Sensorized T-Shirt for Cardiological Patients in Telemonitoring †
Abstract
:1. Introduction
2. Materials and Methods
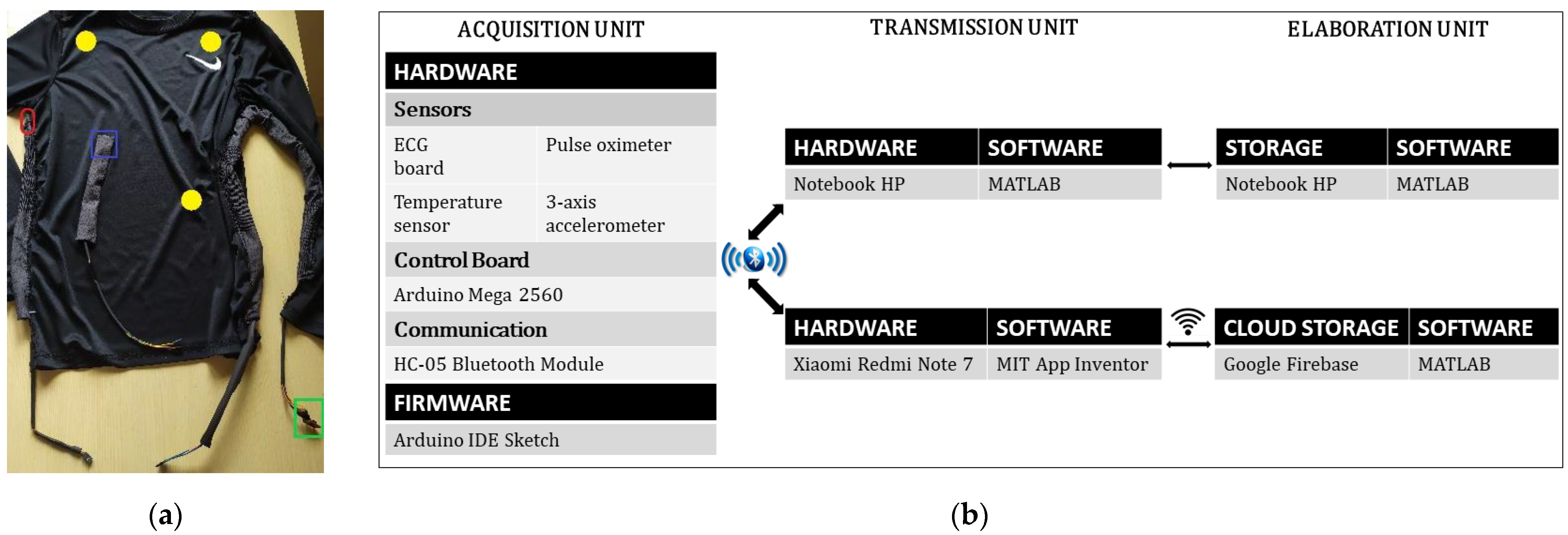
2.1. Sensors
2.2. Control Board and Connectivity
2.3. Acquisition Unit
2.4. Transmission and Elaboration Unit
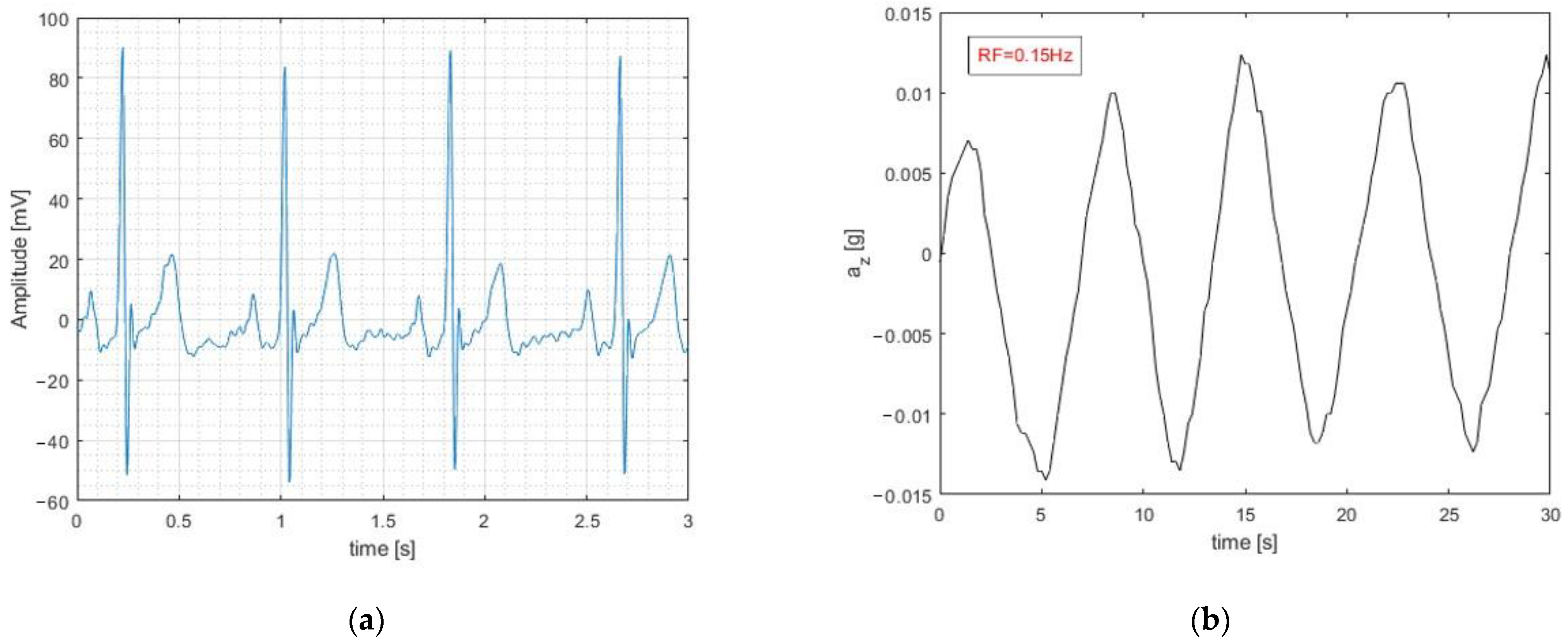
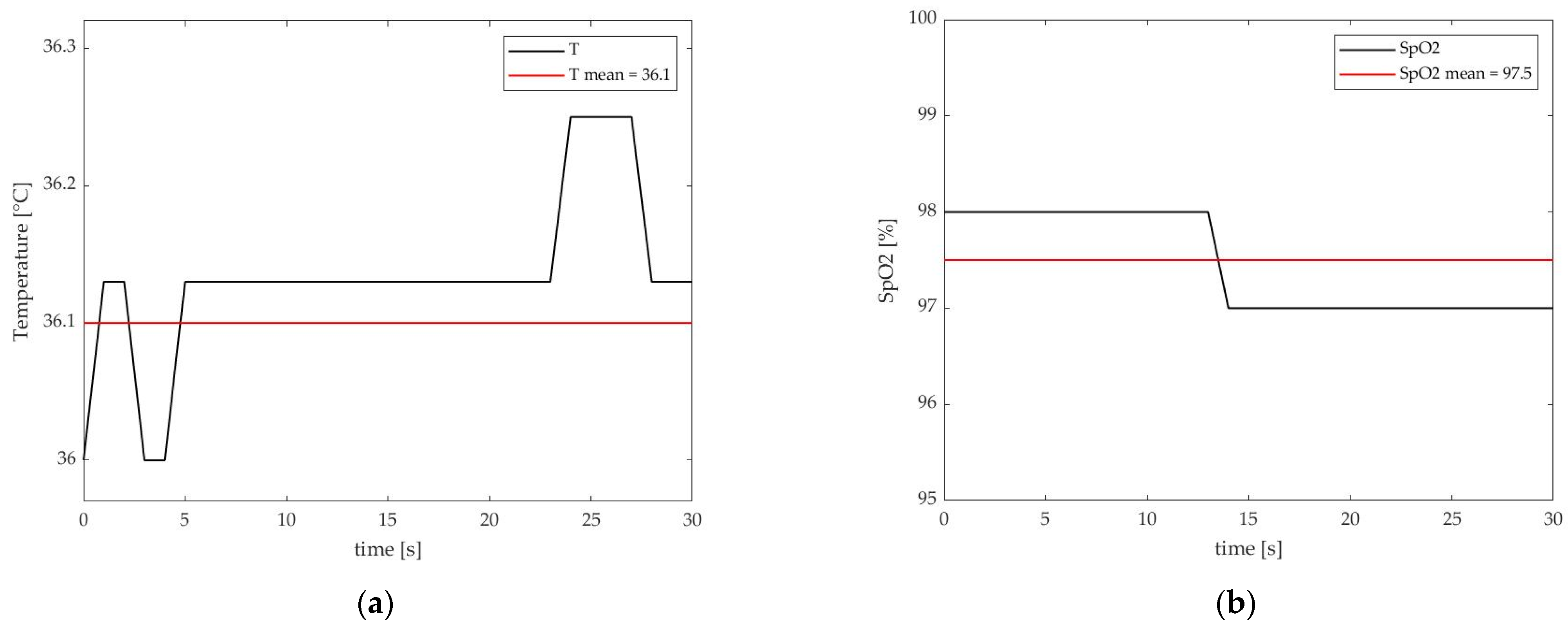
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strehle, E.M.; Shabde, N. One hundred years of telemedicine: Does this new technology have a place in paediatrics? Arch. Dis. Child. 2006, 91, 956–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. A Health Telematics Policy in Support of WHO’s Health-For-All Strategy for Global Health Development: Report of the WHO Group Consultation on Health Telematics, 11–16 December, Geneva, 1997; World Health Organization: Geneva, Switzerland, 1998.
- Deen, M.J. Information and communications technologies for elderly ubiquitous healthcare in a smart home. Pers. Ubiquitous Comput. 2015, 19, 573–599. [Google Scholar] [CrossRef]
- Asua, J.; Orruño, E.; Reviriego, E.; Gagnon, M.P. Healthcare professional acceptance of telemonitoring for chronic care patients in primary care. BMC Med. Inform. Decis. Mak. 2012, 12, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Varnfield, M.; Jayasena, R.; Celler, B. Home telemonitoring for chronic disease management: Perceptions of users and factors influencing adoption. Health Inform. J. 2021, 27, 1460458221997893. [Google Scholar] [CrossRef]
- Queirós, A.; Pereira, L.; Dias, A.; Rocha, N.P. Technologies for Ageing in Place to Support Home Monitoring of Patients with Chronic Diseases. In Proceedings of the 10th International Joint Conference on Biomedical Engineering Systems and Technologies, Porto, Portugal, 21–23 February 2017; pp. 66–76. [Google Scholar] [CrossRef]
- Walker, R.C.; Tong, A.; Howard, K.; Palmer, S.C. Patient expectations and experiences of remote monitoring for chronic diseases: Systematic review and thematic synthesis of qualitative studies. Int. J. Med. Inform. 2019, 124, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Lucangeli, L.; D’Angelantonio, E.; Camomilla, V.; Pallotti, A. SISTINE: Sensorized Socks for Telemonitoring of Vascular Disease Patients. In Proceedings of the 2021 IEEE International Workshop on Metrology for Industry 4.0 & IoT (MetroInd4.0&IoT), Rome, Italy, 7–9 June 2021; pp. 192–197. [Google Scholar] [CrossRef]
- Henriques, J.; Carvalho, P.; Paredes, S.; Rocha, T.; Habetha, J.; Antunes, M.; Morais, J. Prediction of Heart Failure Decompensation Events by Trend Analysis of Telemonitoring Data. IEEE J. Biomed. Health Inform. 2014, 19, 1757–1769. [Google Scholar] [CrossRef]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef] [Green Version]
- Eletter, S.; Yasmin, T.; Elrefae, G.; Aliter, H.; Elrefae, A. Building an Intelligent Telemonitoring System for Heart Failure: The Use of the Internet of Things, Big Data, and Machine Learning. In Proceedings of the 2020 21st International Arab Conference on Information Technology (ACIT), Giza, Egypt, 28–30 November 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Kitsiou, S.; Paré, G.; Jaana, M. Effects of Home Telemonitoring Interventions on Patients with Chronic Heart Failure: An Overview of Systematic Reviews. J. Med. Internet Res. 2015, 17, e63. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Cardiovascular Diseases (CVDs). 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 1 August 2021).
- Khunti, K. Accurate interpretation of the 12-lead ECG electrode placement: A systematic review. Health Educ. J. 2013, 73, 610–623. [Google Scholar] [CrossRef]
- Kwon, S.; Lee, S.-R.; Choi, E.-K.; Ahn, H.-J.; Song, H.-S.; Lee, Y.-S.; Oh, S. Validation of Adhesive Single-Lead ECG Device Compared with Holter Monitoring among Non-Atrial Fibrillation Patients. Sensors 2021, 21, 3122. [Google Scholar] [CrossRef]
- Bayoumy, K.; Gaber, M.; Elshafeey, A.; Mhaimeed, O.; Dineen, E.H.; Marvel, F.A.; Martin, S.S.; Muse, E.D.; Turakhia, M.P.; Tarakji, K.G.; et al. Smart wearable devices in cardiovascular care: Where we are and how to move forward. Nat. Rev. Cardiol. 2021, 18, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. 2016 ESC Guidelines for the Management of Atrial Fibrillation Developed in Collaboration with EACTS. Eur. Heart J. 2016, 37, e636–e683. [Google Scholar] [CrossRef] [Green Version]
- Duncker, D.; Ding, W.Y.; Etheridge, S.; Noseworthy, P.A.; Veltmann, C.; Yao, X.; Jared Bunch, T.; Gupta, D. Smart Wearables for Cardiac Monitoring—Real-World Use beyond Atrial Fibrillation. Sensors 2021, 21, 2539. [Google Scholar] [CrossRef] [PubMed]
- Nicolò, A.; Massaroni, C.; Schena, E.; Sacchetti, M. The Importance of Respiratory Rate Monitoring: From Healthcare to Sport and Exercise. Sensors 2020, 20, 6396. [Google Scholar] [CrossRef] [PubMed]
- Vanegas, E.; Igual, R.; Plaza, I. Sensing Systems for Respiration Monitoring: A Technical Systematic Review. Sensors 2020, 20, 5446. [Google Scholar] [CrossRef] [PubMed]
- Massaroni, C.; Nicolò, A.; Presti, D.L.; Sacchetti, M.; Silvestri, S.; Schena, E. Contact-Based Methods for Measuring Respiratory Rate. Sensors 2019, 19, 908. [Google Scholar] [CrossRef] [Green Version]
- Orphanidou, C.; Fleming, S.; Shah, S.A.; Tarassenko, L. Data Fusion for Estimating Respiratory Rate from a Single-Lead ECG. Biomed. Signal Process. Control 2013, 8, 98–105. [Google Scholar] [CrossRef]
- Majumder, S.; Mondal, T.; Deen, M.J. Wearable Sensors for Remote Health Monitoring. Sensors 2017, 17, 130. [Google Scholar] [CrossRef]
- Masip, J.; Gayà, M.; Páez, J.; Betbesé, A.; Vecilla, F.; Manresa, R.; Ruíz, P. Pulsioximetría en el diagnóstico de insuficiencia cardiaca aguda. Rev. Esp. Cardiol. 2012, 65, 879–884. [Google Scholar] [CrossRef]
- Kirschen, G.W.; Singer, D.D.; Thode, H.C.; Singer, A.J. Relationship between body temperature and heart rate in adults and children: A local and national study. Am. J. Emerg. Med. 2020, 38, 929–933. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.C. Wearable Sensors for Human Activity Monitoring: A Review. IEEE Sens. J. 2014, 15, 1321–1330. [Google Scholar] [CrossRef]
- Reith, J.; Jørgensen, H.; Pedersen, P.; Nakamaya, H.; Jeppesen, L.; Olsen, T.; Raaschou, H. Body temperature in acute stroke: Relation to stroke severity, infarct size, mortality, and outcome. Lancet 1996, 347, 422–425. [Google Scholar] [CrossRef]
- Geurts, M.; on behalf of the DUST investigators; Scheijmans, F.E.V.; van Seeters, T.; Biessels, G.J.; Kappelle, L.J.; Velthuis, B.K.; van der Worp, H.B. Temporal profile of body temperature in acute ischemic stroke: Relation to infarct size and outcome. BMC Neurol. 2016, 16, 233. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, M.H.; Hossain, Q.D.; Saha, P.; Rahaman, M. Design, fabrication and performance evaluation of a three electrode ECG recorder. In Proceedings of the 2016 International Conference on Innovations in Science, Engineering and Technology (ICISET), Dhaka, Bangladesh, 28–29 October 2016; pp. 1–4. [Google Scholar] [CrossRef]
- Guvenc, H. Wireless ECG Device with Arduino. In Proceedings of the 2020 Medical Technologies Congress (TIPTEKNO), Antalya, Turkey, 19–20 November 2020; pp. 1–4. [Google Scholar] [CrossRef]
- Gifari, M.W.; Zakaria, H.; Mengko, R. Design of ECG Homecare:12-lead ECG acquisition using single channel ECG device developed on AD8232 analog front end. In Proceedings of the 2015 International Conference on Electrical Engineering and Informatics (ICEEI), Denpasar, Indonesia, 10–11 August 2015; pp. 371–376. [Google Scholar] [CrossRef]
- Prasad, A.S.; Kavanashree, N. ECG Monitoring System Using AD8232 Sensor. In Proceedings of the 2019 International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 17–19 July 2019; pp. 976–980. [Google Scholar] [CrossRef]
- Ghifari, A.F.; Perdana, R.S. Minimum System Design of The IoT-Based ECG Monitoring. In Proceedings of the 2020 International Conference on ICT for Smart Society (ICISS), Bandung, Indonesia, 19–20 November 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Penica, M.; Mohandas, R.; Bhattacharya, M.; Vancamp, K.; Hayes, M.; O’Connell, E. A Covid-19 viral transmission prevention system for embedded devices utilising deep learning. In Proceedings of the 2021 32nd Irish Signals and Systems Conference (ISSC), Athlone, Ireland, 10–11 June 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Saha, R.; Biswas, S.; Sarmah, S.; Karmakar, S.; Das, P. A Working Prototype Using DS18B20 Temperature Sensor and Arduino for Health Monitoring. SN Comput. Sci. 2021, 2, 33. [Google Scholar] [CrossRef]
- Fajrin, H.R.; Ilahi, M.R.; Handoko, B.S.; Permatasari, I. Body temperature monitoring based on telemedicine. J. Phys. Conf. Ser. 2019, 1381, 012014. [Google Scholar] [CrossRef]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D. Respiratory Rate and Flow Waveform Estimation from Tri-axial Accelerometer Data. In Proceedings of the 2010 International Conference on Body Sensor Networks, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar] [CrossRef]
- Ahsanuzzaman, S.; Ahmed, T.; Rahman, A. Low Cost, Portable ECG Monitoring and Alarming System Based on Deep Learning. In Proceedings of the 2020 IEEE Region 10 Symposium (TENSYMP), Dhaka, Bangladesh, 5–7 June 2020; pp. 316–319. [Google Scholar] [CrossRef]
- Hughes, S.; Liu, H.; Zheng, D. Influences of Sensor Placement Site and Subject Posture on Measurement of Respiratory Frequency Using Triaxial Accelerometers. Front. Physiol. 2020, 11, 823. [Google Scholar] [CrossRef] [PubMed]
- Ferrazza, M.; D’Abbondanza, N.; Piuzzi, E.; Pallotti, A. Statistical classification for the screening of cardiological patients by means of a sensorized T-shirt. In Proceedings of the 2021 International Electronic Conference on Applied Sciences, Online, 15–31 October 2021. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Abbondanza, N.; Ferrazza, M.; Lucangeli, L.; Piuzzi, E.; Pallotti, A. Sensorized T-Shirt for Cardiological Patients in Telemonitoring. Eng. Proc. 2021, 11, 48. https://doi.org/10.3390/ASEC2021-11130
D’Abbondanza N, Ferrazza M, Lucangeli L, Piuzzi E, Pallotti A. Sensorized T-Shirt for Cardiological Patients in Telemonitoring. Engineering Proceedings. 2021; 11(1):48. https://doi.org/10.3390/ASEC2021-11130
Chicago/Turabian StyleD’Abbondanza, Noemi, Martina Ferrazza, Leandro Lucangeli, Emanuele Piuzzi, and Antonio Pallotti. 2021. "Sensorized T-Shirt for Cardiological Patients in Telemonitoring" Engineering Proceedings 11, no. 1: 48. https://doi.org/10.3390/ASEC2021-11130







