A Novel PDMS-Based Microfeature-Size Fabrication Method for Biocompatible and Flexible Devices †
Abstract
:1. Introduction
2. Materials and Methods
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Heath, J.R.; Ratner, M.A. Molecular Electronics; American Institute of Physics: College Park, MD, USA, 2003. [Google Scholar]
- Li, L.; Chin, W.S. Rapid fabrication of a flexible and transparent Ag Nanocubes@ PDMS Film as a SERS substrate with high performance. ACS Appl. Mater. Interfaces 2020, 12, 37538–37548. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Zhang, K.; Tian, G.; Jiang, B.; Huang, Y. Stretchable electronics based on PDMS substrates. Adv. Mater. 2021, 33, 2003155. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Peng, S.; Blanloeuil, P.; Wu, S.; Wang, C.H. Wearable Temperature Sensors with Enhanced Sensitivity by Engineering Microcrack Morphology in PEDOT: PSS–PDMS Sensors. ACS Appl. Mater. Interfaces 2020, 12, 36578–36588. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, Y.; Jiang, W. A stretchable and transparent strain sensor based on sandwich-like PDMS/CNTs/PDMS composite containing an ultrathin conductive CNT layer. Compos. Sci. Technol. 2020, 186, 107938. [Google Scholar] [CrossRef]
- Ko, H.C.; Stoykovich, M.P.; Song, J.; Malyarchuk, V.; Choi, W.M.; Yu, C.J.; Geddes Iii, J.B.; Xiao, J.; Wang, S.; Huang, Y.; et al. A hemispherical electronic eye camera based on compressible silicon optoelectronics. Nature 2008, 454, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.W.; Kim, N.; Kim, S. Fabrication of subretinal 3D microelectrodes with hexagonal arrangement. Micromachines 2020, 11, 467. [Google Scholar] [CrossRef]
- Zhou, M.; Kang, D.H.; Kim, J.; Weiland, J.D. Shape morphable hydrogel/elastomer bilayer for implanted retinal electronics. Micromachines 2020, 11, 392. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.C.; Kim, Y.; Ha, C.S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Ojuroye, O.; Torah, R.; Beeby, S. Modified PDMS packaging of sensory e-textile circuit microsystems for improved robustness with washing. Microsyst. Technol. 2019, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Han, S.I.; Kim, D.; Hyeon, T.; Kim, D.H. High-performance stretchable conductive nanocomposites: Materials, processes, and device applications. Chem. Soc. Rev. 2019, 48, 1566–1595. [Google Scholar] [CrossRef]
- Kim, J.; Lee, M.; Shim, H.J.; Ghaffari, R.; Cho, H.R.; Son, D.; Jung, Y.H.; Soh, M.; Choi, C.; Jung, S.; et al. Stretchable silicon nanoribbon electronics for skin prosthesis. Nat. Commun. 2014, 5, 5747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shanehsazzadeh, F.; Rouhi, S.; Ahmadvand, T.; Namazi, M.; Kiani, S.; Fardmanesh, M. A Novel, Low Cost and Versatile Fabrication Method of Flexible Multi-electrode Array for Spinal Cord Stimulation. In Proceedings of the 2020 27th National and 5th International Iranian Conference on Biomedical Engineering (ICBME), Tehran, Iran, 26–27 November 2020; pp. 138–142. [Google Scholar]
- Hasan, M.N.; Radwan, A.N.; Kim, M.; Kucukal, E.; Maji, D.; Pashaei, V.; Chung, C.Y.; Kakkar, A.; Gurkan, U.A. Emerging micro and nanotechnologies in neuroscience: Devices, fabrication methods, and implementation in monitoring of neural activity and drug delivery. Technology 2019, 7, 57–83. [Google Scholar] [CrossRef]
- Ahmadvand, T.; Mirsadeghi, S.; Shanehsazzadeh, F.; Kiani, S.; Fardmanesh, M. A Novel Low-Cost Method for Fabrication of 2D Multi-Electrode Array (MEA) to Evaluate Functionality of Neuronal Cells. Proceedings 2020, 60, 51. [Google Scholar]
- Ortigoza-Diaz, J.; Scholten, K.; Larson, C.; Cobo, A.; Hudson, T.; Yoo, J.; Baldwin, A.; Weltman Hirschberg, A.; Meng, E. Techniques and considerations in the microfabrication of Parylene C microelectromechanical systems. Micromachines 2018, 9, 422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanzari, I.; Callisti, M.; De Grazia, A.; Evans, D.J.; Polcar, T.; Prodromakis, T. Parylene C topographic micropattern as a template for patterning PDMS and Polyacrylamide hydrogel. Sci. Rep. 2017, 7, 5764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.S.; Mahmood, M.; Lee, Y.; Kim, N.K.; Kwon, S.; Herbert, R.; Kim, D.; Cho, H.C.; Yeo, W.H. All-in-One, wireless, stretchable hybrid electronics for smart, connected, and ambulatory physiological monitoring. Adv. Sci. 2019, 6, 1900939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RTV-2 Silicones; The Durability, Stability and High Temperature Resistance of RTV-2 Silicone Rubber. Available online: https://www.elkem.com/silicones/technologies/elastomers/rtv-2/ (accessed on 28 February 2022).
- Armani, D.; Liu, C.; Aluru, N. Re-configurable fluid circuits by PDMS elastomer micromachining. In Proceedings of the Technical Digest. IEEE International MEMS 99 Conference. Twelfth IEEE International Conference on Micro Electro Mechanical Systems (Cat. No. 99CH36291), Orlando, FL, USA, 21 January 1999. [Google Scholar]
- Suzuki, Y. Micromachined high-aspect-ratio parylene beam and its application to low-frequency seismometer Micro Electro Mechanical Systems. In Proceedings of the Sixteenth Annual International Conference on Micro Electro Mechanical Systems, MEMS-03 Kyoto, Kyoto, Japan, 23 January 2003. [Google Scholar]
- Rousche, P.J.; Pellinen, D.S.; Pivin, D.P.; Williams, J.C.; Vetter, R.J.; Kipke, D.R. Flexible polyimide-based intracortical electrode arrays with bioactive capability. IEEE Trans. Biomed. Eng. 2001, 48, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Terkan, K.; Zurita, F.; Jamal Khalaf, T.; Rinklin, P.; Teshima, T.; Kohl, T.; Wolfrum, B. Soft peripheral nerve interface made from carbon nanotubes embedded in silicone. APL Mater. 2020, 8, 101111. [Google Scholar] [CrossRef]
- Guo, L.; DeWeerth, S.P. Implementation of integratable PDMS-based conformable microelectrode arrays using a multilayer wiring interconnect technology. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009. [Google Scholar]
- Branner, A.; Stein, R.B.; Normann, R.A. Selective stimulation of cat sciatic nerve using an array of varying-length microelectrodes. J. Neurophysiol. 2001, 85, 1585–1594. [Google Scholar] [CrossRef] [Green Version]
- McDonnall, D.; Clark, G.A.; Normann, R.A. Interleaved, multisite electrical stimulation of cat sciatic nerve produces fatigue-resistant, ripple-free motor responses. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 208–215. [Google Scholar] [CrossRef]
- Ecoflex TM 00-30. Available online: https://www.smooth-on.com/products/ecoflex-00-30/ (accessed on 28 February 2022).
- Cho, D.; Park, J.; Kim, T.; Jeon, S. Recent advances in lithographic fabrication of micro-/nanostructured polydimethylsiloxanes and their soft electronic applications. J. Semicond. 2019, 40, 111605. [Google Scholar] [CrossRef]
- Cong, H.; Pan, T. Photopatternable conductive PDMS materials for microfabrication. Adv. Funct. Mater. 2008, 18, 1912–1921. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, J.; Ren, J.; Rong, L.; Cao, P.F.; Advincula, R.C. 3D printed multifunctional, hyperelastic silicone rubber foam. Adv. Funct. Mater. 2019, 29, 1900469. [Google Scholar] [CrossRef]
- Millet, L.J.; Jain, A.; Gillette, M.U. Less is More: Oligomer extraction and hydrothermal annealing increase PDMS bonding forces for new microfluidics assembly and for biological studies. bioRxiv 2017, 150953. [Google Scholar] [CrossRef]
- Lee, J.N.; Park, C.; Whitesides, G.M. Solvent compatibility of poly (dimethylsiloxane)-based microfluidic devices. Anal. Chem. 2003, 75, 6544–6554. [Google Scholar] [CrossRef] [PubMed]
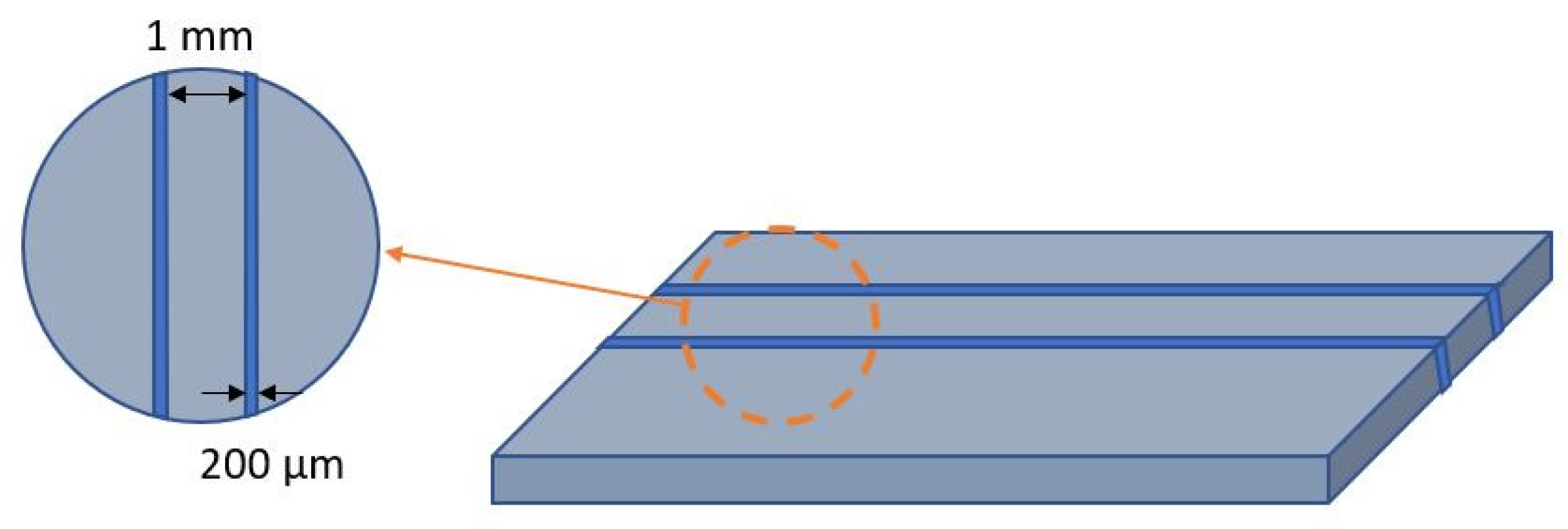
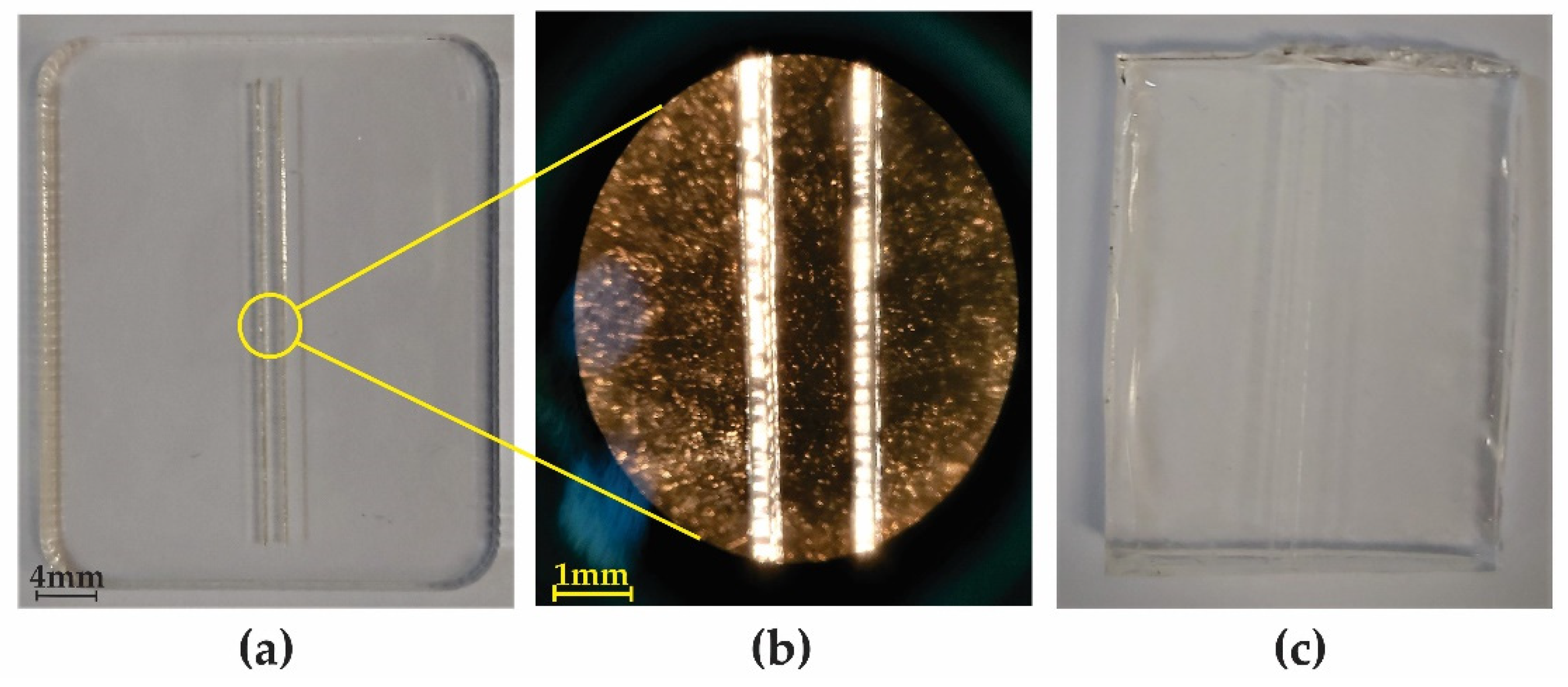
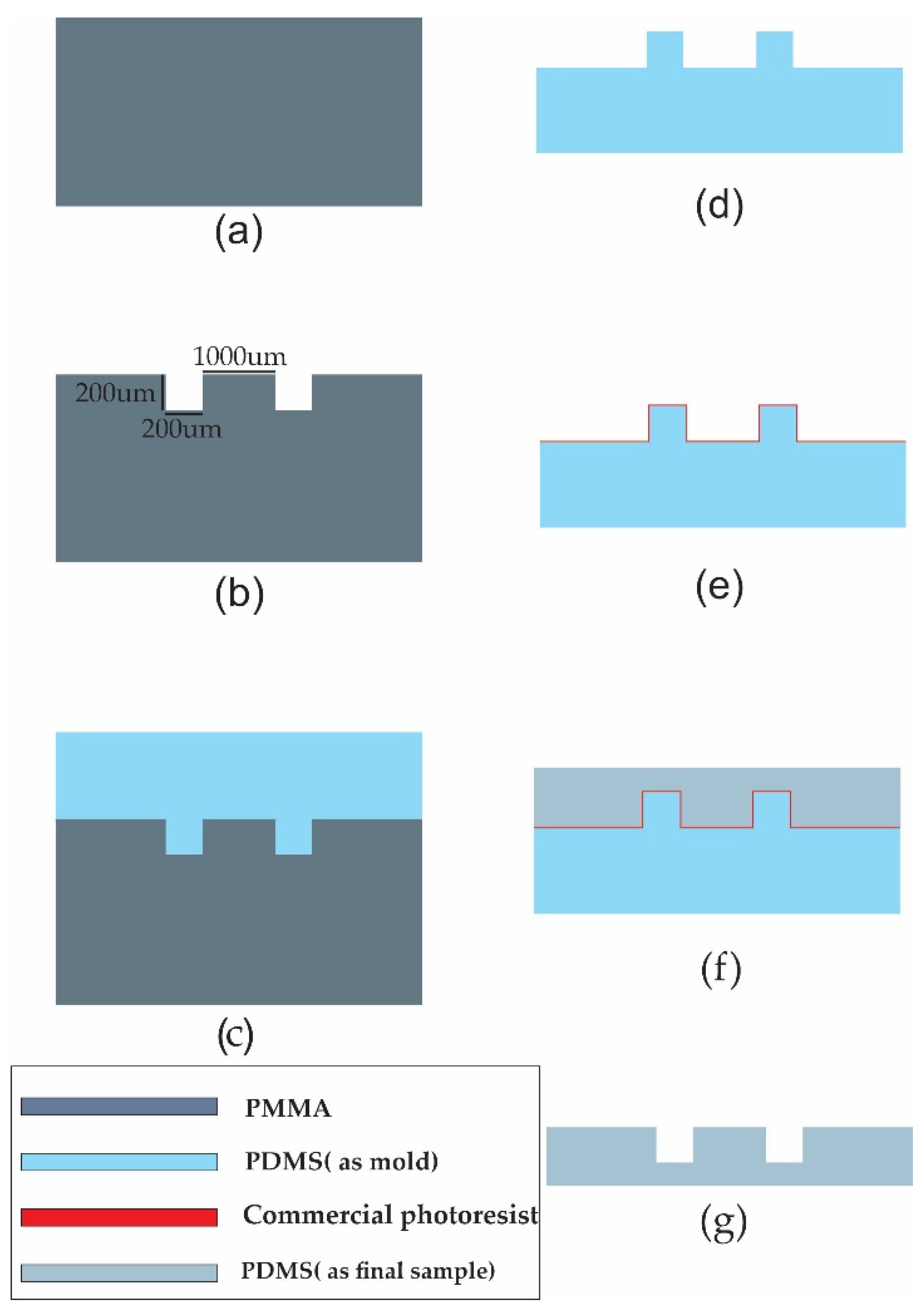

| Mold Materials | PMMA Peeling Off Feasibility | Silicon Spray Anti-Adhesion Layer | Water and Sugar Solution Anti-Adhesion Layer | Commercial Photoresist Anti-Adhesion Layer |
|---|---|---|---|---|
| RTV-2 | ☑ | ⊠ | ⊠ | ⊠ |
| Ecoflex00-30 | ⊠ | ⊠ | ⊠ | ⊠ |
| PDMS | ☑ | ☑ | ☑ | ☑ |
| Micropatterning Technique | Advantages | Fabrication Challenges |
|---|---|---|
| photolithography methods |
|
|
| Molding techniques |
|
|
| PDMS-based-molding technique |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashayekhi, F.; Shanehsazzadeh, F.; Fardmanesh, M. A Novel PDMS-Based Microfeature-Size Fabrication Method for Biocompatible and Flexible Devices. Eng. Proc. 2021, 11, 36. https://doi.org/10.3390/ASEC2021-11132
Mashayekhi F, Shanehsazzadeh F, Fardmanesh M. A Novel PDMS-Based Microfeature-Size Fabrication Method for Biocompatible and Flexible Devices. Engineering Proceedings. 2021; 11(1):36. https://doi.org/10.3390/ASEC2021-11132
Chicago/Turabian StyleMashayekhi, Fatemeh, Faezeh Shanehsazzadeh, and Mehdi Fardmanesh. 2021. "A Novel PDMS-Based Microfeature-Size Fabrication Method for Biocompatible and Flexible Devices" Engineering Proceedings 11, no. 1: 36. https://doi.org/10.3390/ASEC2021-11132






