Study of the Stabilizing Agent Influence in the Catalytic Degradation of Methylene Blue Using Silver Nanoparticles †
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of AgNPs
2.2. Methylene Blue Degradation
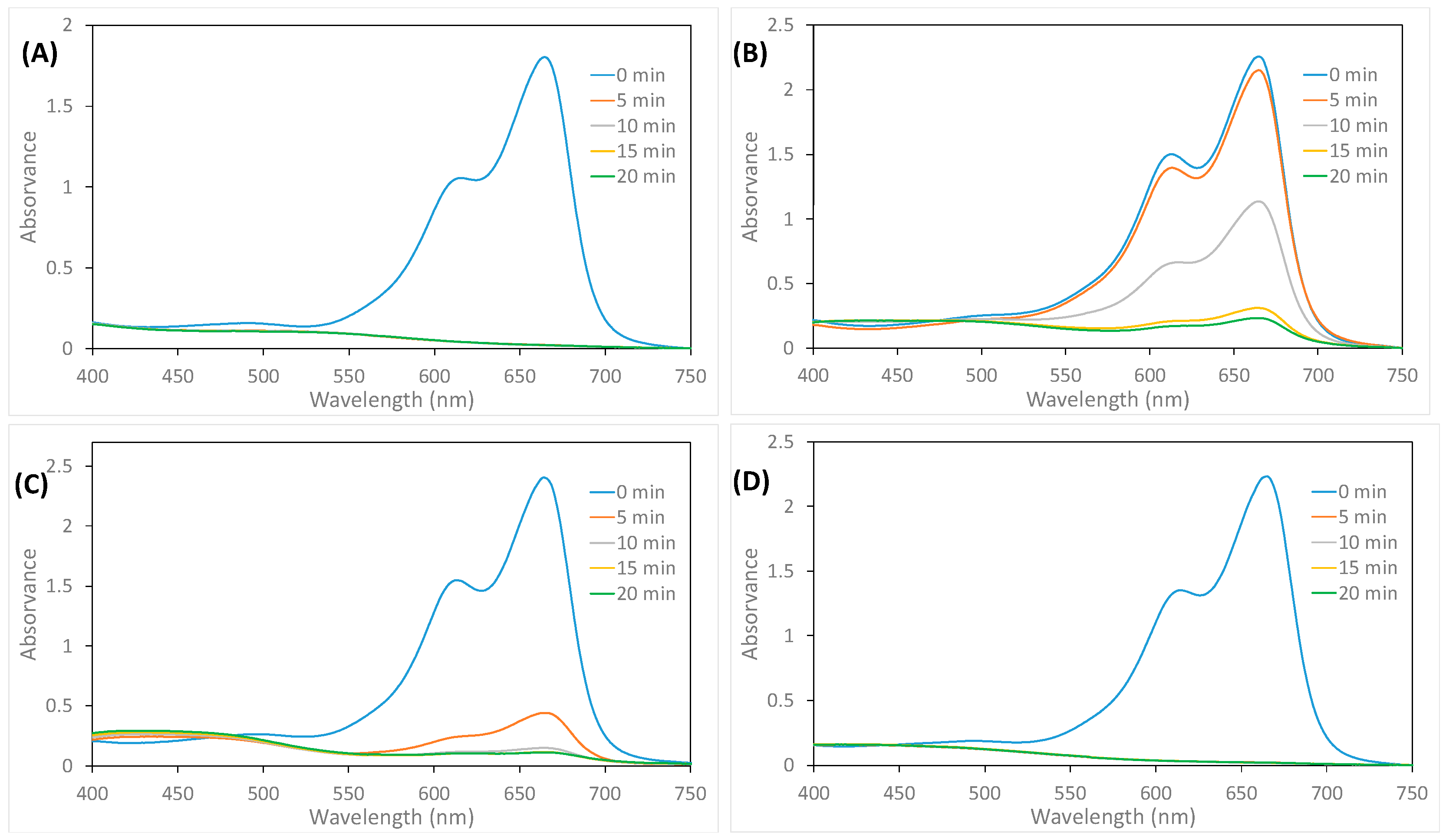
3. Results
3.1. Preparation of AgNPs
3.2. Methylene Blue Degradation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adem Esmail, B.; Suleiman, L. Analyzing evidence of sustainable urban water management systems: A review through the lenses of sociotechnical transitions. Sustainability 2020, 12, 4481. [Google Scholar] [CrossRef]
- Slama, H.B.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.; Choi, J.-W.; Jang, H.; Shokouhimehr, M.; Varma, R. Recent Advances in the Nanocatalyst-Assisted NaBH4 Reduction of Nitroaromatics in Water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Lu, Z.-A.; Wu, J.; Chen, M.-X.; Zhang, Y.; Zhang, B.; Gao, G.-L.; Li, S.; Xu, P. Recent Advances in Plasmon-Promoted Organic Transformations Using Silver-Based Catalysts. ACS Appl. Mater. Interfaces 2020, 12, 54266–54284. [Google Scholar] [CrossRef] [PubMed]
- Kästner, C.; Thünemann, A. Catalytic Reduction of 4-Nitrophenol Using Silver Nanoparticles with Adjustable Activity. Langmuir 2016, 32, 7383–7391. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, M.T.A.; Figueiredo, D.M.M.; Marques, N.R.S.; Pereira, G.A.L.; Pereira, G. Study of the Stabilizing Agent Influence in the Catalytic Degradation of Methylene Blue Using Silver Nanoparticles. Eng. Proc. 2023, 31, 19. https://doi.org/10.3390/ASEC2022-13854
Lima MTA, Figueiredo DMM, Marques NRS, Pereira GAL, Pereira G. Study of the Stabilizing Agent Influence in the Catalytic Degradation of Methylene Blue Using Silver Nanoparticles. Engineering Proceedings. 2023; 31(1):19. https://doi.org/10.3390/ASEC2022-13854
Chicago/Turabian StyleLima, Max T. A., Danilo M. M. Figueiredo, Nayally R. S. Marques, Giovannia A. L. Pereira, and Goreti Pereira. 2023. "Study of the Stabilizing Agent Influence in the Catalytic Degradation of Methylene Blue Using Silver Nanoparticles" Engineering Proceedings 31, no. 1: 19. https://doi.org/10.3390/ASEC2022-13854
APA StyleLima, M. T. A., Figueiredo, D. M. M., Marques, N. R. S., Pereira, G. A. L., & Pereira, G. (2023). Study of the Stabilizing Agent Influence in the Catalytic Degradation of Methylene Blue Using Silver Nanoparticles. Engineering Proceedings, 31(1), 19. https://doi.org/10.3390/ASEC2022-13854






