The Relationship between the Degree of Conversion in Dental Dimethacrylate Polymers Determined by Infrared Spectroscopy and Polymerization Shrinkage †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.1. Instrumentation
2.3.2. Calculation of the Degree of Conversion (DCIR)
2.4. Polymerization Shrinkage
2.4.1. Density Measurements
2.4.2. Calculation of the Polymerization Shrinkage
2.4.3. Calculation of the Degree of Conversion (DCS)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajewski, V.E.S.; Pfeifer, C.S.; Fróes-Salgado, N.R.G.; Boaro, L.C.C.; Braga, R.R. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Kannurpatti, A.; Anseth, J.; Bowman, C.H.N. A study of the evolution of mechanical properties and structural heterogeneity of polymer networks formed by photo-polymerizations of multifunctional (meth)acrylates. Polymer 1998, 39, 2507–2513. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Alshali, R.Z.; Silikas, N.; Satterthwaite, J.D. Degree of conversion of bulk-fill compared to conventional resin-composites at two time intervals. Dent. Mater. 2013, 29, e213–e217. [Google Scholar] [CrossRef] [PubMed]
- Dickens, H.; Stansbury, J.W.; Choi, K.M.; Floyd, C.J.E. Photopolymerization kinetics of methacrylate dental resins. Macromolecules 2003, 36, 6043–6053. [Google Scholar] [CrossRef]
- Pfeifer, C.S.; Shelton, Z.R.; Braga, R.R.; Windmoller, D.; Machado, J.C.; Stansbury, J.W. Characterization of dimethacrylate polymeric networks: A study of the crosslinked structure formed by monomers used in dental composites. Eur. Polym. J. 2011, 47, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stansbury, J.W. Dimethacrylate network formation and polymer property evolution as determined by the selection of monomers and curing conditions. Dent. Mater. 2012, 28, 13–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barszczewska-Rybarek, I.; Chladek, G. Studies on the curing efficiency and mechanical properties of Bis-GMA and TEGDMA nanocomposites containing silver nanoparticles. Int. J. Mol. Sci. 2018, 19, 3937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Par, M.; Gamulin, O.; Marovic, D.; Klaric, E.; Tarle, Z. Raman spectroscopic assessment of degree of conversion of bulk-fill resin composites—Changes at 24 hours post cure. Open. Dent. 2015, 40, e92–e101. [Google Scholar] [CrossRef] [PubMed]
- Randolph, L.D.; Palin, W.M.; Bebelman, S.; Devaux, J.; Gallez, B.; Leloup, G.; Leprince, J.G. Ultra-fast light-curing resin composite with increased conversion and reduced monomer elution. Dent. Mater. 2014, 30, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.H.; Erickson, R.L.; Davidson, C.L. The effect of filler and silane content on conversion of resin-based composite. Dent. Mater. 2003, 19, 327–333. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Academic Press: London, UK, 1991; pp. 137–281. [Google Scholar]
- Moraes, L.G.; Rocha, R.S.; Menegazzo, L.M.; de Araújo, E.B.; Yukimito, K.; Moraes, J.C. Infrared spectroscopy: A tool for determination of the degree of conversion in dental composites. J. Appl. Oral. Sci. 2008, 16, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Barszczewska-Rybarek, I.M. Quantitative determination of degree of conversion in photocured poly(urethane-dimethacrylate)s by FTIR spectroscopy. J. Appl. Polym. Sci. 2012, 123, 1604–1611. [Google Scholar] [CrossRef]
- Collares, F.M.; Portella, F.F.; Leitune, V.C.B.; Samuel, S.M.W. Discrepancies in degree of conversion measurements by FTIR. Braz. Oral. Res. 2014, 28, 9–15. [Google Scholar]
- Patel, M.P.; Braden, M.; Davy, K.W.M. Polymerization shrinkage of methacrylate esters. Biomaterials 1987, 8, 53–56. [Google Scholar] [CrossRef]
- ISO 1675:1985; Plastics—Liquid Resins—Determination of Density by the Pyknometer Method. International Standard Organisation: London, UK, 1985.
- Oliveira, K.M.; Lancellotti, A.C.; Ccahuana-Vásquez, R.A.; Consani, S. Shrinkage stress and degree of conversion of dental composite submitted to different photoactivation protocols. Acta. Odontol. Latinoam. 2012, 25, 115–122. [Google Scholar] [PubMed]
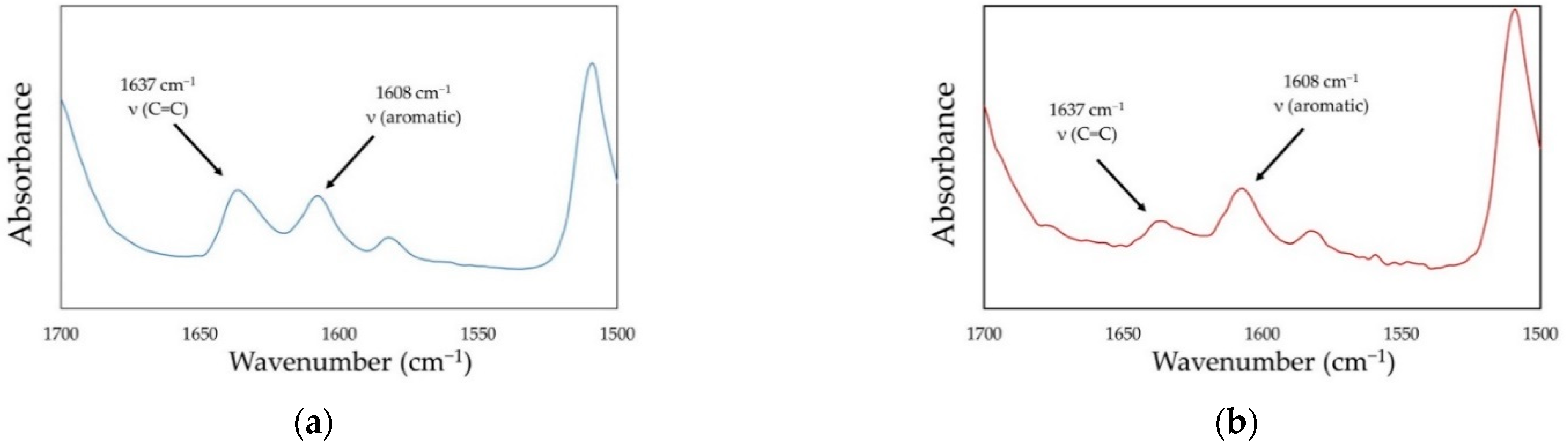
| Sample Name | Weight Ratios | MW (g/mol) | xDB (mol/kg) | dm (g/cm3) | dp (g/cm3) | |||
|---|---|---|---|---|---|---|---|---|
| Bis-GMA | TEGDMA | avg. | SD | avg. | SD | |||
| B20:T80 | 20 | 80 | 314.07 | 6.37 | 1.106 | 0.024 | 1.194 | 0.029 |
| B30:T70 | 30 | 70 | 330.09 | 6.06 | 1.113 | 0.052 | 1.204 | 0.025 |
| B40:T60 | 40 | 60 | 347.78 | 5.75 | 1.121 | 0.072 | 1.207 | 0.061 |
| B50:T50 | 50 | 50 | 367.51 | 5.44 | 1.127 | 0.046 | 1.210 | 0.014 |
| B60:T40 | 60 | 40 | 389.60 | 5.13 | 1.133 | 0.062 | 1.219 | 0.016 |
| B70:T30 | 70 | 30 | 414.50 | 4.83 | 1.141 | 0.094 | 1.233 | 0.033 |
| B80:T20 | 80 | 20 | 442.81 | 4.52 | 1.147 | 0.135 | 1.216 | 0.019 |
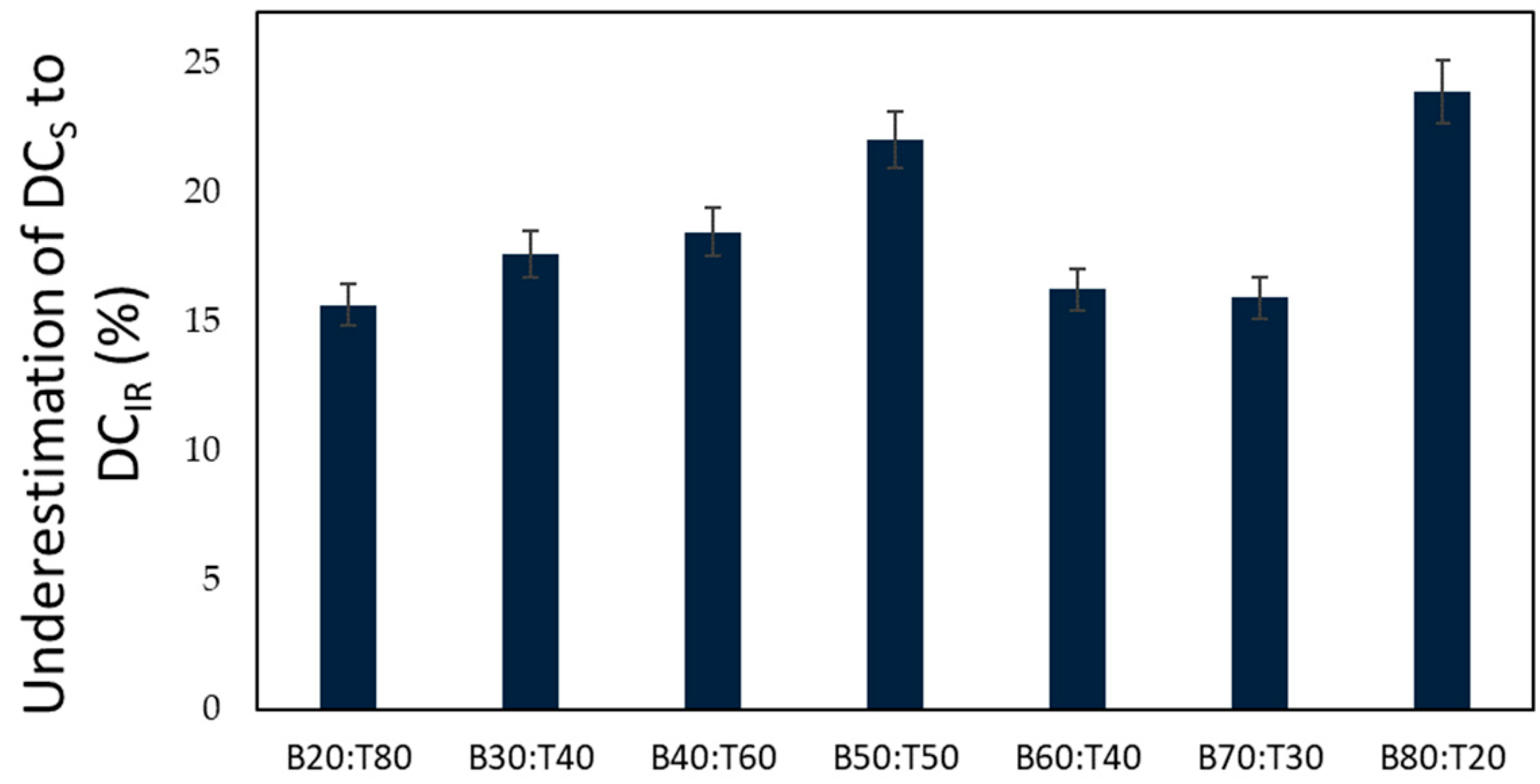
| Sample Name | Stheor (%) | S (%) | DCS (%) | DCIR (%) | |||
|---|---|---|---|---|---|---|---|
| avg. | SD | avg. | SD | avg. | SD | ||
| B20:T80 | 15.85 | 7.39 | 0.45 | 46.64 | 2.32 | 55.30 | 5.23 |
| B30:T70 | 15.17 | 7.55 | 0.52 | 49.81 | 2.56 | 60.47 | 4.78 |
| B40:T60 | 14.49 | 7.21 | 0.41 | 49.75 | 2.31 | 61.01 | 5.23 |
| B50:T50 | 13.80 | 6.89 | 0.46 | 49.88 | 3.04 | 63.97 | 3.56 |
| B60:T40 | 13.09 | 7.11 | 0.39 | 54.33 | 4.34 | 64.87 | 4.87 |
| B70:T30 | 12.38 | 7.57 | 0.55 | 61.23 | 3.45 | 72.83 | 5.44 |
| B80:T20 | 11.66 | 5.44 | 0.25 | 46.68 | 2.98 | 61.34 | 5.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrószcz, M.W.; Barszczewska-Rybarek, I.M.; Wori, P. The Relationship between the Degree of Conversion in Dental Dimethacrylate Polymers Determined by Infrared Spectroscopy and Polymerization Shrinkage. Eng. Proc. 2021, 11, 52. https://doi.org/10.3390/ASEC2021-11151
Chrószcz MW, Barszczewska-Rybarek IM, Wori P. The Relationship between the Degree of Conversion in Dental Dimethacrylate Polymers Determined by Infrared Spectroscopy and Polymerization Shrinkage. Engineering Proceedings. 2021; 11(1):52. https://doi.org/10.3390/ASEC2021-11151
Chicago/Turabian StyleChrószcz, Marta W., Izabela M. Barszczewska-Rybarek, and Promise Wori. 2021. "The Relationship between the Degree of Conversion in Dental Dimethacrylate Polymers Determined by Infrared Spectroscopy and Polymerization Shrinkage" Engineering Proceedings 11, no. 1: 52. https://doi.org/10.3390/ASEC2021-11151







