Abstract
Sensors with electrochemically formed polymeric films as a sensitive layer are of high interest in electroanalysis. Voltammetric sensors based on the glassy carbon electrodes (GCEs) covered with carbon nanomaterials and electropolymerized phenolic acids (gallic and ellagic) or triphenylmethane dyes (thymolphthalein and aluminon) were developed. Conditions of potentiodynamic electropolymerization were optimized. The electrodes were characterized with scanning electron microscopy, cyclic voltammetry, chronoamperometry, and electrochemical impedance spectroscopy (EIS). In the differential pulse mode, sensors provided a sensitive and selective response to different classes of the antioxidants (capsaicinoids, flavanones, and flavonols). The practical applicability of the sensors was demonstrated on food and plant samples.
1. Introduction
Electrochemical sensors based on electropolymerized coverage as a sensitive layer are of high interest in electroanalysis. Various monomers are successfully used for the sensors’ creation in particular compounds with phenolic moiety. As known, their electropolymerization leads to the formation of non-conductive coverages [1,2]. Therefore, carbon nanomaterials (nanotubes, nanofibers) are successfully applied as a platform for the further electropolymerization of a suitable monomer [2]. On the other hand, the presence of carbon nanomaterials increases the electrode surface roughness and electroactive area, providing a higher amount of the polymeric coverages electrodeposited as well as its uniform surface distribution. The presence of phenolic fragments in the polymer structure can provide a sensitive response to the compounds containing similar moieties, particularly natural phenolic antioxidants [2].
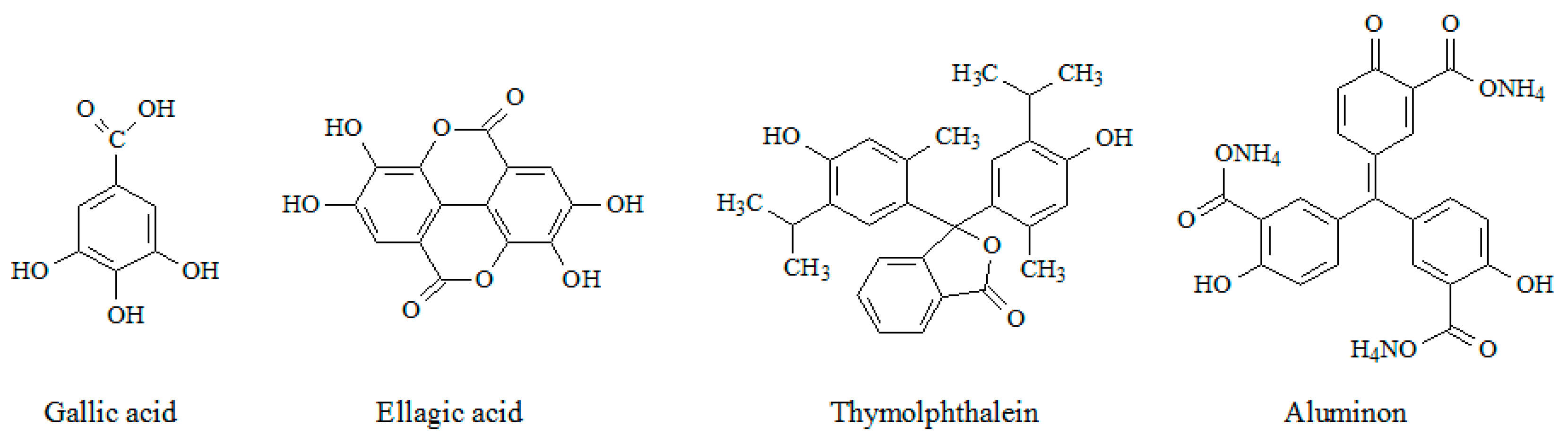
Among a wide range of phenolic monomers, natural phenolic acids (gallic and ellagic) and triphenylmethane dyes (thymolphthalein and aluminon) are of interest (Figure 1) and have been studied in the current work. Their electropolymerization conditions have been optimized and properties of the electrodes created have been characterized by scanning electron microscopy (SEM), cyclic voltammetry, chronoamperometry, and electrochemical impedance spectroscopy (EIS). The electrodes act as sensitive and selective voltammetric sensors to different classes of the antioxidants (capsaicinoids, flavanones (hesperidin and naringin), and flavonols (rutin and quercetin)). The simultaneous quantification of flavanones and flavonols has been achieved using sensors based on the electropolymerized triphenylmethane dyes for the first time. The practical applicability of the sensors has been demonstrated on food and plant samples.
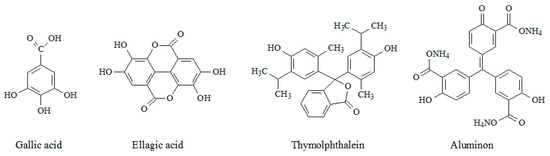
Figure 1.
Structure of monomers under investigation.
2. Materials and Methods
Ellagic acid (95% purity) from Sigma-Aldrich (Darmstadt, Germany); aluminon, thymolphthalein, gallic acid, naringin (95%), hesperidin (94%), and quercetin dihydrate (95%) from Sigma (Steinheim, Germany); capsaicin (95%), dihydrocapsaicin (85%), and nonivamide (97%) from Sigma (Bengaluru, India); and rutin trihydrate (97%) from Alfa Aesar (Heysham, UK) were used. Standard solutions of 0.86 mM of ellagic acid, and 10 mM of aluminon and thymolphthalein were prepared in methanol (c.p. grade) and 10 mM of gallic acid in ethanol (rectificate). Stock solutions of the analytes (10 mM for naringin, rutin and quercetin, 0.40 mM for hesperidin in methanol (c.p. grade), and 10 mM solutions of capsaicinoids in ethanol) were prepared in 5.0 mL flasks. The exact dilution was used for the preparation of less-concentrated solutions.
Multi-walled carbon nanotubes (MWCNTs) (outer diameter 40–60 nm, inner diameter 5–10 nm, and 0.5–500 μm length) from Aldrich (Steinheim, Germany), single-walled carbon nanotubes functionalized with polyaminobenzene sulfonic acid (f-SWCNTs) (d × l is 1.1 nm × 0.5–1.0 μm) from Sigma-Aldrich (Steinheim, Germany), and graphitized (iron-free) carbon nanofibers (CNF) (d × l 100 nm × 20–200 μm and average diameter 130 nm) from Aldrich (St. Louis, MO, USA) were used as electrode surface modifiers. Sonication for 30 min (15 min for CNF) in an ultrasonic bath (WiseClean WUC-A03H (DAIHAN Scientific Co., Ltd., Wonju-si, Republic of Korea) was applied for the preparation of carbon nanomaterials homogeneous 0.5 mg mL−1 of MWCNTs and 1.0 mg mL−1 of f-SWCNTs or CNF suspensions. The 1% sodium dodecylsulfate (Panreac, Barcelona, Spain) and dimethylformamide were used as dispersive media for MWCNTs and f-SWCNTs, respectively. Other chemicals were c.p. graded and used as received.
Potentiostats/galvanostats μAutolab Type III (Eco Chemie B.V., Utrecht, The Netherlands) with GPES 4.9.005 software and Autolab PGSTAT 302N with the FRA 32M module (Eco Chemie B.V., Utrecht, The Netherlands) and NOVA 1.10.1.9 software were used for the electrochemical measurements. The glassy electrochemical cell of 10 mL volume was used. The working glassy carbon electrodes (GCEs) of 3 mm diameter (CH Instruments, Inc., Bee Cave, TX, USA and BASi® Inc., West Lafayette, IN, USA) and 1 mm diameter, or modified electrodes, an Ag/AgCl reference electrode, and a platinum wire as the auxiliary electrode were used.
The pH measurements were performed at “Expert-001” pH meter (Econix-Expert Ltd., Moscow, Russia) using a glassy electrode.
A high-resolution field emission scanning electron microscope MerlinTM (Carl Zeiss, Oberkochen, Germany) operated at an accelerating voltage of 5 kV and emission current of 300 pA was applied for the electrodes’ surface morphology characterization.
3. Results and Discussion
3.1. Electropolymerization of Phenolic Acids and Triphenylmethane Dyes and Electrode Characteristics
A layer-by-layer modification of the GCE surface was performed. Firstly, bare GCE was covered with 4.0 µL of MWCNTs or 2.0 µL of f-SWCNTs or CNF suspensions, and the solvents were evaporated to dryness at room temperature. Then, the electropolymerization of phenolic acids and triphenylmethane dyes was performed in potentiodynamic mode. A poly(gallic acid) layer was obtained at the surface of GCEs with 1 mm diameter, and other polymeric coverage with GCEs of 3 mm diameter. All monomers studied underwent irreversible electrooxidation with one step (two steps for the ellagic acid) for the anodic branches on cyclic voltammograms (Table 1)—this corresponded with the oxidation of phenolic moiety with the formation of phenoxyl radical, of which underwent the following reactions of dimerization and polymerization.

Table 1.
Oxidation potentials of monomers under consideration.
The oxidation currents gradually decreased to full disappearance as the number of cycles increased—this agreed with the reported data for the same and other phenol-containing compounds [2,3,4,5,6] and indicated the formation of insulating polymeric coverage. The electropolymerization conditions (monomer concentration, supporting electrolyte type and pH, potential scan rate and range, number of cycles) were optimized. The corresponding data are summarized in Table 2.

Table 2.
Optimized conditions of phenolic acid and triphenylmethane dye electropolymerization.
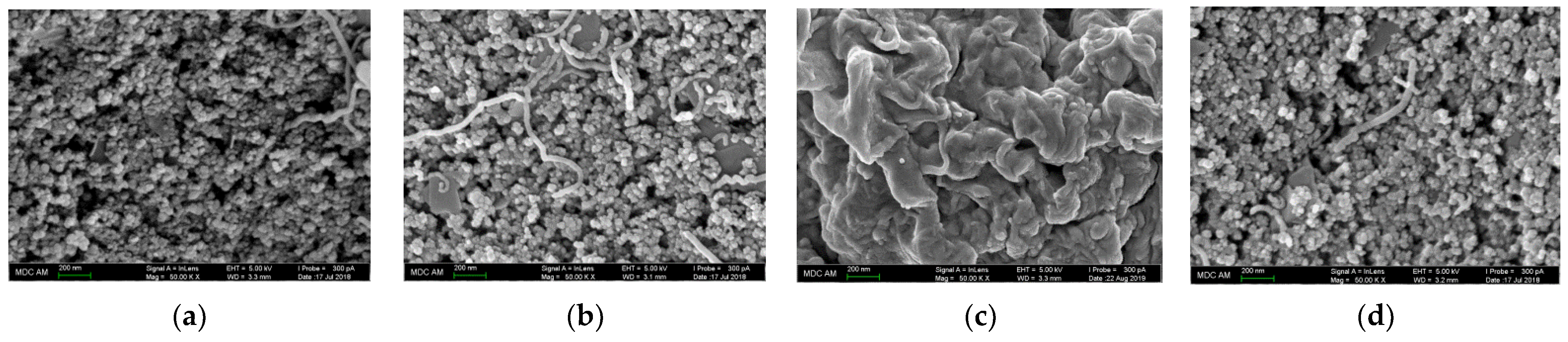
The electrode surface has been characterized with SEM, cyclic voltammetry, chronoamperometry, and EIS. Polymeric coverages exhibit a porous structure with the shape of particles and their aggregates (folded structure with pores and channels in the case of polyaluminon) deposited on the surface of carbon nanomaterials (Figure 2). SEM data confirm the high roughness of the polymer-modified electrodes and the increase in their surface area.

Figure 2.
SEM images of the electrode surface: (a) poly(gallic acid)/MWCNTs/GCE; (b) poly(ellagic acid)/MWCNTs/GCE; (c) polyaluminon/f-SWNTs/GCE; (d) polythymolphthalein/CNF/GCE.
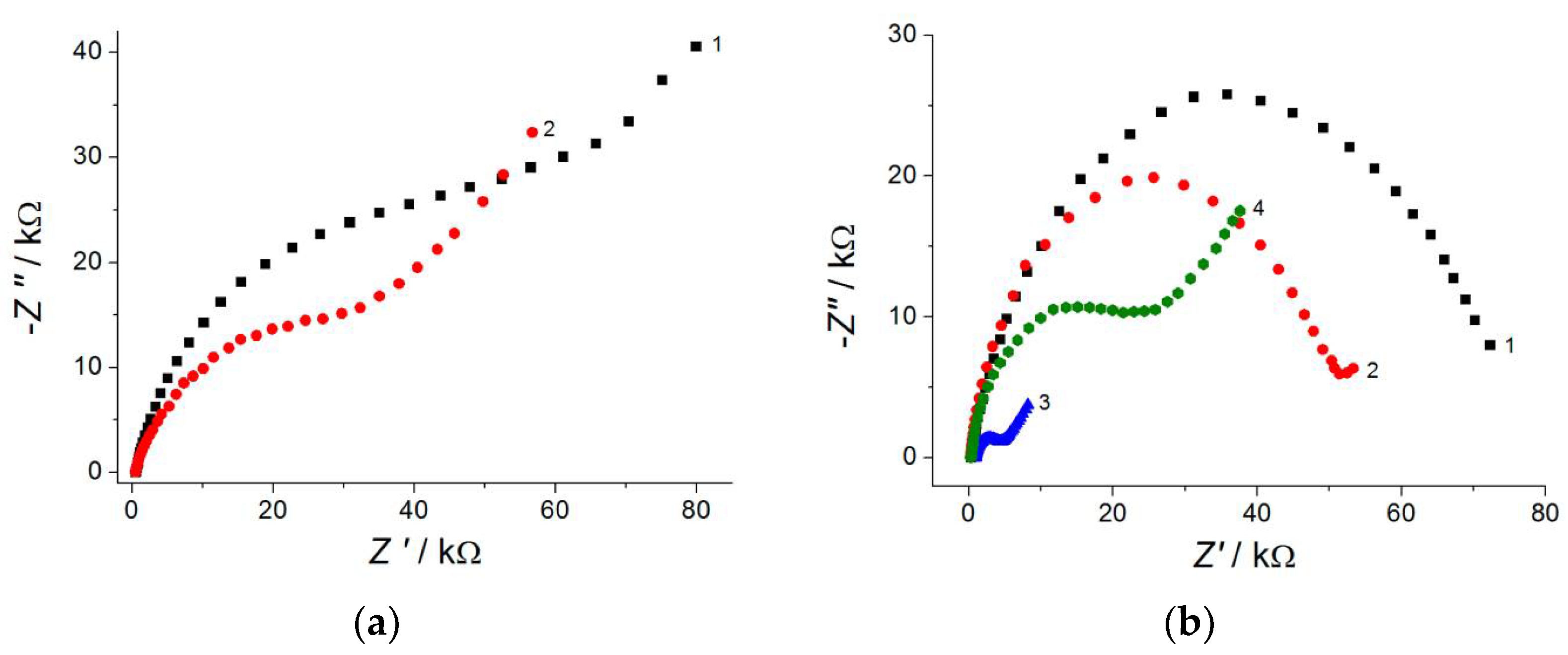
Electrochemical data for [Fe(CN)6]4− ion oxidation indicate a statistically significant (2.3–8.9-fold) increase in the electroactive surface area of the polymer-based electrodes vs. bare GCEs. EIS spectra show (Figure 3) a statistically significant decrease in the semicircle diameter, i.e., the charge-transfer resistance for the modified electrodes confirm a higher electron transfer rate. Thus, the electropolymerized coverages based on the phenolic acids and triphenylmethane dyes are perspective materials for application in electroanalysis.
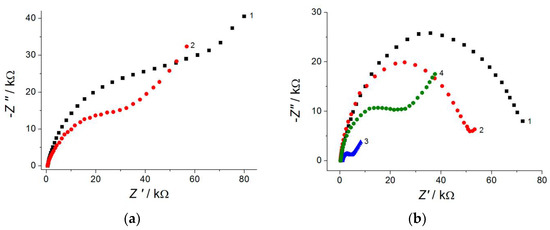
Figure 3.
Electrochemical impedance spectra in the presence of 1.0 mM [Fe(CN)6]3−/4− in neutral medium for bare GCE (1) and the polymer-modified electrode: (a) poly(gallic acid)/MWCNTs/GCE (2); (b) poly(ellagic acid)/MWCNTs/GCE (2).
3.2. Voltammetric Sensing of the Antioxidants
The electrodes have shown a sensitive and selective response to different classes of the antioxidants (capsaicinoids, flavanones (hesperidin and naringin), and flavonols (rutin and quercetin)). The electrooxidation parameters of the antioxidants have been found (Table 3) on the basis of cyclic voltammetry data at various pH levels of the supporting electrolyte and potential scan rate. All analytes are oxidized with the participation of an equal number of protons and electrons. The electrooxidation of capsaicinoids, naringin, and flavonols is a surface-controlled process. The diffusion-driven electrochemical process has been confirmed for hesperidin and naringin at the polyaluminon/f-SWCNTs/GCE. 4-Hydroxy-3-methoxyphenyl fragments of capsaicinoids, 3′-hydroxy-4′-methoxyphenyl fragment of hesperidin, hydroxyl group, and the catechol fragment in the ring B of naringin and flavonols, respectively, undergo electrooxidation with the formation of o-quinone fragments, of which agree with the reported data [7,8,9,10].

Table 3.
Electrooxidation parameters of the capsaicinoids and phenolic antioxidants at the polymer-modified electrodes.
Novel voltammetric sensors based on electropolymerized phenolic acids and triphenylmethane dyes for the quantification of antioxidants worked in the differential pulse mode. The possibility of the selective simultaneous quantification of flavanones (hesperidin and naringin) and flavonols (quercetin and rutin) has been achieved. The analytical characteristics of natural antioxidants (Table 4) are improved vs. those reported earlier for other electrochemical sensors. Furthermore, high selectivity of the sensors’ response to target analytes in the presence of other natural phenolics and ascorbic acid is an important advantage of the sensors developed, allowing their practical application.

Table 4.
Analytical characteristics of natural antioxidant determination using polymer-based sensors.
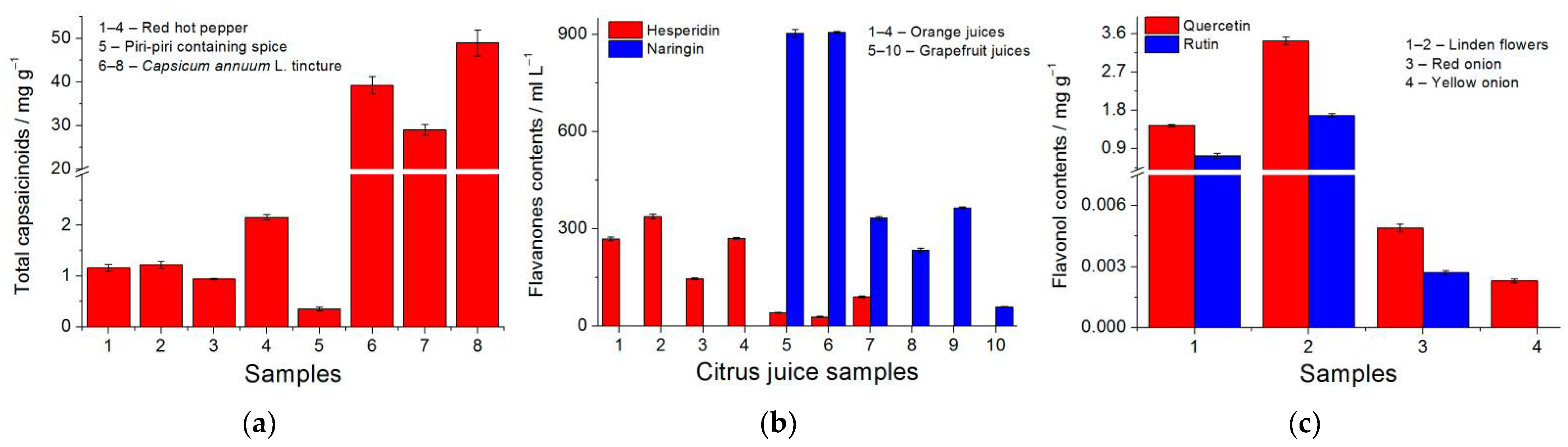
Polymer-based sensors have been successfully tested in the food and plant sample analysis. Total capsaicinoid contents have been evaluated in spices of red hot pepper and Capsicum annuum L. tinctures (Figure 4a). Flavonols (hesperidin and naringin) have been measured in fresh and commercial orange and grapefruit juices. Samples 1–7 were analyzed using a polyaluminon-based sensor for the simultaneous determination of flavanones, while the poly(ellagic acid)-based sensor for naringin was tested on the samples 8–10 (Figure 4b). The quantification of quercetin and rutin has been performed in red and yellow anions and linden (Tilia L.) flowers (Figure 4c).
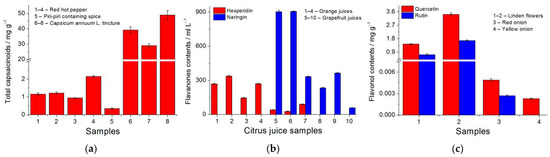
Figure 4.
Quantification of antioxidants in food and plant samples: (a) total capsaicinoids in spices of red hot pepper and Capsicum annuum L. tinctures at poly(gallic acid)/MWCNTs/GCE in the Britton–Robinson buffer pH 2.0; (b) hesperidin and naringin in citrus juices at polyaluminon/f-SWCNTs/GCE in phosphate buffer pH 5.0 and at poly(ellagic ac-id)/MWCNTs/GCE in phosphate buffer pH 6.5; (c) quercetin and rutin in linden (Tilia L.) flowers and onion at polythymolphthalein/CNF/GCE in phosphate buffer pH 7.5.
Thus, electropolymerized phenol-containing compound/carbon nanomaterial composites can be considered as a promising sensing platform in the antioxidant electroanalysis.
Author Contributions
Conceptualization, G.Z.; methodology, G.Z.; validation, G.Z. and E.Y.; investigation, E.Y. and A.Z.; writing—original draft preparation, G.Z.; writing—review and editing, G.Z.; visualization, G.Z., E.Y. and A.Z.; supervision, G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors thank Aleksei Rogov (Laboratory of Scanning Electron Microscopy, Interdisciplinary Center for Analytical Microscopy, Kazan Federal University) for the scanning electron microscopy measurements.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Da Silva, L.V.; de Almeida, A.K.A.; Xavier, J.A.; Lopes, C.B.; dos Santos Silva, F.A.; Lima, P.R.; dos Santos, N.D.; Kubota, L.T.; Goulart, M.O.F. Phenol based redox mediators in electroanalysis. J. Electroanal. Chem. 2018, 827, 230–252. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Guss, E.; Yakupova, E. Electrochemical sensors based on the electropolymerized natural phenolic antioxidants and their analytical application. Sensors 2021, 21, 8385. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, R.; Newair, E.F. Voltammetric determination of polyphenolic content in pomegranate juice using a poly(gallic acid)/multiwalled carbon nanotube modified electrode. Beilstein J. Nanotechnol. 2016, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Saritha, D.; Reddy, A.V.B.; Venu, M.; Madhuri, C.; Kiranmai, S.; Anitha, K.; Madhavi, G. Fabrication, characterization and development of a modified poly(bromocresol purple/multiwalled carbon nanotubes) carbon paste electrode for the determination of sulfanilic acid. Anal. Bioanal. Electrochem. 2019, 11, 123–136. [Google Scholar]
- Ziyatdinova, G.; Guss, E.; Budnikov, H. Selective electrochemical sensor based on the electropolymerized p-coumaric acid for the direct determination of L-cysteine. Electrochim. Acta 2018, 270, 369–377. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Zhupanova, A.; Davletshin, R. Simultaneous determination of ferulic acid and vanillin in vanilla extracts using voltammetric sensor based on electropolymerized bromocresol purple. Sensors 2022, 22, 288. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhang, D.; Tong, Z.; Xu, X.; Yang, X. Voltammetric behavior and the determination of quercetin at a flowerlike Co3O4 nanoparticles modified glassy carbon electrode. J. Appl. Electrochem. 2011, 41, 189–196. [Google Scholar] [CrossRef]
- Yardim, Y. Sensitive detection of capsaicin by adsorptive stripping voltammetry at a boron-doped diamond electrode in the presence of sodium dodecylsulfate. Electroanalysis 2011, 23, 2491–2497. [Google Scholar] [CrossRef]
- Sims, M.J.; Li, Q.; Kachoosangi, R.T.; Wildgoose, G.G.; Compton, R.G. Using multi-walled carbon nanotube modified electrodes for the adsorptive striping voltammetric determination of hesperidin. Electrochim. Acta 2009, 54, 5030–5034. [Google Scholar] [CrossRef]
- Masek, A.; Zaborski, M.; Chrzescijanska, E. Electrooxidation of flavonoids at platinum electrode studied by cyclic voltammetry. Food Chem. 2011, 127, 699–704. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).