Cobalt Detection Using Fluorescent Dye Layers †
Abstract
1. Introduction
2. Materials
2.1. Fluorescense Quenching
2.2. Calcein: Fluorescence Emission and Preparation
2.3. Cobalt Solutions Preparation
2.4. Experimental Set-Up
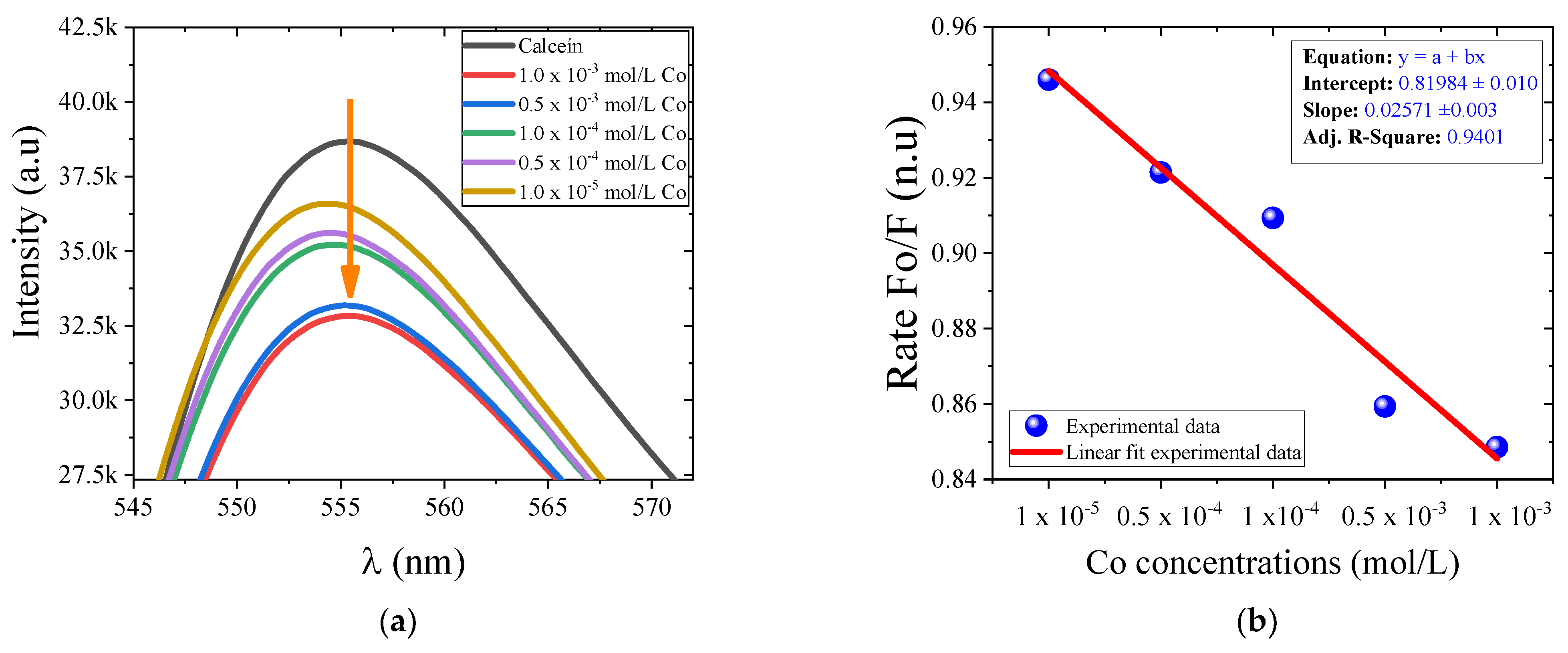
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Michalski, R.; Ficek, A. Environmental pollution by chemical substances used in the shale gas extraction—A review. Desalination Water Treat. 2015, 57, 1336–1343. [Google Scholar] [CrossRef]
- Qiu, Q.; Tan, Z.; Wang, J.; Peng, J.; Li, M.; Zhan, Z. Extraction, enumeration and identification methods for monitoring microplastics in the environment. Estuar. Coast. Shelf Sci. 2016, 176, 102–109. [Google Scholar] [CrossRef]
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar] [CrossRef] [PubMed]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K. Cobalt: Its role in health and disease. Met. Ions. Life Sci. 2013, 13, 295–320. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans-A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Wiederschain, G.Y. The Molecular Probes handbook. A guide to fluorescent probes and labeling technologies. Biochemistry 2011, 76, 1276–1277. [Google Scholar] [CrossRef]
- (ATSDR), U.S. Department of Health and Human Services. Toxicological Profile for Cobalt. 2004. Available online: https://wwwn.cdc.gov/Tsp/ToxProfiles/ToxProfiles.aspx?id=373&tid=64 (accessed on 15 September 2022).


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armenta-Serna, F.A.; Fuentes-Rubio, Y.A.; Domínguez-Cruz, R.F.; García-Garza, L.A.; Baldovino-Pantaleón, O.; Ruiz-Zamarreño, C. Cobalt Detection Using Fluorescent Dye Layers. Eng. Proc. 2022, 27, 67. https://doi.org/10.3390/ecsa-9-13217
Armenta-Serna FA, Fuentes-Rubio YA, Domínguez-Cruz RF, García-Garza LA, Baldovino-Pantaleón O, Ruiz-Zamarreño C. Cobalt Detection Using Fluorescent Dye Layers. Engineering Proceedings. 2022; 27(1):67. https://doi.org/10.3390/ecsa-9-13217
Chicago/Turabian StyleArmenta-Serna, Fernando Arturo, Yadira Aracely Fuentes-Rubio, René Fernando Domínguez-Cruz, Luis Antonio García-Garza, Oscar Baldovino-Pantaleón, and Carlos Ruiz-Zamarreño. 2022. "Cobalt Detection Using Fluorescent Dye Layers" Engineering Proceedings 27, no. 1: 67. https://doi.org/10.3390/ecsa-9-13217
APA StyleArmenta-Serna, F. A., Fuentes-Rubio, Y. A., Domínguez-Cruz, R. F., García-Garza, L. A., Baldovino-Pantaleón, O., & Ruiz-Zamarreño, C. (2022). Cobalt Detection Using Fluorescent Dye Layers. Engineering Proceedings, 27(1), 67. https://doi.org/10.3390/ecsa-9-13217








