Formulation of Effervescent Compact Detergent Tablets with Unique Chemical Compositions †
Abstract
:1. Introduction
2. Materials and Methods
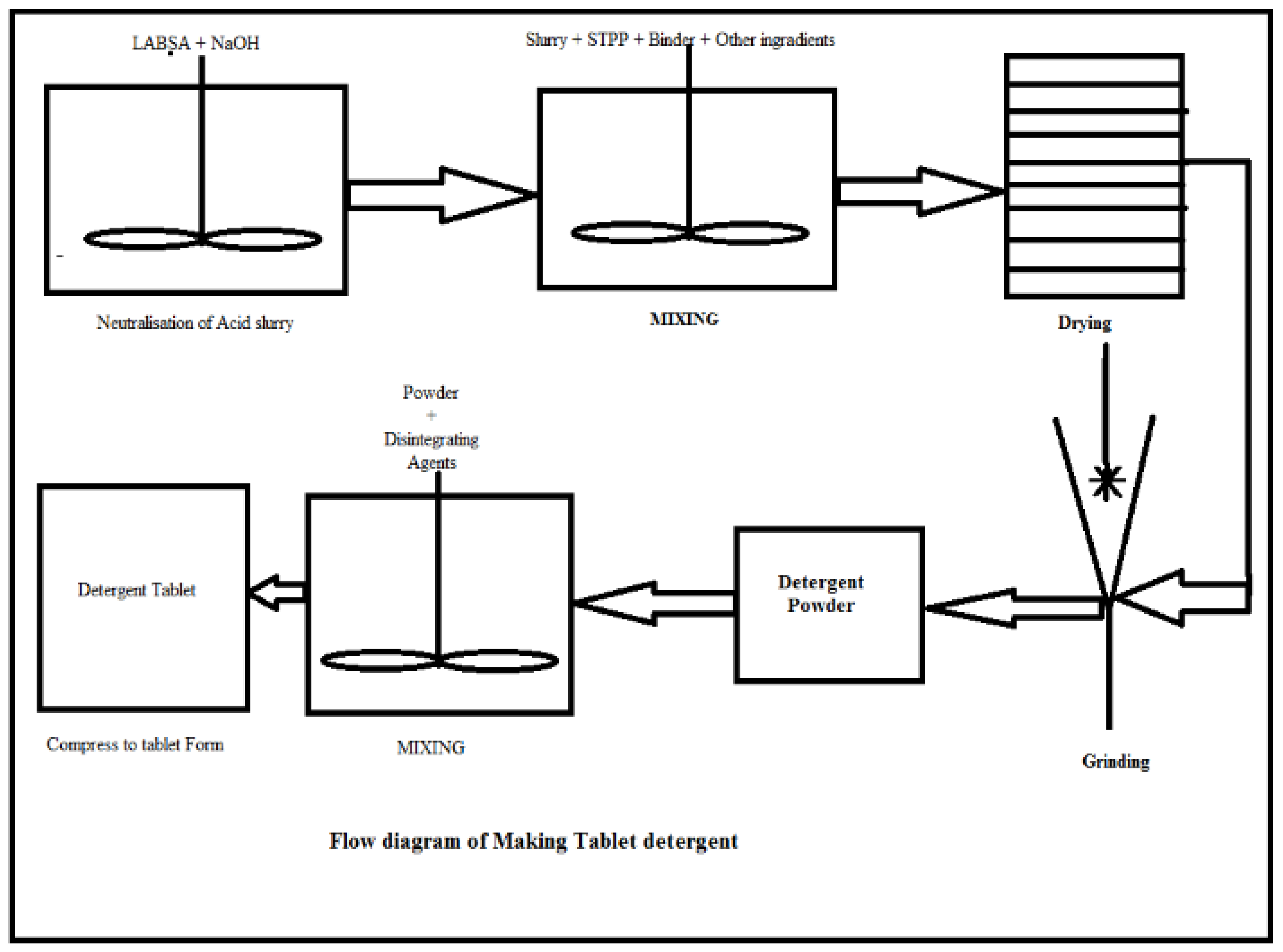
2.1. Formulation of Detergent Tablets
- (i)
- Neutralization of Acid Slurry of LABSALABSA has an acid value of 225 , which was neutralized by NaOH solution. As per stoichiometry, LABSA (100 g) was neutralized with 16 g NaOH (Table 1).
- (ii)
- Formulation of DetergentNeutralized LABSA was then mixed with sodium silicate, sodium sulphate, water and STPP at 55 °C using an overhead stirrer. The quantity of sodium silicate and STPP depended upon active matter and hardness of water [3,4,5,6]. This mixture of surfactant, binder and other ingredients were dried using a vacuum oven at 70 °C under a pressure of 350 mm for 4 to 5 h. The dried mixture was converted in fine powder form with the help of a mixer or grinder or ball mill.
- (iii)
- Compressing to Tablet FormThe fine powder was then mixed with various disintegrating agents such as starch, silicic acid, citric acid, and sodium bicarbonate. This mixture of detergent powder and disintegrating agents were compressed with the help of the tablet machine for converting detergents to tablet form. When this tablet was contacted with enough water, the CO2 was released from disintegrating agents, which facilitated dispersion of the tablet in water.
2.2. Detergency Test
2.2.1. Fabric Soiling
2.2.2. Washing
3. Results and Discussion
3.1. Disintegration Time
3.2. Cleaning Performance of Detergents and Their Tablets
3.3. Foam Stability and Height
3.4. Wetting Ability
3.5. Tablet Friability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, A.; Sharma, A.; Bansal, S.; Sharma, P. Comparative interaction study of amylase and surfactants for potential detergent formulation. J. Mol. Liq. 2018, 261, 397–401. [Google Scholar] [CrossRef]
- Cheng, K.C.; Khoo, Z.S.; Lo, N.W.; Tan, W.J.; Chemmangattuvalappil, N.G. Design and performance optimisation of detergent product containing a binary mixture of anionic-nonionic surfactants. Heliyon 2020, 6, e03861. [Google Scholar] [CrossRef] [PubMed]
- Chateau, M.E.; Galet, L.; Soudais, Y.; Fages, J. Processing a detergent powder formulation: Direct compression, and high shear wet granulation followed by compression. Powder Technol. 2005, 157, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Ahmadian, H.; Ghadiri, M. Analysis of enzyme dust formation in detergent manufacturing plants. Adv. Powder Technol. 2007, 18, 53–67. [Google Scholar] [CrossRef]
- Tai, X.M.; Song, J.Y.; Du, Z.P.; Liu, X.; Wang, T.; Wang, G. The performance test of fatty acid methyl ester sulfonates and application in the dishwashing liquid detergent. J. Dispers. Sci. Technol. 2018, 39, 1422–1426. [Google Scholar] [CrossRef]
- Ponnusamy, T.; Dubal, S.A.; Momin, S.A. Studies in detergency: Influence of different factors for removing motor oil stain from the cotton fabric. J. Dispers. Sci. Technol. 2008, 29, 1123–1128. [Google Scholar] [CrossRef]
- Kogawa, A.C.; Cernic, B.G.; do Couto, L.G.D.; Salgado, H.R.N. Synthetic detergents: 100 years of history. Saudi Pharm. J. 2017, 25, 934–938. [Google Scholar] [CrossRef] [PubMed]

| Sr. No. | Sample | Weight of Sample | B.R. | Acid Value |
|---|---|---|---|---|
| 1 | LABSA | 2.25 g | 90 mL | |
| 2 | LABSA after neutralization | 2.5 g | 0.0 |
| Sr. No. | Detergent Powder (%) | Disintegrating Agents (g) | Volume of Water (mL) | Disintegration Time | |||
|---|---|---|---|---|---|---|---|
| Starch | Silicic Acid | Citric Acid | Sodium Bicarbonate | ||||
| 1 | 100 | - | - | - | - | 100 | 78 h |
| 2 | 70 | 30 | - | - | - | 100 | 50 h |
| 3 | 70 | 15 | 15 | - | - | 100 | 2 h |
| 4 | 60 | 15 | 25 | - | - | 100 | 2 h |
| 5 | 60 | 15 | 5 | 8 | 7 | 100 | 3 min |
| 6 | 70 | - | - | 15 | 15 | 100 | 2 h |
| 7 | 60 | 15 | - | 15 | 10 | 100 | 3 min |
| 8 | 60 | 10 | 18 | 12 | 100 | 1 min | |
| 9 | 60 | 10 | 10 | 10 | 10 | 100 | 3.5 min |
| 10 | 60 | 15 | 5 | 10 | 10 | 100 | 1 min |
| 11 | 50 | 15 | 5 | 15 | 15 | 100 | 0.5 min |
| Sample | Concentration | % Detergency |
|---|---|---|
| Detergent Powder | 0.1 | 63.19 |
| 0.25 | 64.8 | |
| 0.5 | 72.59 | |
| Detergent tablet having disintegrating time 0.5 min | 0.1 | 62.45 |
| 0.25 | 65.12 | |
| 0.5 | 72.43 | |
| Detergent tablet having disintegrating time 2.0 min | 0.1 | 59.32 |
| 0.25 | 61.29 | |
| 0.5 | 63.84 | |
| Commercial detergent powder | 0.1 | 44.19 |
| 0.25 | 48.32 | |
| 0.5 | 53.11 |
| Sample | Concentration | Time (min) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | ||
| Height (cm) | |||||||
| Detergent Powder | 0.1 | 26.5 | 26.5 | 26.5 | 26.4 | 25.6 | 24.2 |
| 0.25 | 26.5 | 26.5 | 26.5 | 26.1 | 24.8 | 24.0 | |
| 0.5 | 26.5 | 26.5 | 26.5 | 26.3 | 24.6 | 23.8 | |
| Detergent tablet having disintegrating time 0.5 min | 0.1 | 26.5 | 26.5 | 26.5 | 25.6 | 25.0 | 23.8 |
| 0.25 | 26.5 | 26.5 | 26.5 | 25.8 | 24.2 | 23.4 | |
| 0.5 | 26.5 | 26.5 | 26.5 | 24.5 | 24.0 | 22.8 | |
| Detergent tablet having disintegrating time 2.0 min | 0.1 | 26.5 | 26.5 | 26.5 | 24.5 | 24.5 | 24 |
| 0.25 | 26.5 | 26.5 | 26.5 | 25.4 | 24 | 24 | |
| 0.5 | 26.5 | 26.5 | 26.5 | 25 | 25 | 24.5 | |
| Commercial detergent powder | 0.1 | 23.2 | 23.2 | 22.4 | 22 | 22 | 21 |
| 0.25 | 23.2 | 23.2 | 22.3 | 22 | 22 | 21.5 | |
| 0.5 | 23.0 | 23.0 | 22.3 | 22 | 22 | 21.5 | |
| Sr. No. | Batch | Concentration (%) | ||
|---|---|---|---|---|
| 0.5 | 1 | 1.5 | ||
| 1 | Detergent Powder | 55 s | 38 s | 32 s |
| Detergent tablet | 63 s | 41 s | 38 s | |
| 2 | Commercial detergent powder | 24 s | 25 s | 23 s |
| Sr. No. | Batch No. | % Friability of Tablet |
|---|---|---|
| 1 | Detergent tablet having disintegrating time 2 min | 26.91 |
| 2 | Detergent tablet having disintegrating time 30 s | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mestri, R.; Mali, S.N.; Pratap, A. Formulation of Effervescent Compact Detergent Tablets with Unique Chemical Compositions. Eng. Proc. 2021, 11, 38. https://doi.org/10.3390/ASEC2021-11186
Mestri R, Mali SN, Pratap A. Formulation of Effervescent Compact Detergent Tablets with Unique Chemical Compositions. Engineering Proceedings. 2021; 11(1):38. https://doi.org/10.3390/ASEC2021-11186
Chicago/Turabian StyleMestri, Rohan, Suraj N. Mali, and Amit Pratap. 2021. "Formulation of Effervescent Compact Detergent Tablets with Unique Chemical Compositions" Engineering Proceedings 11, no. 1: 38. https://doi.org/10.3390/ASEC2021-11186







