An FTIR Spectroscopy Investigation on Different Methods of Lipid Extraction from HepG2 Cells †
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Growth
2.2. Lipid Extraction Methods
2.3. FTIR Measurements
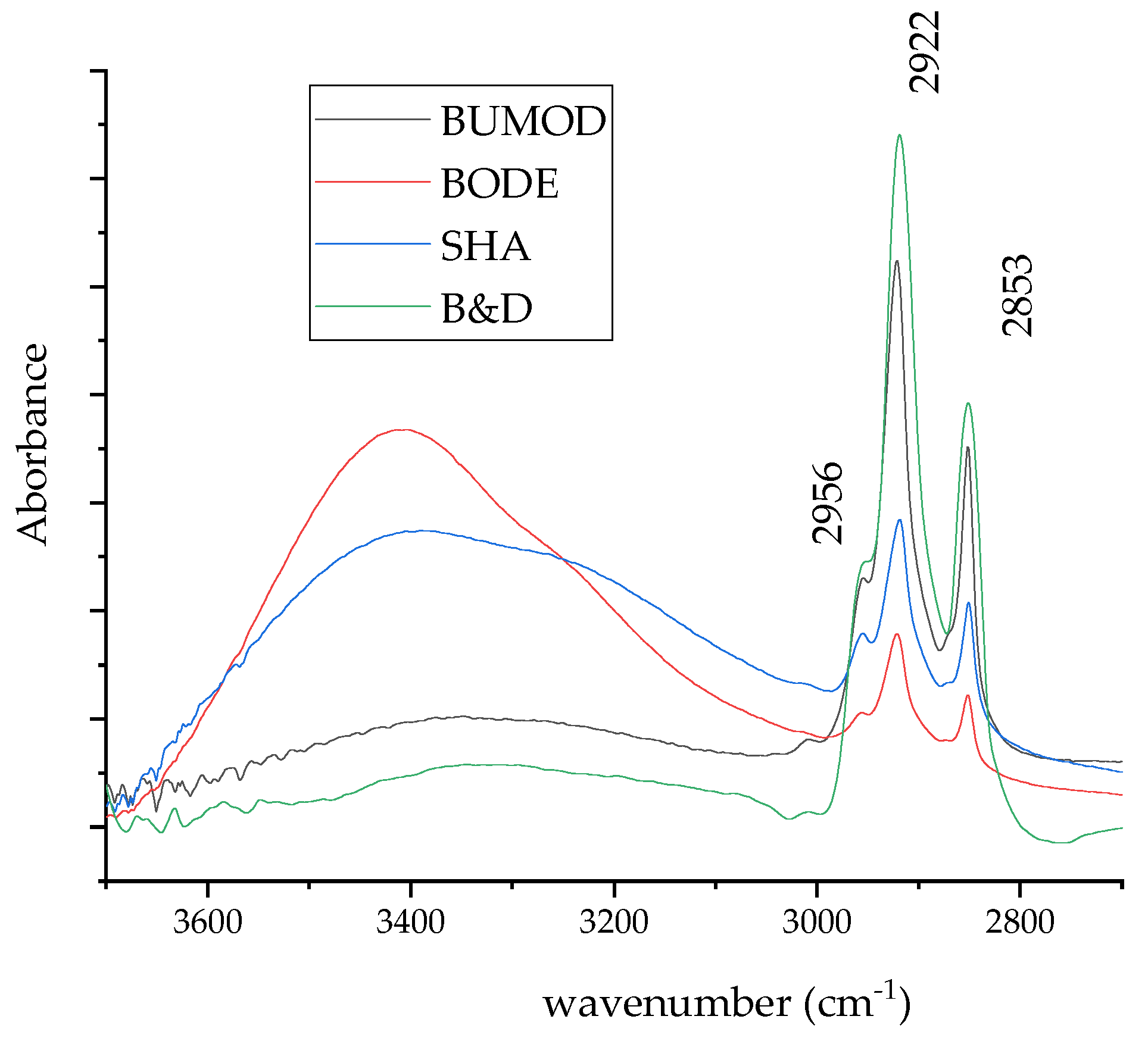
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Muro, E.; Atilla-Gokcumen, G.E.; Eggert, U.S. Lipids in cell biology: How can we understand them better? Mol. Biol. Cell 2014, 25, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.J.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.J.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; Heys, K.A.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.; Wang, M.; Frisch-Daiello, J.; Han, X. Improved butanol–methanol (BUME) method by replacing acetic acid for lipid extraction of biological samples. Lipids 2016, 51, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.S. Advances in Lipid Extraction Methods—A Review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef] [PubMed]
- Shaner, R.L.; Allegood, J.C.; Park, H.; Wang, E.; Kelly, S.; Haynes, C.A.; Sullards, M.C.; Merrill, A.H. Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J. Lipid Res. 2009, 50, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.T.; Tolosa, L.; Gómez-Lechón, M.J. Culture and functional characterization of human hepatoma HepG2 cells. In Protocols in In Vitro Hepatocyte Research; Springer: Berlin/Heidelberg, Germany, 2015; pp. 77–93. [Google Scholar]
- Gautam, R.; Vanga, S.; Ariese, F.; Umapathy, S. Review of multidimensional data processing approaches for Raman and infrared spectroscopy. EPJ Tech. Instrum. 2015, 2, 1–38. [Google Scholar] [CrossRef]
- Talari, A.C.S.; Martinez, M.A.G.; Movasaghi, Z.; Rehman, S.; Rehman, I.U. Advances in Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2017, 52, 456–506. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; ur Rehman, D.I. Fourier transform infrared (FTIR) spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2008, 43, 134–179. [Google Scholar] [CrossRef]
- Strug, I.; Utzat, C.; Cappione, A.; Gutierrez, S.; Amara, R.; Lento, J.; Capito, F.; Skudas, R.; Chernokalskaya, E.; Nadler, T. Development of a univariate membrane-based mid-infrared method for protein quantitation and total lipid content analysis of biological samples. J. Anal. Methods Chem. 2014, 2014, 657079. [Google Scholar] [CrossRef] [PubMed]
- Merck Millipore. Simplified Analysis of Lipid or Detergent Content in Biological Samples Using the IR-Based Direct Detect® Spectrometer; Application Note; Merck Millipore: Burlington, MA, USA, 2013. [Google Scholar]
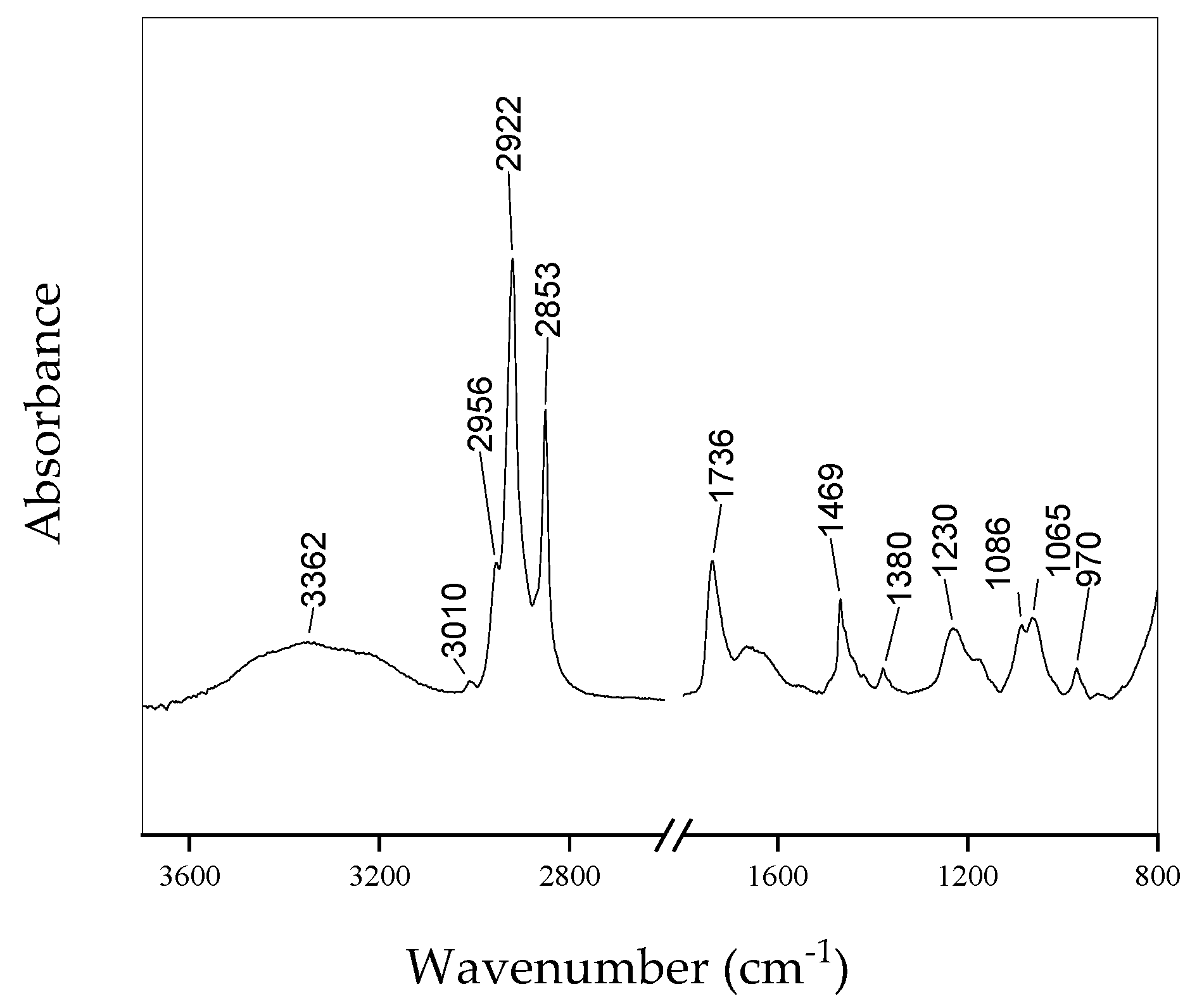
| Peak Position (cm−1) | Assignment |
|---|---|
| 3362 | O-H, N-H, C-H stretching |
| 3010 | CH=CH asymmetric stretching |
| 2956 | CH3 Asymmetric stretching |
| 2922 | CH2 asymmetric stretching |
| 2853 | CH2 symmetric stretching |
| 1736 | C=O stretching |
| 1469 | CH2 bending |
| 1380 | CH3 bending |
| 1230 | Asymmetric stretching |
| 1086 | Symmetric stretching |
| 1065 | CO stretching |
| 970 | CO stretching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faramarzi, B.; Moggio, M.; Cardamuro, V.; Portaccio, M.; Diano, N.; Manti, L.; Lepore, M. An FTIR Spectroscopy Investigation on Different Methods of Lipid Extraction from HepG2 Cells. Eng. Proc. 2022, 27, 39. https://doi.org/10.3390/ecsa-9-13263
Faramarzi B, Moggio M, Cardamuro V, Portaccio M, Diano N, Manti L, Lepore M. An FTIR Spectroscopy Investigation on Different Methods of Lipid Extraction from HepG2 Cells. Engineering Proceedings. 2022; 27(1):39. https://doi.org/10.3390/ecsa-9-13263
Chicago/Turabian StyleFaramarzi, Bahar, Martina Moggio, Valeria Cardamuro, Marianna Portaccio, Nadia Diano, Lorenzo Manti, and Maria Lepore. 2022. "An FTIR Spectroscopy Investigation on Different Methods of Lipid Extraction from HepG2 Cells" Engineering Proceedings 27, no. 1: 39. https://doi.org/10.3390/ecsa-9-13263
APA StyleFaramarzi, B., Moggio, M., Cardamuro, V., Portaccio, M., Diano, N., Manti, L., & Lepore, M. (2022). An FTIR Spectroscopy Investigation on Different Methods of Lipid Extraction from HepG2 Cells. Engineering Proceedings, 27(1), 39. https://doi.org/10.3390/ecsa-9-13263








