Synthesis of Doped Sol-Gel Glasses as Adsorbents for Water Treatment †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Plain and Doped Sol-Gel Glasses
2.3. Characterization of the Prepared Sol-Gel Glasses
2.4. Adsorption Experiments
3. Results and Discussion
3.1. Physical Characteristics of the Prepared Nanocomposites
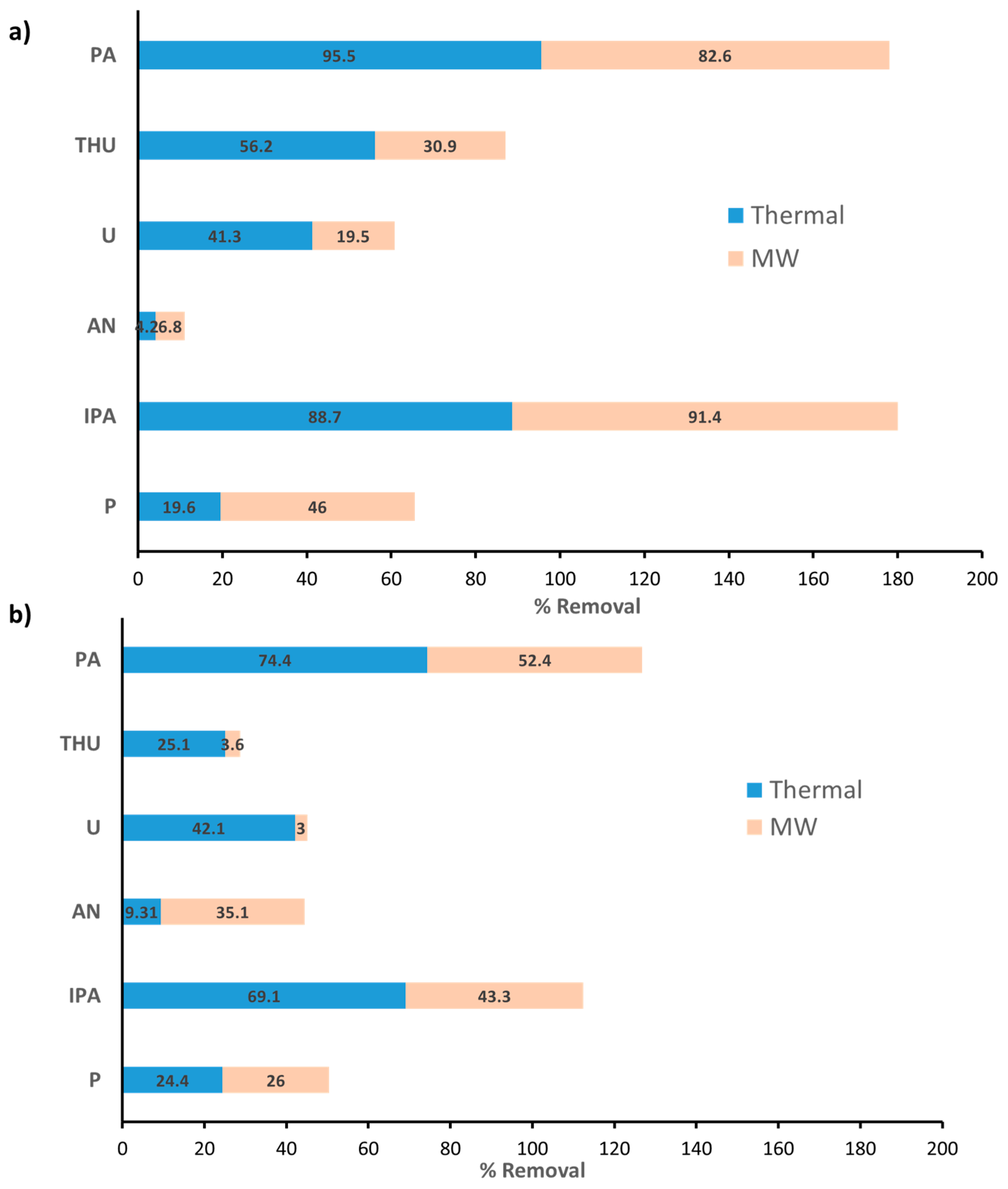
3.2. Adsorption Study on MB
3.3. Chemical Characteristics of the Prepared Sol-Gel Glasses
3.4. Mechanism of Adsorption
3.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, X.-Y.; Wang, Q.; Wang, Y.; Fu, Z.-J.; Wang, Z.-Y.; Mamba, B.; Sun, S.-P. Designing durable self-cleaning nanofiltration membranes via sol-gel assisted interfacial polymerization for textile wastewater treatment. Sep. Purif. Technol. 2022, 289, 120752. [Google Scholar] [CrossRef]
- Dehaghi, R.; Behpour, M.; Mir, N. Purification of textile wastewater by using coated Sr/S/N doped TiO2 nanolayers on glass orbs. Korean J. Chem. Eng. 2018, 35, 1441–1449. [Google Scholar] [CrossRef]
- Irfan, M.; Zaheer, F.; Hussain, H.; Naz, M.Y.; Shukrullah, S.; Legutko, S.; Mahnashi, M.H.; Alsaiari, M.A.; Ghanim, A.A.J.; Rahman, S.; et al. Kinetics and adsorption isotherms of amine-functionalized magnesium ferrite produced using sol-gel method for treatment of heavy metals in wastewater. Materials 2022, 15, 4009. [Google Scholar] [CrossRef] [PubMed]
- Lamy-Mendes, A.; Torres, R.B.; Vareda, J.P.; Lopes, D.; Ferreira, M.; Valente, V.; Girão, A.V.; Valente, A.J.M.; Durães, L. Amine modification of silica aerogels/xerogels for removal of relevant environmental pollutants. Molecules 2019, 24, 3701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beagan, A.; Alotaibi, K.; Almakhlafi, M.; Algarabli, W.; Alajmi, N.; Alanazi, M.; Alwaalah, H.; Alharbi, F.; Alshammari, R.; Alswieleh, A. Amine and sulfonic acid functionalized mesoporous silica as an effective adsorbent for removal of methylene blue from contaminated water. J. King Saud Univ.-Sci. 2022, 34, 101762. [Google Scholar] [CrossRef]
- Chueachot, R.; Wongkhueng, S.; Khankam, K.; Lakrathok, A.; Kaewnon, T.; Naowanon, W.; Amnuaypanich, S.; Nakhowong, R. Adsorption efficiency of methylene blue from aqueous solution with amine-functionalized mesoporous silica nanospheres by co-condensation biphasic synthesis: Adsorption condition and equilibrium studies. Mater. Proc. 2018, 5, 14079–14085. [Google Scholar] [CrossRef]
- Yu, Z.-H.; Zhai, S.-R.; Guo, H.; Lv, T.-M.; Song, Y.; Zhang, F.; Ma, H.-C. Removal of methylene blue over low-cost mesoporous silicananoparticles prepared with naturally occurring diatomite. J. Sol-Gel Sci. Technol. 2018, 88, 541–550. [Google Scholar] [CrossRef]
- El-Sayed, M.; Elsayed, R.; Attia, A.; Farghal, H.; Azzam, R.; Madkour, T. Novel nanoporous membranes of bio-based cellulose acetate, poly(lactic acid) and biodegradable polyurethane in-situ impregnated with catalytic cobalt nanoparticles for the removal of Methylene Blue and Congo Red dyes from wastewater. Carbohydr. Polym. Technol. Appl. 2021, 2, 100123. [Google Scholar] [CrossRef]
- Bayomie, O.; Kandeel, H.; Shoeib, T.; Yang, H.; Youssef, N.; El-Sayed, M. Novel approach for effective removal of methylene blue dye from water using fava bean peel waste. Sci. Rep. 2020, 10, 7824. [Google Scholar] [CrossRef] [PubMed]
- Werkneh, A.; Habtu, N.; Beyene, H. Removal of hexavalent chromium from tannery wastewater using activated carbon primed from sugarcane bagasse. Adsorpt./Desorption Stud. 2014, 2, 128–135. [Google Scholar]
- El-Sayed, H.E.M.; El-Sayed, M.M.H. Assessment of Food Processing and Pharmaceutical Industrial Wastes as Potential Biosorbents: A Review. BioMed Res. Int. 2014, 2014, 146769. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, S.C.; Yoshimura, T.M.; Ribeiro, M.S.; Junqueira, H.C.; Maciel, C.; Coutinho-Neto, M.D.; Baptista, M.S. Urea enhances the photodynamic efficiency of methylene blue. J. Photochem. Photobiol. B Biol. 2015, 150, 31–37. [Google Scholar] [CrossRef] [PubMed]

| Sol-Gel Glass | Conventional Thermal Treatment | Microwave Irradiation | ||||
|---|---|---|---|---|---|---|
| Color | Transparency | Particle Size | Color | Transparency | Particle Size | |
| P | Colorless | Transparent | 500–630 μm | Colorless | Transparent | 300–500 μm |
| U | Orange | Transparent | 500–630 μm | White | Opaque | 250–500 μm |
| THU | Yellow | Opaque | <250 μm | White | Opaque | <250 μm |
| PA | Orange | Opaque | 250 μ–2 mm | Orange | Opaque | 250–630 μm |
| IPA | Orange | Opaque | 500 μ–2 mm | Orange | Opaque | 250–630 μm |
| AN | Black | Opaque | 500–630 μm | Black | Opaque | 250–630 μm |
| Wavenumber (cm−1) | Functional Group | Thermally-Treated | MW-Treated | ||
|---|---|---|---|---|---|
| PA | IPA | PA | IPA | ||
| 3500–3400 | O-H stretch | ✓ | ✓ | ✓ broad | ✓ broad |
| 2800–3000 | sp3 C-H stretch | ✓ | ✓ | ✓ | ✓ |
| 2350 | Si-H | ✓ | --- | ✓ small doublet | --- |
| 1500–1550 | N-H bending | ✓ | ✓ | ✓ | ✓ |
| 1600 | C=O | ✓ | ✓ | ✓ | --- |
| 1380 | CH3 bending | ✓ | ✓ sharp | ✓ sharp | ✓ |
| 1075–1100 | Si-O-Si | ✓ | ✓ sharp | ✓ sharp | ✓ sharp |
| 942–972 | Si-OH stretch | ✓ | ✓ | ✓ small | ✓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, H.F.; Emam, S.; El-Sayed, M.M.H.; Awad, B.M. Synthesis of Doped Sol-Gel Glasses as Adsorbents for Water Treatment. Eng. Proc. 2023, 31, 46. https://doi.org/10.3390/ASEC2022-13838
Mohamed HF, Emam S, El-Sayed MMH, Awad BM. Synthesis of Doped Sol-Gel Glasses as Adsorbents for Water Treatment. Engineering Proceedings. 2023; 31(1):46. https://doi.org/10.3390/ASEC2022-13838
Chicago/Turabian StyleMohamed, Hayam F., Shahinaz Emam, Mayyada M. H. El-Sayed, and Boshra M. Awad. 2023. "Synthesis of Doped Sol-Gel Glasses as Adsorbents for Water Treatment" Engineering Proceedings 31, no. 1: 46. https://doi.org/10.3390/ASEC2022-13838
APA StyleMohamed, H. F., Emam, S., El-Sayed, M. M. H., & Awad, B. M. (2023). Synthesis of Doped Sol-Gel Glasses as Adsorbents for Water Treatment. Engineering Proceedings, 31(1), 46. https://doi.org/10.3390/ASEC2022-13838






