Abstract
Viral detection has been studied predominantly in the last few years, along with the morbidity of COVID-19. Biosensors have been widely used for the detection of various biological molecules, showing a high potential for miniaturization and a friendly approach towards detection. Nanomaterials play a significant role in the development of biosensing devices due to their distinct morphological, optical, electrical, chemical, and physical properties, which improve their sensing efficiency. Therefore, the present work reports the fabrication of an electrochemical immunosensor adorned with gold nanoparticles coupled to a redox indicator-labeled antibody conjugate for the rapid detection of SARS-CoV-2 antibodies. The fabricated immunosensor can detect SARS-CoV-2 antibodies within a linear detection range of 10–100 ngmL−1 and offer a sensitivity of 0.013 × 10−3 mA ng−1mLmm−2. The adopted concept can be extended further for the detection of other viral antibodies with high sensitivity and display high prospects for miniaturization, hence offering tremendous commercialization potential.
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a causative agent of the coronavirus disease (COVID-19), poses a grave impact on individual health. It is a single-stranded RNA virus belonging to the Sarbecovirus subgenus of the Betacoronavirus genus. The basic structural proteins of the virus are spike protein(S), envelope protein(E), and nucleocapsid protein(N) [1]. The receptor-binding domain of the spike protein attacks the angiotensin-converting enzyme 2(ACE-2) present in the lung, resulting in endocytosis causing fever, cold, cough in mild cases, severe lung infection, myocardial infection, kidney, heart, or multiple organ failure in severe cases [2,3].
Although RT-PCR remains the gold standard for the confirmation of the disease, specific laboratory requirements, such as sub-zero temperature, long reaction time, need for a trained technician, etc., hurdle the scrutiny of the disease among the masses. Thus, for the initial screening of patients, point-of-care (PoC) devices, such as lateral flow immunoassays based on reversed affinity between receptor-ligand interactions, have been developed for rapid, on-site, self-monitoring by people [4,5,6]. However, these devices are qualitative and have a high limit of detection due to which they are prone to show false-negative and false-positive results in cases with a lower concentration of antibodies in the sample and display cross-reactivity.
Recently, biosensors with optical, colorimetric, piezoelectric, and electrochemical readouts have been reported for the detection of antibodies in the blood, serum, saliva, and nasopharyngeal and oropharyngeal samples. Electrochemical biosensors have a well-established reputation for high accuracy, sensitivity, and precise diagnosis [7,8,9]. Furthermore, upon modification with nanomaterials, they tend to show an overall enhancement in the diagnosis [10,11,12,13]. Gold nanomaterials (nanoparticles, nano-flowers, magnetic nanobeads, nanocomposites, etc.) have been widely reported to improve the efficiency of various sensing technologies. Apart from improving the interfacial properties, they can also be easily functionalized and conjugated to biomolecules to serve as reporters [14,15,16,17,18].
Thus, to monitor and meet the global exigency for rapid, reliable, and early-stage diagnosis of COVID-19 in the present study, we have fabricated an electrochemical immunosensor decorated with gold nanoparticles and an electroactive probe conjugate for the quantitative estimation of SARS-CoV-2 antibodies. The sensor offers a linear detection range of 10 to 100 ngmL−1, a low limit of detection (LOD = 3.59 ngmL−1), high linearity R2 = 0.96, and sensitivity of 0.013 × 10−3 mAng−1mLmm−2. The immunosensor shows excellent stability for a month. The quantitative estimation for SARS-CoV-2 antibody can be useful for sero-surveillance studies in the future.
2. Materials
For immunosensor fabrication and characterization, the following were purchased: Nafion (5%, SRL), SARS-CoV-2 spike protein (Sigma Aldrich, St. Louis, MO, USA), a monoclonal antibody against SARS-CoV-2 (Sigma Aldrich), BSA (Bovine Serum Albumin, SRL), horseradish peroxidase tagged with a secondary antibody (HRP-pAb) (Sigma Aldrich), hydrogen peroxide (H2O2) (30%, Fisher Scientific, Waltham, MA, USA), chloroauric acid (HAuCl4) (Sigma Aldrich), trisodium citrate dihydrate (SRL), potassium ferrocyanide (Fisher Scientific), and potassium ferricyanide (Fisher Scientific).
3. Methodology
The gold nanoparticles were first prepared using the Turkevich method [19,20,21]. The immunosensor was fabricated onto the conducting ITO-coated glass as a base electrode. The electrodes were washed via ultrasonication with acetone, ethanol, and water (10 min each). A polymeric solution of 1% Nafion was spin-coated onto the conducting surface of the ITO to form a uniform layer (nf/ITO), which was eventually modified with a layer of synthesized gold nanoparticles (AuNPs) to obtain the AuNPs/nf/ITO surface. Following this, the capture probe was immobilized on the surface of the AuNPs/nf/ITO. The concentration and incubation time of the capture probe (Spro) were optimized prior to immobilization. Non-specific binding sites of the capture probe were then blocked by incubating Spro/AuNPs/nf/ITO with BSA(1 mg/mL) for 45 min. The fabricated immunosensor probe BSA/Spro/AuNPs/nf/ITO was then used to detect specific antibodies (CoV2-Ab) against SARS-CoV-2 in the sample. A signal probe, i.e., HRP-tagged secondary antibody (HRP-pAb), was incubated with CoV2-Ab/BSA/Spro/AuNPs/nf/ITO to form the immunosensor (HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO). After each modification, the electrodes were washed with PBS (phosphate buffer saline) to remove the unbound molecules, and finally, the electrodes were stored at 4 °C until further use. The electrodes were electrochemically characterized for each layer. A response study of BSA/Spro/AuNPs/nf/ITO was performed using different concentrations of CoV2-Ab. H2O2 was used as a substrate to activate the HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO immunosensor. Therefore, before the response study, the concentration and volume of H2O2 (5%, 200 µL) were optimized. The electrochemical response of HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO was recorded for different concentrations of CoV2-Ab and a calibration curve was formed. To study the stability of the immunosensor, thirty immunosensors (HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO) were prepared and stored at 4 °C and their electrochemical responses were recorded at regular intervals for 30 days.
4. Characterization
UV-visible spectroscopy was performed using a Hitachi U3300 spectrophotometer for the optical characterization of the gold nanoparticles. A stand-alone potentiostat/galvanostat, an Auto-Lab workstation (MAC90135) procured from Metrohm and guided by NOVA 2.1 software, was used to perform differential pulse voltammetry for electrochemical characterization and response study of the fabricated HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO immunosensor. To perform the electrochemistry, a three-electrode electrochemical cell was utilized, wherein the fabricated immunosensor probe was used as the working electrode, the platinum electrode (Pt) as the counter electrode, and Ag/AgCl as the reference electrode. The testing was performed in a PBS solution with 5 mM ferro/ferricyanide solution [Fe (CN)6]3−/4−, and the changes in the current response were noted for the analysis.
5. Result and Discussion
5.1. Gold Nanoparticles Characterization
5.1.1. UV-visible Spectroscopy
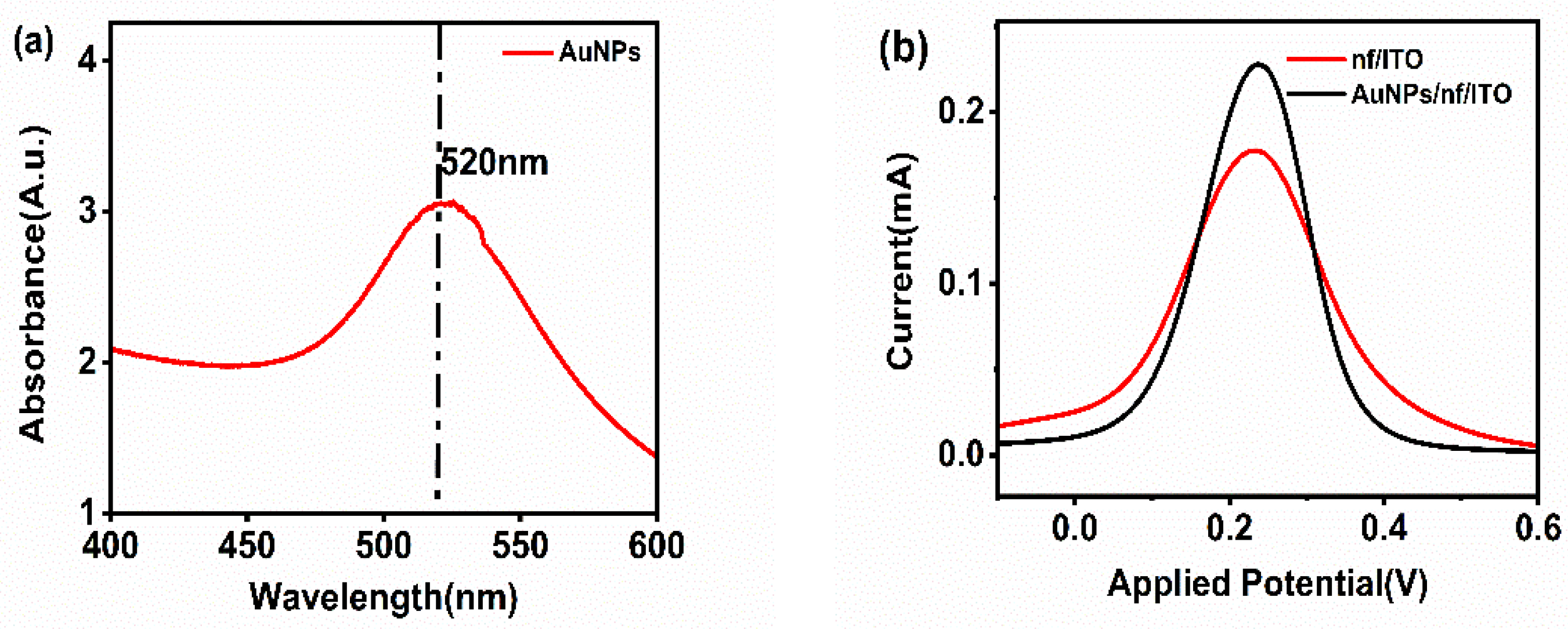
Figure 1a shows the characterization of gold nanoparticles using UV-visible spectroscopy. The UV-visible spectra of the synthesized gold nanoparticles display a peak at 520 nm, which is specific for spherical gold nanoparticles due to the localized surface plasmon resonance (LSPR) occurring in the conduction band when the light of a specific wavelength strikes the surface of these particles.
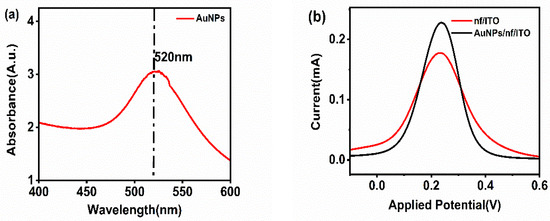
Figure 1.
(a) UV-visible spectroscopy of gold nanoparticles. (b) Electrochemical characterization of nf/ITO and AuNPs/nf/ITO.
5.1.2. Electrochemical Characterization of Gold Nanoparticles
For the confirmation of the activity of the gold nanoparticles, nf/ITO was spin-coated with a uniform layer of gold nanoparticles (AuNPs), and the electrochemical response was recorded. The anodic peak current of nf/ITO (0.165 mA) increased to 0.212 mA for the AuNPs/nf/ITO surface, indicating the presence of highly conductive gold nanoparticles on the electrode (Figure 1b).
5.2. Electrochemical Characterization of the Fabricated Immunosensor
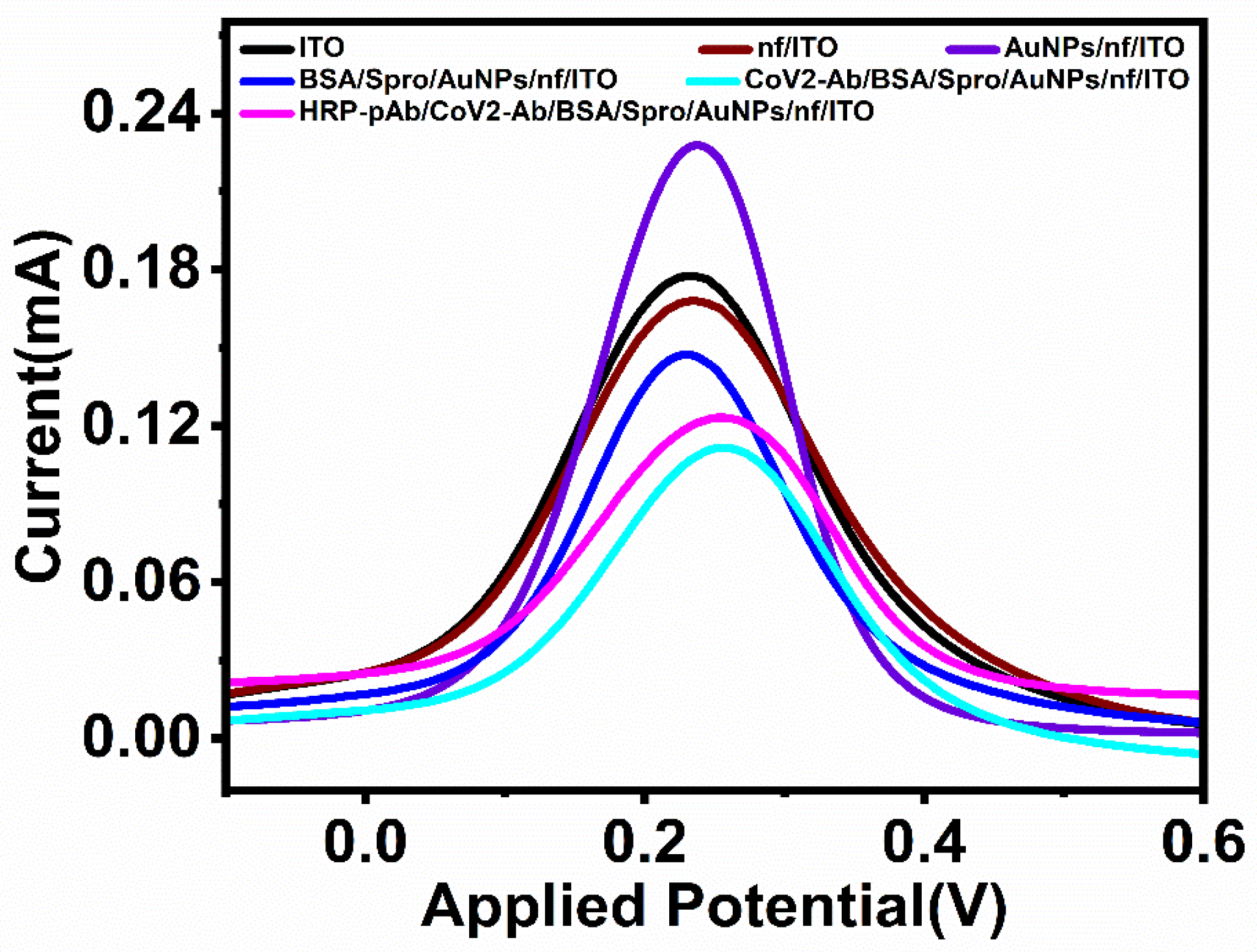
For each deposited layer, the electrode was electrochemically characterized using differential pulse voltammetry carried out at a voltage range of −0.3 V to 0.6 V, with a step potential of 0.005 V and a scan rate of 0.1 V/s in 5 mM ferro/ferricyanide solution [Fe (CN)6]3−/4− prepared in PBS. Due to the conductive nature of bare ITO, it provides a peak current of 0.18 mA. Upon the deposition of the biopolymeric layer on the electrode, the current decreases to 0.16 mA. To improve the overall conductivity and charge transfer, a layer of synthesized gold nanoparticles (AuNPs) was spin-coated onto the electrode to form AuNPs/nf/ITO, which exhibited an electrochemical response of 0.21 mA. AuNPs/nf/ITO was then considered as a base for the immobilization of the optimized concentration (600 ngmL−1) of the capture probe (Spro) for 60 min to form Spro/AuNPs/nf/ITO. Following the immobilization of Spro, the electrode was incubated with BSA (Bovine Serum Albumin) for 45 min to block the non-specific binding sites and form BSA/Spro/AuNPs/nf/ITO, which decreased the electrochemical response to 0.154 mA. The BSA/Spro/AuNPs/nf/ITO probe was used to detect the analyte, CoV2-Ab. To enhance the detection range and sensitivity of the electrode, HRP-pAb was used as a signal probe in the immunosensor HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO, which showed an increase in its electrochemical response (Figure 2).
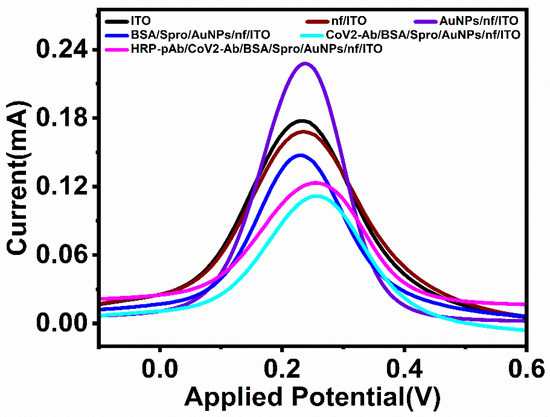
Figure 2.
Electrochemical characterization of the fabricated, HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO immunosensor.
5.3. Analytical Performance of the Immunosensor
5.3.1. Optimization of H2O2
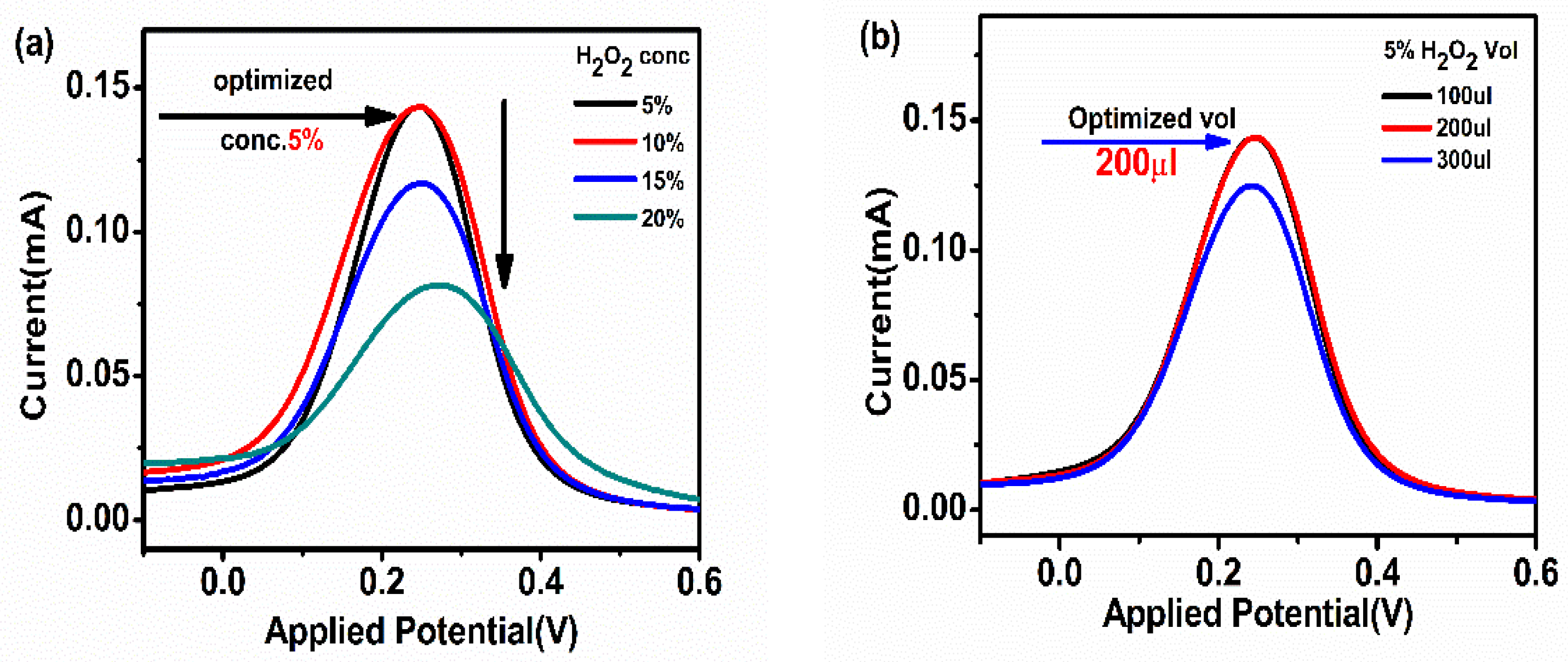
H2O2 was used as a substrate for the activation of HRP in HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO. To optimize the concentration and volume of H2O2, the immunosensor was electrochemically analyzed by spiking various concentrations (5–20%) and volumes (100–300 µL) of H2O2 to obtain a maximum response at 200 µL of 5% H2O2 concentration, as shown in Figure 3a,b.
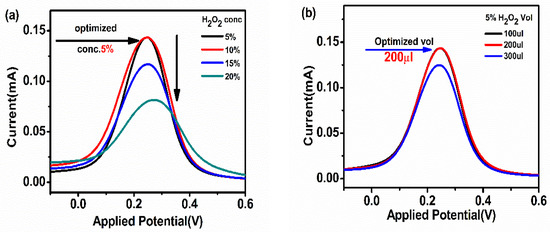
Figure 3.
(a) Concentration; (b) volume optimization of the substrate H2O2 used to activate HRP in HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO.
5.3.2. Response Study
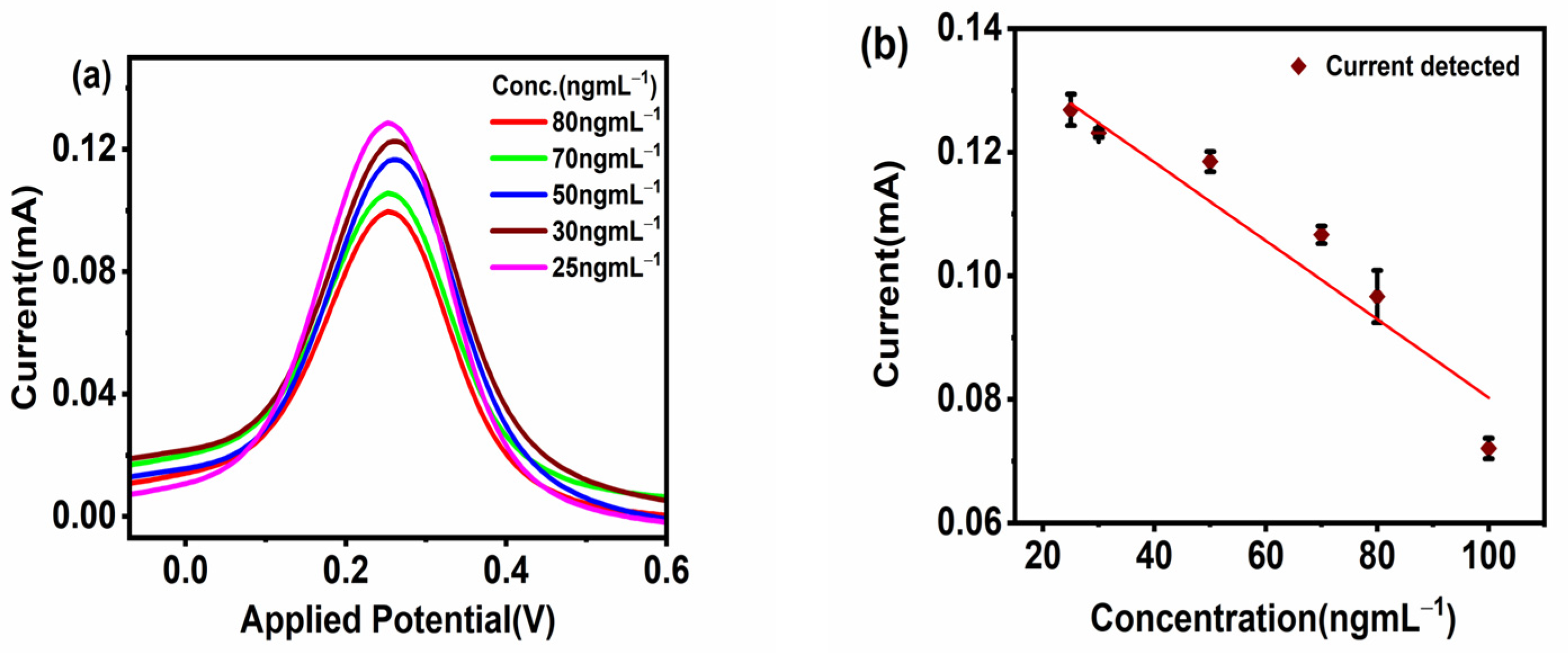
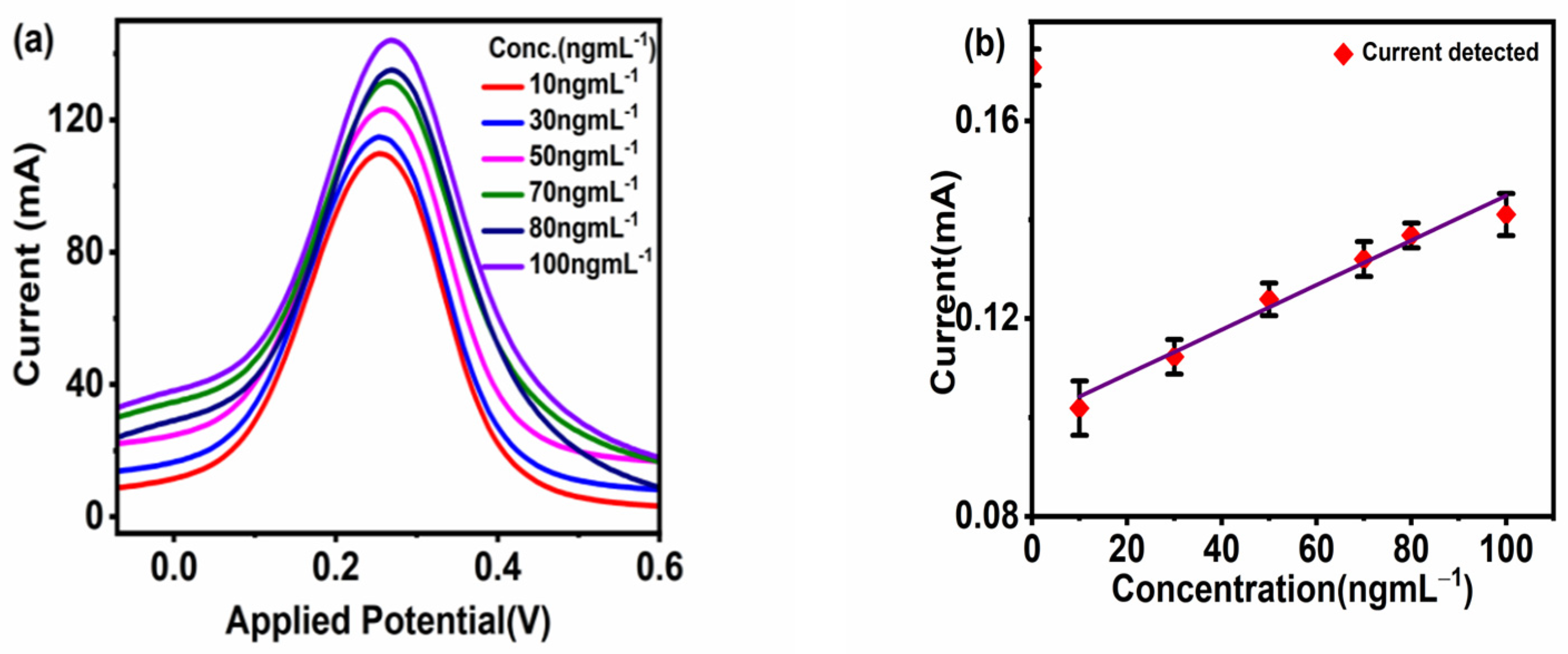
The sensor applicability was tested for the detection of different concentrations of CoV2-Ab. The electrochemical response for BSA/Spro/AuNPs/nf/ITO was recorded, and a decrease in current was recorded upon increasing the concentration of CoV2-Ab within a linear range of 25–80 ngmL−1, above which no further change was observed in the electrochemical response, as shown in Figure 4a,b. Furthermore, to enhance the range of detection and sensitivity, CoV2-Ab/BSA/Spro/AuNPs/nf/ITO was incubated with an electro-active probe-antibody conjugate (HRP-pAb). Initially, a blank reading was recorded for the BSA/Spro/AuNPs/nf/ITO probe with HRP-pAb, giving an electrochemical response of 0.17 mA. Following this, a varied concentration of CoV2-Ab was tested on HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO, which displayed an increase in the anodic peak current within a linear range of 10–100 ngmL−1, above which no significant changes were observed with a further increase in CoV2-Ab concentrations. A calibration curve was prepared for the anodic peak response as a function of concentration. The anodic peak current for the blank was higher for the HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO immunosensor, as in the case of blank analysis, the polyclonal antibody HRP-pAb, interacted directly with the capture probe Spro. HRP-pAb binds to a larger number of Spro, generating a high electrochemical response. However, during the analysis, the presence of an analyte (CoV2-Ab), which is a biomolecule, creates a hindrance in the charge transfer. Hence, a decrease in the conductivity of the immunosensor was observed when compared to the blank. The immunosensor displayed a detection range of 10–100 ngmL−1, with linearity R2 of 0.96, high sensitivity of 0.013 × 10−3 mAng−1mLmm−2,, a low limit of detection (LOD) of 3.59 ngmL−1, and quantification (LOQ) of 11.84 ngmL−1 (Figure 5a,b).
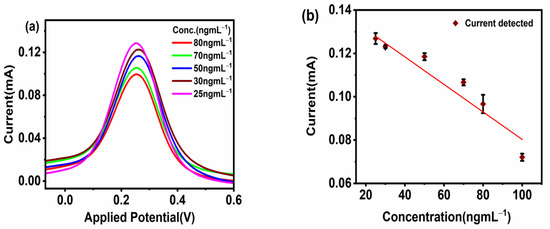
Figure 4.
Response studies. (a) Differential Pulse Voltammetry. (b) Calibration curve of the fabricated BSA/Spro/AuNPs/nf/ITO against CoV2-Ab. The red diamond symbol represents the current corresponding to the given concentration(x-axis) of CoV2-Ab, on interaction with fabrictaed BSA/Spro/AuNPs/nf/ITO immunosensor along with the black error bar indicating the standard deviation.
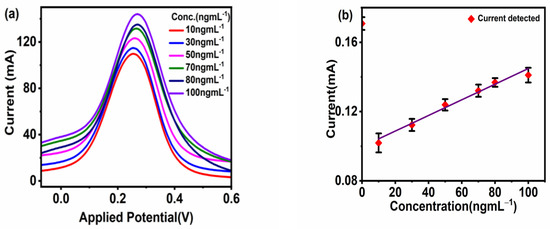
Figure 5.
Response studies. (a) Differential pulse voltammetry. (b) Calibration curve of the fabricated HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO immunosensor against CoV2-Ab. The red diamond symbol represents the current corresponding to the given concentration(x-axis) of CoV2-Ab, on interaction with fabrictaed HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO immunosensor along with the black error bar indicating the standard deviation.
5.4. Stability Study
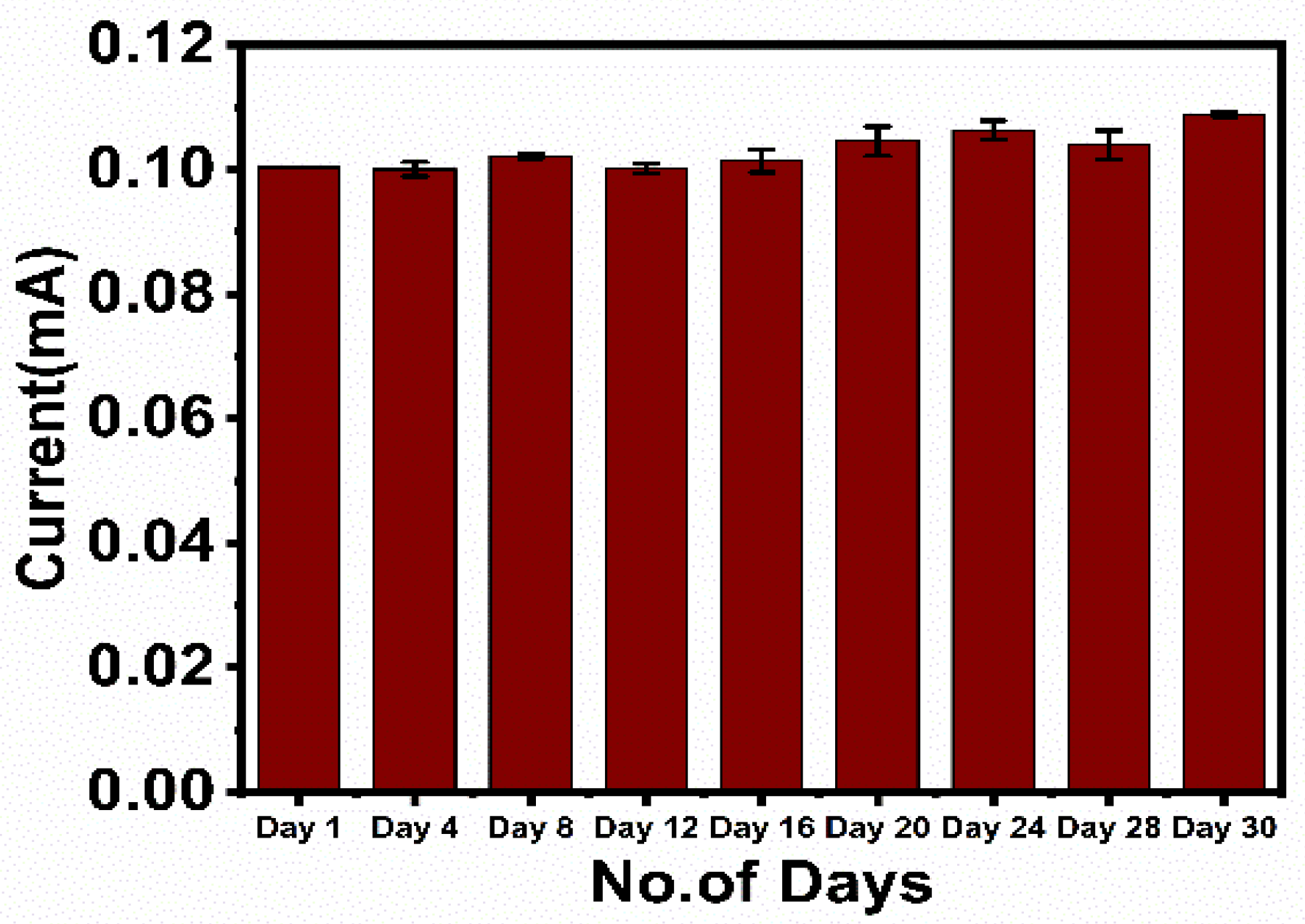
The electrochemical response of the fabricated immunosensor electrodes was recorded for 30 days at a regular interval of 4 days. The results indicated the electrodes were highly stable for 30 days, as only a 2–4% deviation of results was observed till the 20th day, which consecutively increased up to 10% till the 30th day due to the denaturation of the protein structure (Figure 6).
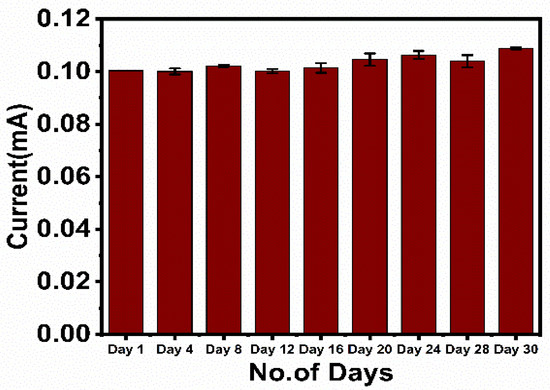
Figure 6.
Stability study of the fabricated immunosensor, HRP-pAb/CoV2-Ab/BSA/Spro/AuNPs/nf/ITO.
6. Conclusions
A nanostructure-decorated electrochemical immunosensor for the rapid quantification of the SARS-CoV-2 antibody was fabricated. The strategy employed for the detection of the viral antibody is the specific interaction of the SARS-CoV-2 spike receptor-binding protein to its antibody. For the fabrication of the immunosensor, the surface properties of the electrodes were enhanced using gold nanoparticles. Furthermore, in the presence of the conjugated secondary antibody, the detection of the antibody concentration ranged from 10–100 ngmL−1 with high linearity (R2 = 0.96) and sensitivity of 0.013 × 10−3 mAng−1mLmm−2 with a limit of detection (LOD = 3.59 ngmL−1), quantification (LOQ = 11.84 ngmL−1), and stability (30 days). The fabricated immunosensor has a high potential for miniaturization and can be configured with a mobile phone-based interface for on-the-spot, rapid, and quantitative detection.
Author Contributions
Conceptualization, T.B. and C.; methodology, T.B. and C.; software, C. and A.G.; validation, T.B., C. and A.G.; formal analysis: T.B., C. and A.G.; investigation, C. and A.G.; resources, T.B.; data curation: A.G.; writing—original draft preparation: C. and A.G.; writing—review and editing, T.B. and C.; visualization: C.; supervision, T.B.; project administration, T.B.; funding acquisition, T.B. All authors have read and agreed to the published version of the manuscript.
Funding
The project was funded by BRNS. Project reference no. 54/14/12/2020-BRNS/37089.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank BRNS for funding this research and Amity University Uttar Pradesh for the laboratory support.
Conflicts of Interest
There are no conflicts of interest among the authors.
References
- He, F.; Deng, Y.; Li, W. Coronavirus disease 2019: What we know? J. Med. Virol. 2020, 92, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Ma, Y.; Zhai, M.; Xia, L.; Liu, J.; Yu, J.; Duan, W. The role of SARS-CoV-2 target ACE2 in cardiovascular diseases. J. Cell. Mol. Med. 2021, 25, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Critical Care 2020, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Leech, D. Affinity Biosensors. 1994. Available online: https://pubs.rsc.org/en/content/articlelanding/1994/cs/cs9942300205/unauth (accessed on 21 May 2023).
- Charles, J.; Janeway, A.; Travers, P.; Walport, M.; Shlomchik, M.J. The Interaction of the Antibody Molecule with Specific Antigen. 2001. Available online: https://www.ncbi.nlm.nih.gov/books/NBK27160/ (accessed on 5 April 2023).
- Payandehpeyman, J.; Parvini, N.; Moradi, K.; Hashemian, N. Detection of SARS-CoV-2 Using Antibody−Antigen Interactions with Graphene-Based Nanomechanical Resonator Sensors. ACS Appl. Nano Mater. 2021, 2021, 6189–6200. [Google Scholar] [CrossRef]
- Xu, L.; Li, D.; Ramadan, S.; Li, Y.; Klein, N. Facile biosensors for rapid detection of COVID-19. Biosens. Bioelectron. 2020, 170, 112673. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Michalek, P.; Ilieva, L.; Adam, V.; Zitka, O. COVID-19: A challenge for electrochemical biosensors. TrAC Trends Anal. Chem. 2021, 136, 116192. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Navale, G.R.; Dharne, M.S. Biosensors: Frontiers in rapid detection of COVID-19. 3 Biotech 2020, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- ZRahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. Electrochemical immunosensor with Cu2O nanocube coating for detection of SARS-CoV-2 spike protein. Microchimica Acta 2021, 188, 1–9. [Google Scholar] [CrossRef]
- Sundeep, D.; Varadharaj, E.K.; Umadevi, K.; Jhansi, R. Role of Nanomaterials in Screenprinted Electrochemical Biosensors for Detection of COVID-19 and for Post-Covid Syndromes. Ecs Adv. 2023, 2, 016502. [Google Scholar] [CrossRef]
- Vadlamani, B.S.; Uppal, T.; Verma, S.C.; Misra, M. Functionalized TiO2 nanotube-based electrochemical biosensor for rapid detection of SARS-CoV-2. Sensors 2020, 20, 5871. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajjadi, M.; Soufi, G.J.; Iravani, S.; Varma, R.S. Nanomaterials and nanotechnology-associated in-novations against viral infections with a focus on coronaviruses. Nanomaterials 2020, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Beduk, T.; Beduk, D.; de Oliveira Filho, J.I.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; Turhan, K.; Salama, K.N.; et al. Rapid Point-of-Care COVID-19 Diagnosis with a Gold-Nanoarchitecture-Assisted Laser-Scribed Graphene Biosensor. Anal. Chem. 2021, 93, 8585–8594. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, S.; Gopinath, S.C.; Ismail, Z.H.; Arshad, M.M.; Poopalan, P. Aptasensing nucleocapsid protein on nanodiamond assembled gold interdigitated electrodes for impedimetric SARS-CoV-2 infectious disease assessment. Biosens. Bioelectron. 2022, 197, 113735. [Google Scholar] [CrossRef] [PubMed]
- Funari, R.; Chu, K.Y.; Shen, A.Q. Detection of antibodies against SARS-CoV-2 spike protein by gold nanospikes in an opto-microfluidic chip. Biosens. Bioelectron. 2020, 169, 112578. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, S.; Razmi, Z.; Ghaedi, M.; Asfaram, A.; Javadian, H. Ultrasound-accelerated synthesis of gold nanoparticles modified choline chloride functionalized graphene oxide as a novel sensitive bioelectrochemical sensor: Optimized meloxicam detection using CCD-RSM design and application for human plasma sample. Ultrason. Sonochem. 2018, 42, 776–786. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarlou, M.; İlk, S.; Hammamchi, H.; Kıraç, F.; Okan, M.; Güven, O.; Barsbay, M. Green and Facile Synthesis of Pul-lulan-Stabilized Silver and Gold Nanoparticles for the Inhibition of Quorum Sensing. ACS Appl. Bio Mater. 2022, 5, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.W.; Skeete, Z.R.; Crew, E.R.; Shan, S.; Luo, J.; Zhong, C.J. Synthesis of Gold Nanoparticles. Compr. Anal. Chem. 2014, 66, 37–79. [Google Scholar] [CrossRef]
- Dong, J.; Carpinone, P.L.; Pyrgiotakis, G.; Demokritou, P.; Moudgil, B.M. Synthesis of precision gold nanoparticles using Turkevich method. KONA Powder Part. J. 2020, 37, 224–232. [Google Scholar] [CrossRef]
- Shi, L.; Buhler, E.; Boué, F.; Carn, F. How does the size of gold nanoparticles depend on citrate to gold ratio in Turkevich synthesis? Final answer to a debated question. J. Colloid Interface Sci. 2017, 492, 191–198. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).