, J.C.A., B.A., T.-W.T. and C.C.W.; Visualization, I.K.B.-R., S.R.F., P.G.-F. and C.C.W.; Writing—original draft, I.K.B.-R. and C.C.W.; Writing—review & editing, S.R.F., S.M.K., M.T., J.C.A., P.G.-F., B.A. and T.-W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by NIH 1R01AG070987 awarded to Craig Weinkauf, and private research funding from Dick and Jan Highberger. No funding source had input into study design, data analysis or plan to publish.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at the University of Arizona (IRB approval number: 1606633762, 7 August 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The deidentified data utilized in the present study will be made available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Varma, P.; Stineman, M.G.; Dillingham, T.R. Epidemiology of limb loss. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moxey, P.W.; Gogalniceanu, P.; Hinchliffe, R.J.; Loftus, I.M.; Jones, K.J.; Thompson, M.M.; Holt, P.J. Lower extremity amputations—A review of global variability in incidence. Diabet. Med. 2011, 28, 1144–1153. [Google Scholar] [CrossRef]
- Dillingham, T.R.; Pezzin, L.E.; Shore, A.D. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch. Phys. Med. Rehabil. 2005, 86, 480–486. [Google Scholar] [CrossRef]
- Lin, C.W.; Armstrong, D.G.; Lin, C.H.; Liu, P.H.; Hung, S.Y.; Lee, S.R.; Huang, C.H.; Huang, Y.Y. Nationwide trends in the epidemiology of diabetic foot complications and lower-extremity amputation over an 8-year period. BMJ Open Diabetes Res. Care 2019, 7, e000795. [Google Scholar] [CrossRef]
- Beckman, J.A.; Duncan, M.S.; Damrauer, S.M.; Wells, Q.S.; Barnett, J.V.; Wasserman, D.H.; Bedimo, R.J.; Butt, A.A.; Marconi, V.C.; Sico, J.J.; et al. Microvascular Disease, Peripheral Artery Disease, and Amputation. Circulation 2019, 140, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Behroozian, A.; Beckman, J.A. Microvascular Disease Increases Amputation in Patients With Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 534–540. [Google Scholar] [CrossRef]
- Olesen, K.K.W.; Anand, S.S.; Gyldenkerne, C.; Thim, T.; Maeng, M. Microvascular disease, peripheral artery disease, and the risk of lower limb amputation. Eur. Heart J. 2020, 41 (Suppl. S2), ehaa946-2354. [Google Scholar] [CrossRef]
- Guven, G.; Hilty, M.P.; Ince, C. Microcirculation: Physiology, Pathophysiology, and Clinical Application. Blood Purif. 2020, 49, 143–150. [Google Scholar] [CrossRef]
- Abularrage, C.J.; Sidawy, A.N.; Aidinian, G.; Singh, N.; Weiswasser, J.M.; Arora, S. Evaluation of the microcirculation in vascular disease. J. Vasc. Surg. 2005, 42, 574–581. [Google Scholar] [CrossRef]
- Jett, S.; Thompson, M.R.; Awasthi, S.; Cuccia, D.J.; Tan, T.W.; Armstrong, D.G.; Mazhar, A.; Weinkauf, C.C. Stratification of Microvascular Disease Severity in the Foot Using Spatial Frequency Domain Imaging. J. Diabetes Sci. Technol. 2023, 17, 25–34. [Google Scholar] [CrossRef]
- Kaze, A.D.; Santhanam, P.; Erqou, S.; Ahima, R.S.; Bertoni, A.; Echouffo-Tcheugui, J.B. Microvascular Disease and Incident Heart Failure Among Individuals With Type 2 Diabetes Mellitus. J. Am. Heart Assoc. 2021, 10, e018998. [Google Scholar] [CrossRef]
- Hillege, H.L.; Janssen, W.M.; Bak, A.A.; Diercks, G.F.; Grobbee, D.E.; Crijns, H.J.; Van Gilst, W.H.; De Zeeuw, D.; De Jong, P.E. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 2001, 249, 519–526. [Google Scholar] [CrossRef]
- Tomita, M.; Kabeya, Y.; Okisugi, M.; Katsuki, T.; Oikawa, Y.; Atsumi, Y.; Matsuoka, K.; Shimada, A. Diabetic Microangiopathy Is an Independent Predictor of Incident Diabetic Foot Ulcer. J. Diabetes Res. 2016, 2016, 5938540. [Google Scholar] [CrossRef]
- Bolakale-Rufai, I.K. Disparity in Microvascular and Macrovascular Disease Risk in Diabetics. Master’s Thesis, The University of Arizona, Tucson, AZ, USA, 2023. [Google Scholar]
- Guirguis-Blake, J.M.; Evans, C.V.; Redmond, N.; Lin, J.S. Screening for Peripheral Artery Disease Using the Ankle-Brachial Index: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 184–196. [Google Scholar] [CrossRef]
- Swaminathan, A.; Vemulapalli, S.; Patel, M.R.; Jones, W.S. Lower extremity amputation in peripheral artery disease: Improving patient outcomes. Vasc. Health Risk Manag. 2014, 10, 417–424. [Google Scholar] [CrossRef]
- Barnes, J.A.; Eid, M.A.; Creager, M.A.; Goodney, P.P. Epidemiology and Risk of Amputation in Patients With Diabetes Mellitus and Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1808–1817. [Google Scholar] [CrossRef]
- Bolakale-Rufai, I.K.; Thompson, M.R.; Concha-Moore, K.; Jett, S.; Awasthi, S.; Cuccia, D.J.; Mazhar, A.; Weinkauf, C.C. Assessment of revascularization impact on microvascular oxygenation and perfusion using spatial frequency domain imaging. J. Surg. Case Rep. 2023, 2023, rjad382. [Google Scholar] [CrossRef]
- McGill, M.; Molyneaux, L.; Yue, D.K. Use of the Semmes-Weinstein 5.07/10 gram monofilament: The long and the short of it. Diabet. Med. 1998, 15, 615–617. [Google Scholar] [CrossRef]
- Feng, Y.; Schlösser, F.J.; Sumpio, B.E. The Semmes Weinstein monofilament examination is a significant predictor of the risk of foot ulceration and amputation in patients with diabetes mellitus. J. Vasc. Surg. 2011, 53, 220–226.e5. [Google Scholar] [CrossRef]
- Feuer, D.S.; Handberg, E.M.; Mehrad, B.; Wei, J.; Bairey Merz, C.N.; Pepine, C.J.; Keeley, E.C. Microvascular Dysfunction as a Systemic Disease: A Review of the Evidence. Am. J. Med. 2022, 135, 1059–1068. [Google Scholar] [CrossRef]
- Marshall, S.M.; Flyvbjerg, A. Prevention and early detection of vascular complications of diabetes. BMJ 2006, 333, 475–480. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and Macrovascular Complications of Diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Armstrong, D.G. The 10-g monofilament: The diagnostic divining rod for the diabetic foot? Diabetes Care 2000, 23, 887. [Google Scholar] [CrossRef]
- The R Project for Statistical Computing. 2021. Available online: https://www.r-project.org/ (accessed on 6 January 2025).
- Nugent, L.; Mehta, P.K.; Bairey Merz, C.N. Gender and microvascular angina. J. Thromb. Thrombolysis 2011, 31, 37–46. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, L.; Hamilton, O.K.L.; Clancy, U.; Backhouse, E.V.; Stewart, C.R.; Stringer, M.S.; Doubal, F.N.; Wardlaw, J.M. Sex Differences in Cerebral Small Vessel Disease: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 756887. [Google Scholar] [CrossRef]
- Kwan, A.C.; Wei, J.; Ouyang, D.; Ebinger, J.E.; Merz, C.N.B.; Berman, D.; Cheng, S. Sex differences in contributors to coronary microvascular dysfunction. Front. Cardiovasc. Med. 2023, 10, 1085914. [Google Scholar] [CrossRef]
- Ozkardes, C.; Harman, J. Association of a Patient’s Type of Insurance With Preventive Service Delivery. Cureus 2023, 15, e44927. [Google Scholar] [CrossRef]
- Scioli, M.G.; Bielli, A.; Arcuri, G.; Ferlosio, A.; Orlandi, A. Ageing and microvasculature. Vasc. Cell 2014, 6, 19. [Google Scholar] [CrossRef]
- Deen, J.F.; Adams, A.K.; Fretts, A.; Jolly, S.; Navas-Acien, A.; Devereux, R.B.; Buchwald, D.; Howard, B.V. Cardiovascular Disease in American Indian and Alaska Native Youth: Unique Risk Factors and Areas of Scholarly Need. J. Am. Heart Assoc. 2017, 6, e007576. [Google Scholar] [CrossRef]
- Haas, A.V.; Rosner, B.A.; Kwong, R.Y.; Rao, A.D.; Garg, R.; Di Carli, M.F.; Adler, G.K. Sex Differences in Coronary Microvascular Function in Individuals With Type 2 Diabetes. Diabetes 2019, 68, 631–636. [Google Scholar] [CrossRef]
- Jalali, Z.; Khademalhosseini, M.; Soltani, N.; Esmaeili Nadimi, A. Smoking, alcohol and opioids effect on coronary microcirculation: An update overview. BMC Cardiovasc. Disord. 2021, 21, 185. [Google Scholar] [CrossRef]
- Yuan, C.M.; Nee, R.; Ceckowski, K.A.; Knight, K.R.; Abbott, K.C. Diabetic nephropathy as the cause of end-stage kidney disease reported on the medical evidence form CMS2728 at a single center. Clin. Kidney J. 2017, 10, 257–262. [Google Scholar] [CrossRef]
- Association, A.D. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44 (Suppl. S1), S151–S167. [Google Scholar] [CrossRef]
- Neriyanuri, S.; Pardhan, S.; Gella, L.; Pal, S.S.; Ganesan, S.; Sharma, T.; Raman, R. Retinal sensitivity changes associated with diabetic neuropathy in the absence of diabetic retinopathy. Br. J. Ophthalmol. 2017, 101, 1174–1178. [Google Scholar] [CrossRef]
- Mauricio, D.; Gratacòs, M.; Franch-Nadal, J. Diabetic microvascular disease in non-classical beds: The hidden impact beyond the retina, the kidney, and the peripheral nerves. Cardiovasc. Diabetol. 2023, 22, 314. [Google Scholar] [CrossRef]
- Sharma, S.; Vas, P.; Rayman, G. Small Fiber Neuropathy in Diabetes Polyneuropathy: Is It Time to Change? J. Diabetes Sci. Technol. 2022, 16, 321–331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).