Novel Insights of Lithium Chloride Therapeutic Approach for Managing Type 2 Diabetic Kidney Disease: Crosslinking Tau Hyperphosphorylation and TGF Beta Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Power Analysis and Sample Size Justification
2.2. Animals and Treatments
2.3. Kidney Sieving
2.4. Lithium Chloride Administration
2.5. Biochemical Assessment
2.6. Histopathological Analysis
2.7. Immunohistochemistry
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Metabolic Characteristics of T2DM Rats
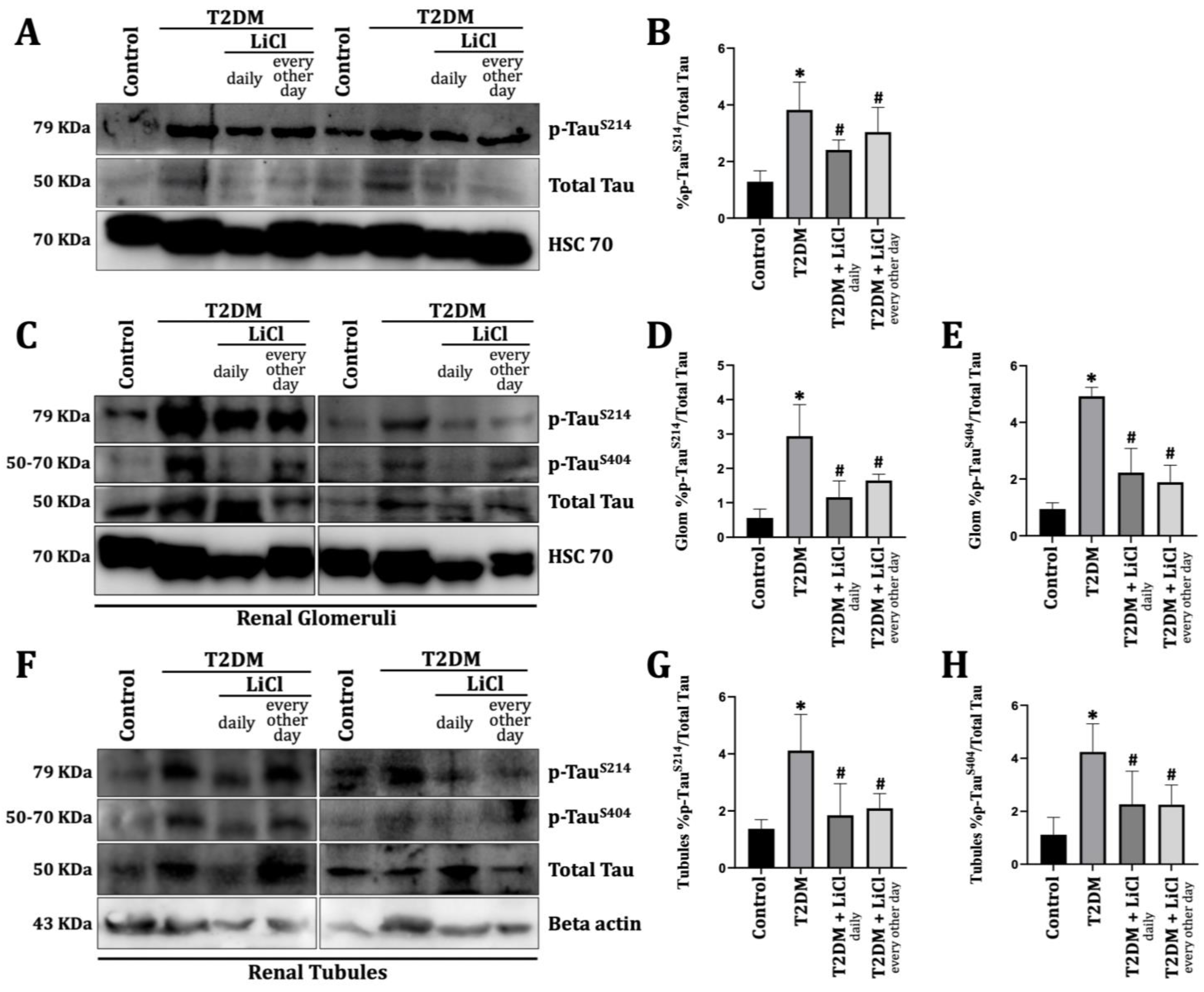
3.2. T2DM Induces Tau Protein Hyperphosphorylation in the Rats’ Kidneys
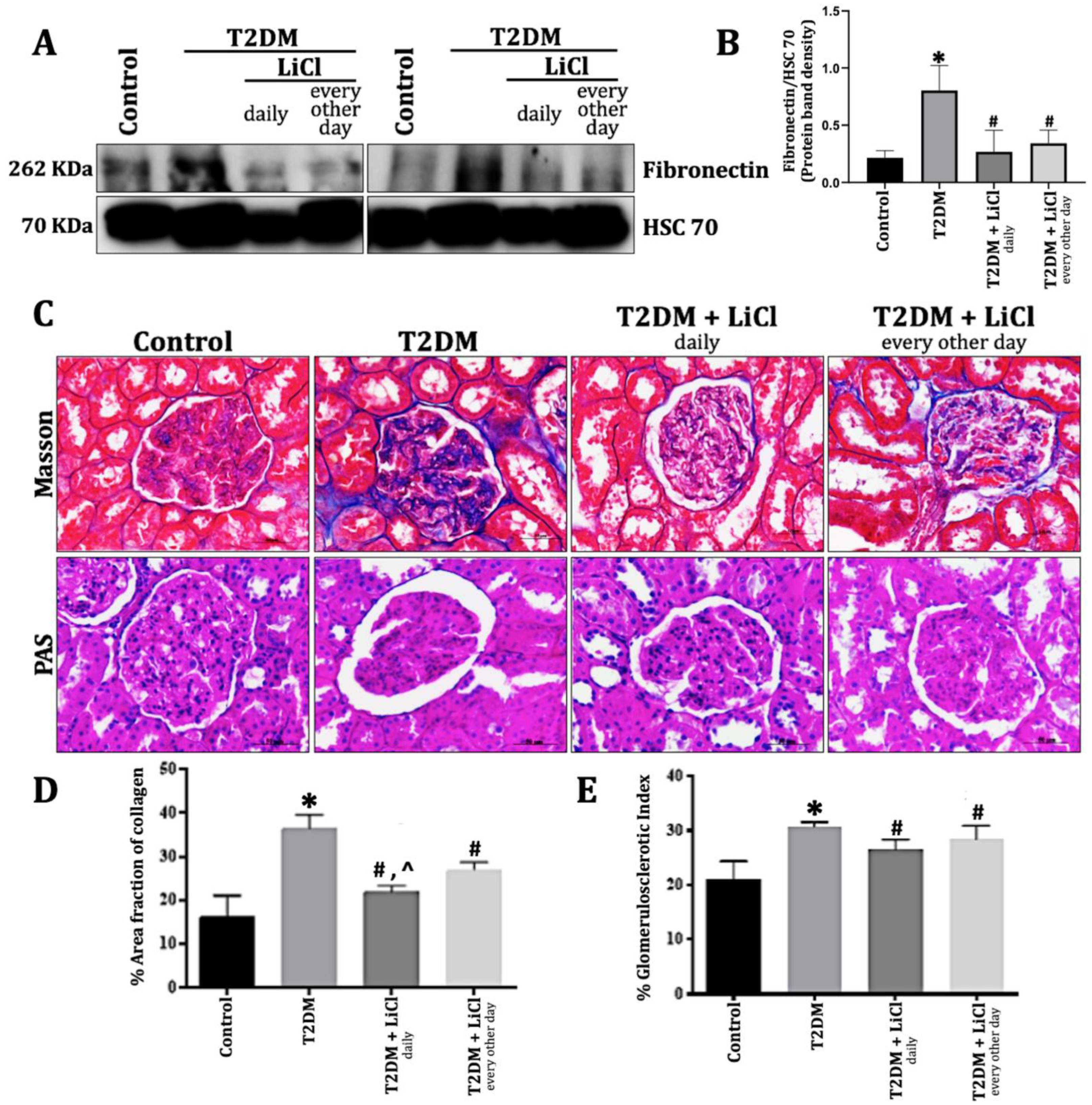
3.3. LiCl Treatment Reduces ECM Accumulation and Kidney Fibrosis in T2DM
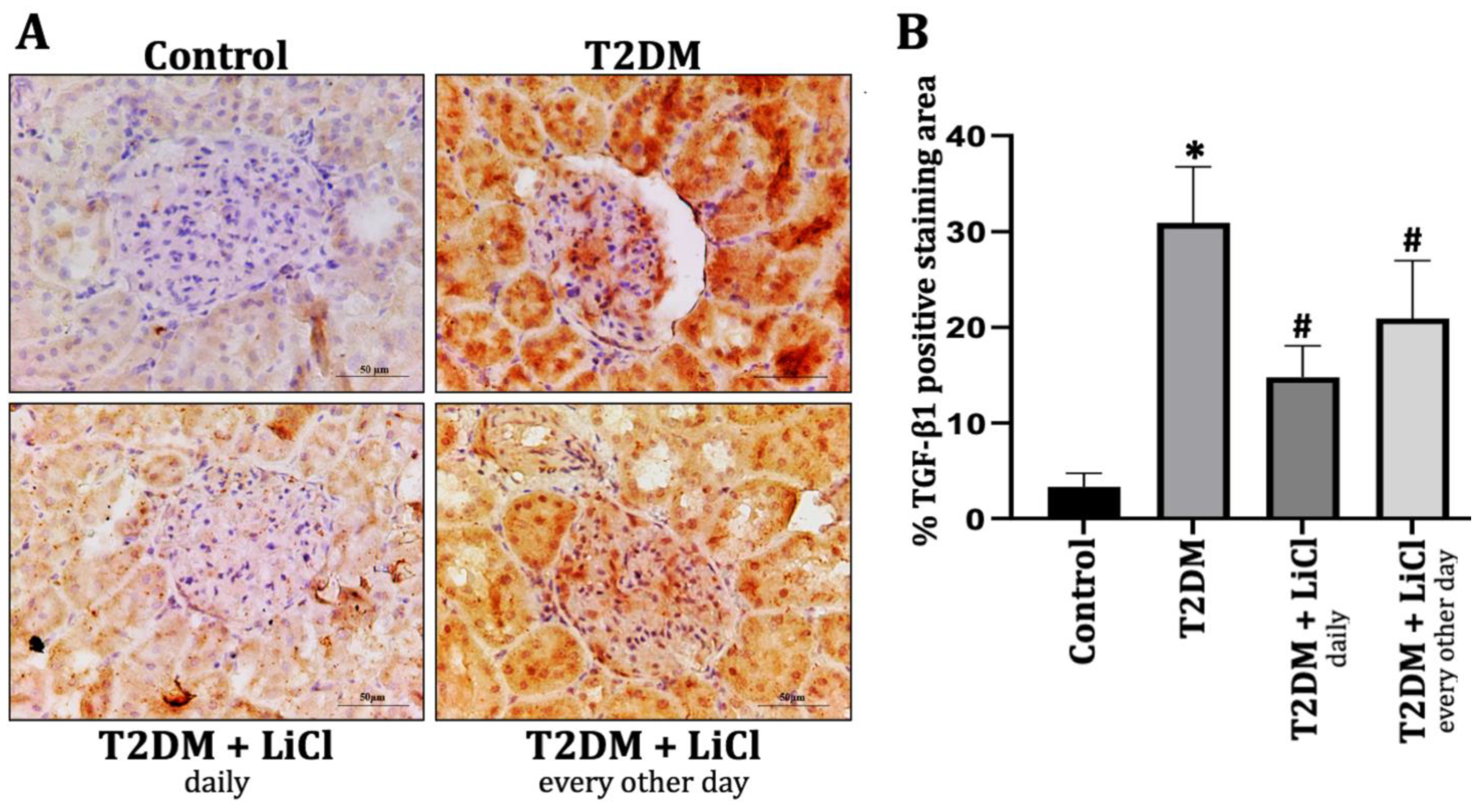
3.4. Tau Hyperphosphorylation Induced Kidney Inflammation via TGF-β1 Overexpression in T2DM Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and Regional Diabetes Prevalence Estimates for 2019 and Projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th Edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Samsu, N. Diabetic Nephropathy: Challenges in Pathogenesis, Diagnosis, and Treatment. BioMed Res. Int. 2021, 2021, 1497449. [Google Scholar] [CrossRef]
- Liman, M.N.P.; Jialal, I. Physiology, Glycosuria; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://www.ncbi.nlm.nih.gov/books/NBK557441 (accessed on 13 March 2023).
- Alsaad, K.O.; Herzenberg, A.M. Distinguishing Diabetic Nephropathy from Other Causes of Glomerulosclerosis: An Update. J. Clin. Pathol. 2007, 60, 18–26. [Google Scholar] [CrossRef]
- Murray, I.; Paolini, M.A. Histology, Kidney and Glomerulus; StatPearls Publishing: Treasure Island, FL, USA, 2023; Available online: https://pubmed.ncbi.nlm.nih.gov/32119431/ (accessed on 17 April 2023).
- Pérez-Morales, R.E.; Del Pino, M.D.; Valdivielso, J.M.; Ortiz, A.; Mora-Fernández, C.; Navarro-González, J.F. Inflammation in Diabetic Kidney Disease. Nephron 2018, 143, 12–16. [Google Scholar] [CrossRef]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-β Signaling in the Kidney: Profibrotic and Protective Effects. Am. J. Physiol.-Ren. Physiol. 2016, 310, F596–F606. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y. TGF-β in Fibrosis by Acting as a Conductor for Contractile Properties of Myofibroblasts. Cell Biosci. 2019, 9, 98. [Google Scholar] [CrossRef]
- Bülow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More than Just a Scaffold. J. Histochem. Cytochem. 2019, 67, 643–661. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Gu, Y.; Oyama, F.; Ihara, Y. τ Is Widely Expressed in Rat Tissues. J. Neurochem. 2002, 67, 1235–1244. [Google Scholar] [CrossRef]
- Kenner, L.; El-Shabrawi, Y.; Hutter, H.; Forstner, M.; Zatloukal, K.; Hoefler, G.; Preisegger, K.-H.; Kurzbauer, R.; Denk, H. Expression of Three- and Four-Repeat Tau Isoforms in Mouse Liver. Hepatology 1994, 20, 1086–1089. [Google Scholar] [CrossRef]
- Hanger, D.P.; Noble, W. Functional Implications of Glycogen Synthase Kinase-3-Mediated Tau Phosphorylation. Int. J. Alzheimer’s Dis. 2011, 2011, 352805. [Google Scholar] [CrossRef]
- Pei, J.-J.; Braak, E.; Braak, H.; Grundke-Iqbal, I.; Iqbal, K.; Winblad, B.; Cowburn, R.F. Distribution of Active Glycogen Synthase Kinase 3β (GSK-3β) in Brains Staged for Alzheimer Disease Neurofibrillary Changes. J. Neuropathol. Exp. Neurol. 1999, 58, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, L.; Creighton, J.; Alexeyev, M.; Strada, S.J.; Stevens, T. Protein Kinase a Phosphorylation of Tau-Serine 214 Reorganizes Microtubules and Disrupts the Endothelial Cell Barrier. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 299, L493–L501. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, J.-Y.; Li, H.; Fang, Z.; Wang, Q.; Deng, H.; Gong, C.-X.; Grundke-Iqbal, I.; Iqbal, K.; Wang, J.-Z. Tau Becomes a More Favorable Substrate for GSK-3 When It Is Prephosphorylated by PKA in Rat Brain. J. Biol. Chem. 2004, 279, 50078–50088. [Google Scholar] [CrossRef]
- Morfini, G.; Szebenyi, G.; Elluru, R.; Ratner, N.; Brady, S.T. Glycogen Synthase Kinase 3 Phosphorylates Kinesin Light Chains and Negatively Regulates Kinesin-Based Motility. EMBO J. 2002, 21, 281–293. [Google Scholar] [CrossRef]
- Biernat, J.; Mandelkow, E.-M. The Development of Cell Processes Induced by Tau Protein Requires Phosphorylation of Serine 262 and 356 in the Repeat Domain and Is Inhibited by Phosphorylation in the Proline-Rich Domains. Mol. Biol. Cell 1999, 10, 727–740. [Google Scholar] [CrossRef]
- Combs, B.; Mueller, R.L.; Morfini, G.; Brady, S.T.; Kanaan, N.M. Tau and Axonal Transport Misregulation in Tauopathies. Adv. Exp. Med. Biol. 2019, 1184, 81–95. [Google Scholar] [CrossRef]
- Mueller, R.L.; Combs, B.; Alhadidy, M.M.; Brady, S.T.; Morfini, G.; Kanaan, N.M. Tau: A Signaling Hub Protein. Front. Mol. Neurosci. 2021, 14, 647054. [Google Scholar] [CrossRef]
- Ho, K.-H.; Yang, X.; Osipovich, A.B.; Cabrera, O.; Hayashi, M.L.; Magnuson, M.A.; Gu, G.; Kaverina, I. Glucose Regulates Microtubule Disassembly and the Dose of Insulin Secretion via Tau Phosphorylation. Diabetes 2020, 69, 1936–1947. [Google Scholar] [CrossRef]
- Ozturk, Z. High-Fat Diet and Low-Dose Streptozotocin Induced Type 2 Diabetes: A Methodological Critique. Appl. Med. Res. 2016, 2, 41. [Google Scholar] [CrossRef][Green Version]
- Rush, B.M.; Small, S.A.; Stolz, D.B.; Tan, R.J. An Efficient Sieving Method to Isolate Intact Glomeruli from Adult Rat Kidney. J. Vis. Exp. 2018, e58162. [Google Scholar] [CrossRef]
- Hong, M.; Chen, D.C.; Klein, P.S.; Lee, V.M. Lithium Reduces Tau Phosphorylation by Inhibition of Glycogen Synthase Kinase-3. J. Biol. Chem. 1997, 272, 25326–25332. [Google Scholar] [CrossRef]
- Farris, A.B.; Alpers, C.E. What Is the Best Way to Measure Renal Fibrosis?: A Pathologist’s Perspective. Kidney Int. Suppl. 2014, 4, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mcmanus, J.F.A. Histological Demonstration of Mucin after Periodic Acid. Nature 1946, 158, 202. [Google Scholar] [CrossRef]
- Ngokere, A.; Choji, T.; Ogenyi, S.; Kumbish, P.; Moses, G.; Ahmed, J.; Suleiman, I.; Zamfara, R.; Bukar, S.; Gwong, V. Periodic Acid Schiff Reactions and General Tissue Morphology of Conventionally-Processed versus Two Rapid Microwave-Processed Tissues. Br. J. Appl. Sci. Technol. 2016, 12, 1. [Google Scholar] [CrossRef]
- Heyderman, E. Immunoperoxidase Technique in Histopathology: Applications, Methods, and Controls. J. Clin. Pathol. 1979, 32, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Yang, P.-C.; Mahmood, T. Western Blot: Technique, Theory, and Trouble Shooting. N. Am. J. Med. Sci. 2012, 4, 429. [Google Scholar] [CrossRef]
- Amorim, R.G.; Guedes, G.D.S.; Vasconcelos, S.M.D.L.; Santos, J.C.D.F. Kidney Disease in Diabetes Mellitus: Cross-Linking between Hyperglycemia, Redox Imbalance and Inflammation. Arq. Bras. Cardiol. 2019, 112, 577–587. [Google Scholar] [CrossRef]
- De Cosmo, S.; Menzaghi, C.; Prudente, S.; Trischitta, V. Role of Insulin Resistance in Kidney Dysfunction: Insights into the Mechanism and Epidemiological Evidence. Nephrol. Dial. Transplant. 2012, 28, 29–36. [Google Scholar] [CrossRef]
- Deng, Y.; Li, B.; Liu, Y.; Iqbal, K.; Grundke-Iqbal, I.; Gong, C.-X. Dysregulation of Insulin Signaling, Glucose Transporters, O-GlcNAcylation, and Phosphorylation of Tau and Neurofilaments in the Brain. Am. J. Pathol. 2009, 175, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, E.; Leboucher, A.; Caron, E.; Ahmed, T.; Tailleux, A.; Dumont, J.; Issad, T.; Gerhardt, E.; Pagesy, P.; Vileno, M.; et al. Tau Deletion Promotes Brain Insulin Resistance. J. Exp. Med. 2017, 214, 2257–2269. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Toledo, J.B.; Lee, E.B.; Arvanitakis, Z.; Kazi, H.; Han, L.-Y.; Louneva, N.; Lee, V.M.-Y.; Kim, S.F.; Trojanowski, J.Q.; et al. Abnormal Serine Phosphorylation of Insulin Receptor Substrate 1 Is Associated with Tau Pathology in Alzheimer’s Disease and Tauopathies. Acta Neuropathol. 2014, 128, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Grillo, C.A. Insulin Resistance as a Therapeutic Target in the Treatment of Alzheimer’s Disease: A State-of-The-Art Review. Front. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef]
- Mull, A.J.; Berhanu, T.K.; Roberts, N.W.; Heydemann, A. The Murphy Roths Large (MRL) Mouse Strain Is Naturally Resistant to High Fat Diet-Induced Hyperglycemia. Metab. Clin. Exp. 2014, 63, 1577–1586. [Google Scholar] [CrossRef][Green Version]
- Zhang, M.; Feng, L.; Zhu, M.; Gu, J.-F.; Jiang, J.; Zhang, H.; Ding, S.; Wu, C.; Jia, X. The Anti-Inflammation Effect of Moutan Cortex on Advanced Glycation End Products-Induced Rat Mesangial Cells Dysfunction and High-Glucose–Fat Diet and Streptozotocin-Induced Diabetic Nephropathy Rats. J. Ethnopharmacol. 2014, 151, 591–600. [Google Scholar] [CrossRef]
- Wu, D.; Wen, W.; Qi, C.-L.; Zhao, R.-X.; Lü, J.-H.; Zhong, C.-Y.; Chen, Y.-Y. Ameliorative Effect of Berberine on Renal Damage in Rats with Diabetes Induced by High-Fat Diet and Streptozotocin. Phytomedicine 2012, 19, 712–718. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Grebenchikov, O.A.; Babenko, V.A.; Pevzner, I.B.; Zorova, L.D.; Likhvantsev, V.V.; Zorov, D.B. Nephroprotective Effect of GSK-3β Inhibition by Lithium Ions and δ-Opioid Receptor Agonist Dalargin on Gentamicin-Induced Nephrotoxicity. Toxicol. Lett. 2013, 220, 303–308. [Google Scholar] [CrossRef]
- Xu, W.; Ge, Y.; Liu, F.; Gong, R. Glycogen Synthase Kinase 3β Orchestrates Microtubule Remodeling in Compensatory Glomerular Adaptation to Podocyte Depletion. J. Biol. Chem. 2015, 290, 1348–1363. [Google Scholar] [CrossRef]
- Xu, W.; Ge, Y.; Liu, F.; Gong, R. Glycogen Synthase Kinase 3β Dictates Podocyte Motility and Focal Adhesion Turnover by Modulating Paxillin Activity. Am. J. Pathol. 2014, 184, 2742–2756. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Ge, Y.; Wang, Z.; Zhuang, S.; Dworkin, L.D.; Peng, A.; Gong, R. Delayed Administration of a Single Dose of Lithium Promotes Recovery from AKI. J. Am. Soc. Nephrol. 2014, 25, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Kalita-De Croft, P.; Bedford, J.J.; Leader, J.P.; Walker, R.J. Amiloride Modifies the Progression of Lithium-Induced Renal Interstitial Fibrosis. Nephrology 2018, 23, 20–30. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, W.; Wang, C.; Tsai, C.; Chen, C.; Chang, Y.; Kai, J.; Lin, C. Inhibiting Glycogen Synthase Kinase-3 Reduces Endotoxaemic Acute Renal Failure by Down-Regulating Inflammation and Renal Cell Apoptosis. Br. J. Pharmacol. 2009, 157, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Sinha, D.; Wang, Z.; Ruchalski, K.L.; Levine, J.S.; Krishnan, S.; Lieberthal, W.; Schwartz, J.H.; Borkan, S.C. Lithium Activates the Wnt and Phosphatidylinositol 3-Kinase Akt Signaling Pathways to Promote Cell Survival in the Absence of Soluble Survival Factors. Am. J. Physiol.-Ren. Physiol. 2005, 288, F703–F713. [Google Scholar] [CrossRef]
- Alsady, M.; Baumgarten, R.; Deen, P.M.T.; de Groot, T. Lithium in the Kidney: Friend and Foe? J. Am. Soc. Nephrol. 2015, 27, 1587–1595. [Google Scholar] [CrossRef]
- Noble, W.; Planel, E.; Zehr, C.; Olm, V.; Meyerson, J.; Suleman, F.; Gaynor, K.; Wang, L.; LaFrancois, J.; Feinstein, B.; et al. Inhibition of Glycogen Synthase Kinase-3 by Lithium Correlates with Reduced Tauopathy and Degeneration in Vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 6990–6995. [Google Scholar] [CrossRef]
- Freland, L.; Beaulieu, J.-M. Inhibition of GSK3 by Lithium, from Single Molecules to Signaling Networks. Front. Mol. Neurosci. 2012, 5, 14. [Google Scholar] [CrossRef]
- Krauss, R.M. Lipids and Lipoproteins in Patients with Type 2 Diabetes. Diabetes Care 2004, 27, 1496–1504. [Google Scholar] [CrossRef]
- Bao, H.-F.; Ge, Y.; Peng, A.; Gong, R. Fine-Tuning of NFκB by Glycogen Synthase Kinase 3β Directs the Fate of Glomerular Podocytes upon Injury. Kidney Int. 2015, 87, 1176–1190. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Fassi, A.; Ilieva, A.P.; Bruno, S.; Iliev, I.P.; Brusegan, V.; Rubis, N.; Gherardi, G.; Arnoldi, F.; Ganeva, M.; et al. Preventing Microalbuminuria in Type 2 Diabetes. N. Engl. J. Med. 2004, 351, 1941–1951. [Google Scholar] [CrossRef]
- Sharma, S.; Smyth, B. From Proteinuria to Fibrosis: An Update on Pathophysiology and Treatment Options. Kidney Blood Press. Res. 2021, 46, 411–420. [Google Scholar] [CrossRef]
- Naaman, S.; Bakris, G.L. Diabetic Nephropathy: Update on Pillars of Therapy Slowing Progression. Diabetes Care 2023, 46, 1574–1586. [Google Scholar] [CrossRef] [PubMed]
- Varghese, R.T.; Jialal, I. Diabetic Nephropathy; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://pubmed.ncbi.nlm.nih.gov/30480939/ (accessed on 9 January 2025).
- Wolf, G.; Reinking, R.; Zahner, G.; Stahl, K.; Shankland, S.J. Erk 1,2 Phosphorylates P27 Kip1: Functional Evidence for a Role in High Glucose-Induced Hypertrophy of Mesangial Cells. Diabetologia 2003, 46, 1090–1099. [Google Scholar] [CrossRef] [PubMed]
- Azancot, M.A.; Moreso, F.; Salcedo, M.; Cantarell, C.; Perello, M.; Torres, I.B.; Montero, A.; Trilla, E.; Sellarés, J.; Morote, J.; et al. The Reproducibility and Predictive Value on Outcome of Renal Biopsies from Expanded Criteria Donors. Kidney Int. 2014, 85, 1161–1168. [Google Scholar] [CrossRef]
- Cohen, A.H. Masson’s Trichrome Stain in the Evaluation of Renal Biopsies: An Appraisal. Am. J. Clin. Pathol. 1976, 65, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Farris, A.B.; Adams, C.D.; Brousaides, N.; Della Pelle, P.A.; Collins, A.B.; Moradi, E.; Smith, R.N.; Grimm, P.C.; Colvin, R.B. Morphometric and Visual Evaluation of Fibrosis in Renal Biopsies. J. Am. Soc. Nephrol. 2010, 22, 176–186. [Google Scholar] [CrossRef]
- Lin, C.L.; Wang, J.Y.; Huang, Y.T.; Kuo, Y.H.; Surendran, K.; Wang, F.S. Wnt/β-Catenin Signaling Modulates Survival of High Glucose–Stressed Mesangial Cells. J. Am. Soc. Nephrol. 2006, 17, 2812–2820. [Google Scholar] [CrossRef]
- Ho, C.; Lee, P.-H.; Hsu, Y.-C.; Huang, Y.-T.; Wang, F.-S.; Lin, C.-L. Sustained Wnt/β-Catenin Signaling Rescues High Glucose Induction of Transforming Growth Factor-β1-Mediated Renal Fibrosis. Am. J. Med. Sci. 2012, 344, 374–382. [Google Scholar] [CrossRef]
- Wolf, G.; Ziyadeh, F.N. Cellular and Molecular Mechanisms of Proteinuria in Diabetic Nephropathy. Nephron Physiol. 2007, 106, p26–p31. [Google Scholar] [CrossRef]
- Zhang, J.; Anshul, F.; Malhotra, D.K.; Jaume, J.; Dworkin, L.D.; Gong, R. Microdose Lithium Protects against Pancreatic Islet Destruction and Renal Impairment in Streptozotocin-Elicited Diabetes. Antioxidants 2021, 10, 138. [Google Scholar] [CrossRef]
- Vega, M.E.; Kastberger, B.; Wehrle-Haller, B.; Schwarzbauer, J.E. Stimulation of Fibronectin Matrix Assembly by Lysine Acetylation. Cells 2020, 9, 655. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhanan, J.; Suresh, G.; Devanathan, V. Neurodegeneration in Type 2 Diabetes: Alzheimer’s as a Case Study. Brain Behav. 2020, 10, e01577. [Google Scholar] [CrossRef]
- Wijesekara, N.; Ahrens, R.; Sabale, M.; Wu, L.; Ha, K.; Verdile, G.; Fraser, P.E. Amyloid-β and Islet Amyloid Pathologies Link Alzheimer’s Disease and Type 2 Diabetes in a Transgenic Model. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2017, 31, 5409–5418. [Google Scholar] [CrossRef] [PubMed]
- Miklossy, J.; Qing, H.; Radenovic, A.; Kis, A.; Vileno, B.; Làszló, F.; Miller, L.; Martins, R.N.; Waeber, G.; Mooser, V.; et al. Beta Amyloid and Hyperphosphorylated Tau Deposits in the Pancreas in Type 2 Diabetes. Neurobiol. Aging 2010, 31, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Leng, S.; Zhang, W.; Zheng, Y.; Liberman, Z.; Rhodes, C.J.; Eldar-Finkelman, H.; Sun, X.J. Glycogen Synthase Kinase 3β Mediates High Glucose-Induced Ubiquitination and Proteasome Degradation of Insulin Receptor Substrate 1. J. Endocrinol. 2010, 206, 171–181. [Google Scholar] [CrossRef]
- Sacco, F.; Seelig, A.; Humphrey, S.J.; Krahmer, N.; Volta, F.; Reggio, A.; Marchetti, P.; Gerdes, J.; Mann, M. Phosphoproteomics Reveals the GSK3-PDX1 Axis as a Key Pathogenic Signaling Node in Diabetic Islets. Cell Metab. 2019, 29, 1422–1432.e3. [Google Scholar] [CrossRef]
- Muñoz-Montaño, J.R.; Moreno, F.J.; Avila, J.; Díaz-Nido, J. Lithium Inhibits Alzheimer’s Disease-like Tau Protein Phosphorylation in Neurons. FEBS Lett. 1997, 411, 183–188. [Google Scholar] [CrossRef]
- Khanna, M.R.; Kovalevich, J.; Lee, V.M.-Y.; Trojanowski, J.Q.; Brunden, K.R. Therapeutic Strategies for the Treatment of Tauopathies: Hopes and Challenges. Alzheimer’s Dement. 2016, 12, 1051–1065. [Google Scholar] [CrossRef]
- Stepanov, A.; Karelina, T.; Markevich, N.; Demin, O.; Nicholas, T. A Mathematical Model of Multisite Phosphorylation of Tau Protein. PLoS ONE 2018, 13, e0192519. [Google Scholar] [CrossRef]
- Gong, C.-X.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K. Post-Translational Modifications of Tau Protein in Alzheimer’s Disease. J. Neural Transm. 2004, 112, 813–838. [Google Scholar] [CrossRef]
- Hoffmann, R.; Lee, V.M.-Y.; Leight, S.; Varga, I.; Otvos, L. Unique Alzheimer’s Disease Paired Helical Filament Specific Epitopes Involve Double Phosphorylation at Specific Sites†. Biochemistry 1997, 36, 8114–8124. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.B.; Rank, K.B.; Bhattacharya, K.; Thomsen, D.R.; Gurney, M.E.; Sharma, S.K. Tau Phosphorylation at Serine 396 and Serine 404 by Human Recombinant Tau Protein Kinase II Inhibits Tau’s Ability to Promote Microtubule Assembly. J. Biol. Chem. 2000, 275, 24977–24983. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.-H.; Johnson, G.V.W. Glycogen Synthase Kinase 3beta Phosphorylates Tau at Both Primed and Unprimed Sites. Differential Impact on Microtubule Binding. J. Biol. Chem. 2003, 278, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Garrido, J.J.; Wandosell, F.G. Modulation of GSK-3 as a Therapeutic Strategy on Tau Pathologies. Front. Mol. Neurosci. 2011, 4, 24. [Google Scholar] [CrossRef]
- Tondo, L.; Alda, M.; Bauer, M.; Bergink, V.; Grof, P.; Hajek, T.; Lewitka, U.; Licht, R.W.; Manchia, M.; Müller-Oerlinghausen, B.; et al. Clinical Use of Lithium Salts: Guide for Users and Prescribers. Int. J. Bipolar Disord. 2019, 7, 16. [Google Scholar] [CrossRef]
- Forlenza, O.V.; De-Paula, V.J.R.; Diniz, B.S.O. Neuroprotective Effects of Lithium: Implications for the Treatment of Alzheimer’s Disease and Related Neurodegenerative Disorders. ACS Chem. Neurosci. 2014, 5, 443–450. [Google Scholar] [CrossRef]
- Mudher, A.; Shepherd, D.; Newman, T.A.; Mildren, P.; Jukes, J.P.; Squire, A.; Mears, A.; Berg, S.; MacKay, D.; Asuni, A.A.; et al. GSK-3β Inhibition Reverses Axonal Transport Defects and Behavioural Phenotypes in Drosophila. Mol. Psychiatry 2004, 9, 522–530. [Google Scholar] [CrossRef]
- Takahashi, M.; Yasutake, K.; Tomizawa, K. Lithium Inhibits Neurite Growth and Tau Protein Kinase I/Glycogen Synthase Kinase-3beta-Dependent Phosphorylation of Juvenile Tau in Cultured Hippocampal Neurons. J. Neurochem. 1999, 73, 2073–2083. [Google Scholar]
- Ziyadeh, F.N. Mediators of Diabetic Renal Disease: The Case for TGF- as the Major Mediator. J. Am. Soc. Nephrol. 2004, 15, S55–S57. [Google Scholar] [CrossRef]
- Langham, R.G.; Kelly, D.J.; Gow, R.M.; Zhang, Y.; Cordonnier, D.J.; Pinel, N.; Zaoui, P.; Gilbert, R.E. Transforming Growth Factor-β in Human Diabetic Nephropathy. Diabetes Care 2006, 29, 2670–2675. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, O.; López-Hernández, F.J.; López-Novoa, J.M. An Integrative View on the Role of TGF-β in the Progressive Tubular Deletion Associated with Chronic Kidney Disease. Kidney Int. 2010, 77, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Carmen Iglesias-de la Cruz, M.; Jim, B.; Hong, S.W.; Isono, M.; Ziyadeh, F.N. Reversibility of Established Diabetic Glomerulopathy by Anti-TGF-β Antibodies in Db/Db Mice. Biochem. Biophys. Res. Commun. 2003, 300, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Jin, Y.; Guo, J.; Ziyadeh, F.N. Neutralization of TGF-β by Anti-TGF-β Antibody Attenuates Kidney Hypertrophy and the Enhanced Extracellular Matrix Gene Expression in STZ-Induced Diabetic Mice. Diabetes 1996, 45, 522–530. [Google Scholar] [CrossRef]
- Ziyadeh, F.N.; Hoffman, B.B.; Han, D.C.; Iglesias-De la Cruz, M.C.; Hong, S.W.; Isono, M.; Chen, S.; McGowan, T.A.; Sharma, K. Long-Term Prevention of Renal Insufficiency, Excess Matrix Gene Expression, and Glomerular Mesangial Matrix Expansion by Treatment with Monoclonal Antitransforming Growth Factor-β Antibody in Db/Db Diabetic Mice. Proc. Natl. Acad. Sci. USA 2000, 97, 8015–8020. [Google Scholar] [CrossRef]
| Parameter | Control | T2DM | T2DM Treated with LiCl Daily | T2DM Treated with LiCl Every Other Day |
|---|---|---|---|---|
| Metabolic Parameters | ||||
| Body weight (g) | 350.10 ± 62.07 | 456.40 ± 20.59 * | 347.20 ± 24.82 # | 491.3 ± 4.93 *^ |
| Blood glucose (mg/dL) | 114.58 ± 5.81 | 355.45 ± 22.69 * | 302.04 ± 40.03 * | 347.93 ± 34.20 * |
| Insulin (pmol/L) | 120 ± 16 | 207 ± 21 * | 167 ± 18 *# | 158 ± 16 *# |
| Free Fatty Acids (mmol/L) | 1.7 ± 0.05 | 5.2 ± 0.12 * | 3.4 ± 0.09 *# | 3.7 ± 0.07 *# |
| Triglycerides (mmol/L) | 1.2 ± 0.02 | 9.9 ± 0.36 * | 5.3 ± 0.44 *# | 4.9 ± 0.33 *# |
| Renal Parameters | ||||
| Kidney weight (g) | 1.94 ± 0.10 | 2.50 ± 0.14 * | 1.73 ± 0.11 # | 2.09 ± 0.13 |
| KW/BW ratio | 3.26 × 10−3 ± 2.1 × 10−4 | 4.33 × 10−3 ± 2.3 × 10−4 * | 3.27 × 10−3 ± 1.4 × 10−4 # | 3.7 × 10−3 ± 2 × 10−4 |
| Proteinuria (mg/g) | 228.92 ± 25.10 | 1099.10 ± 359.79 * | 377.92 ± 103.71 # | 611.85 ± 72.06 # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Assi, L.; Saleh, F.A.; Khalil, M.I.; Eid, A.A. Novel Insights of Lithium Chloride Therapeutic Approach for Managing Type 2 Diabetic Kidney Disease: Crosslinking Tau Hyperphosphorylation and TGF Beta Signaling. Diabetology 2025, 6, 26. https://doi.org/10.3390/diabetology6040026
Abou Assi L, Saleh FA, Khalil MI, Eid AA. Novel Insights of Lithium Chloride Therapeutic Approach for Managing Type 2 Diabetic Kidney Disease: Crosslinking Tau Hyperphosphorylation and TGF Beta Signaling. Diabetology. 2025; 6(4):26. https://doi.org/10.3390/diabetology6040026
Chicago/Turabian StyleAbou Assi, Layal, Fatima A. Saleh, Mahmoud I. Khalil, and Assaad A. Eid. 2025. "Novel Insights of Lithium Chloride Therapeutic Approach for Managing Type 2 Diabetic Kidney Disease: Crosslinking Tau Hyperphosphorylation and TGF Beta Signaling" Diabetology 6, no. 4: 26. https://doi.org/10.3390/diabetology6040026
APA StyleAbou Assi, L., Saleh, F. A., Khalil, M. I., & Eid, A. A. (2025). Novel Insights of Lithium Chloride Therapeutic Approach for Managing Type 2 Diabetic Kidney Disease: Crosslinking Tau Hyperphosphorylation and TGF Beta Signaling. Diabetology, 6(4), 26. https://doi.org/10.3390/diabetology6040026







