Antibody Immobilization in ZnO-Thin Film Transistors for Low-Cost Biosensors Applications †
Abstract
:1. Introduction
2. Materials and Methods
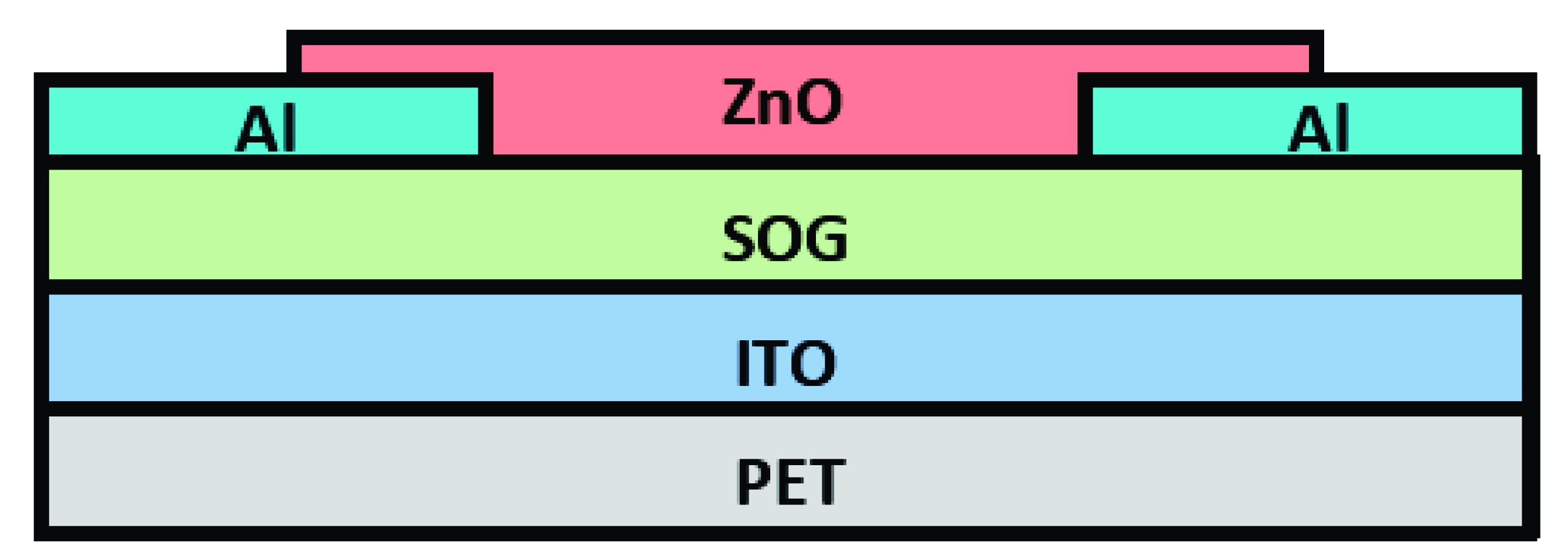
2.1. Fabrication and Characterization of ZnO Thin Films Transistors
2.2. ZnO Thin Film Transistors Functionalization
2.3. Bacterial Detection
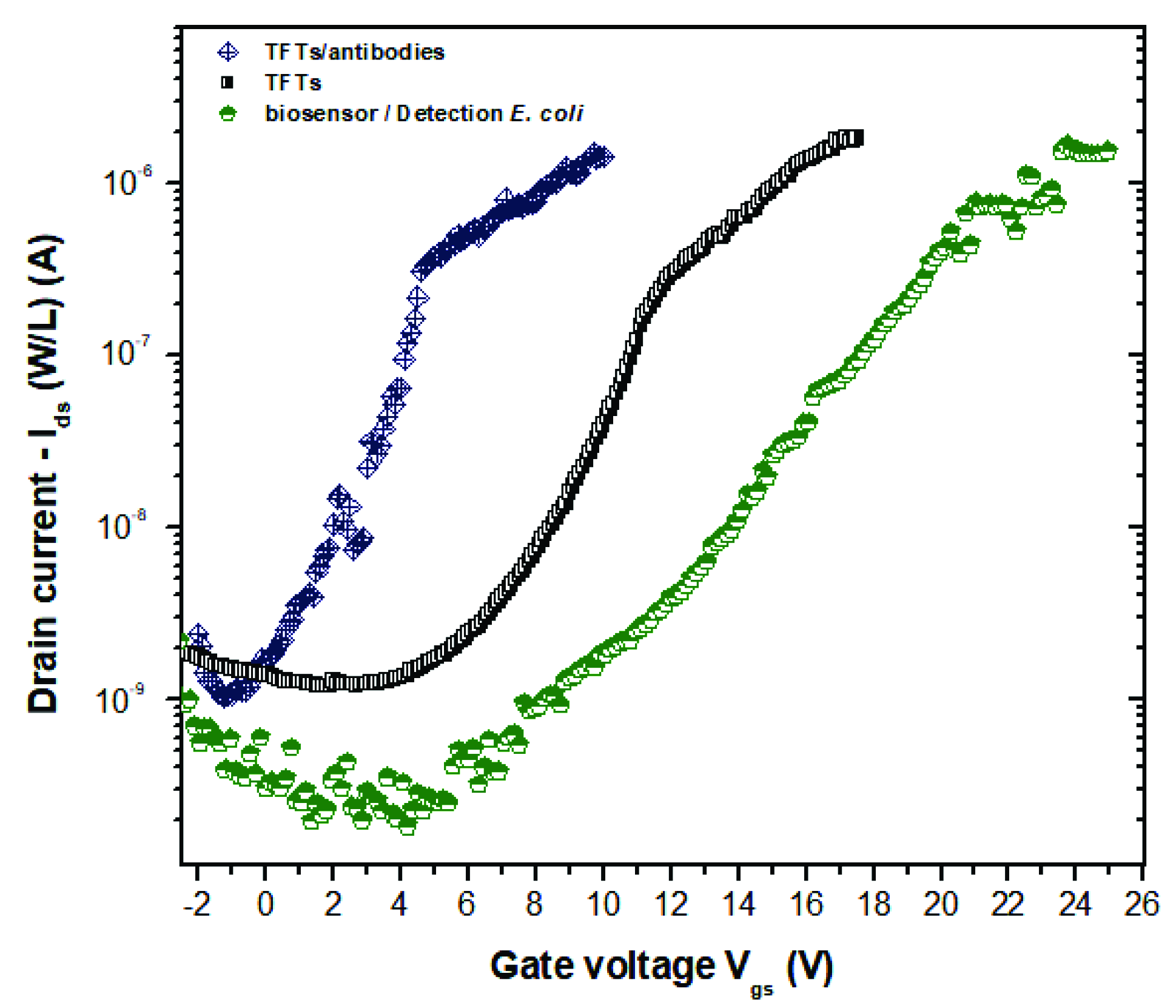
3. Results and Discussion
Antibody Immobilization on ZnO TFTs
4. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velusamy, V.; Arshak, K.; Korostynska, O.; Oliwa, K.; Adley, C. An overview of foodborne pathogen detection: In the perspective of biosensors. Biotechnol. Adv. 2010, 28, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Law, J.W.-F.; Ab Mutalib, N.-S.; Chan, K.-G.; Lee, L.-H. Rapid methods for the detection of foodborne bacterial pathogens: Principles, applications, advantages and limitations. Front. Microbiol. 2015, 5, 770. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Vigneshvar, S.; Sudhakumari, C.C.; Senthilkumaran, B.; Prakash, H. Recent Advances in Biosensor Technology for Potential Applications—An Overview. Front. Bioeng. Biotechnol. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.; Mateo, C.; Guisán, J.; Fernández-Lafuente, R. Preparation of inert magnetic nano-particles for the directed immobilization of antibodies. Biosens. Bioelectron. 2005, 20, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Marco, M.-P.; De Alda, M.J.L.; Barceló, D. Biosensors for environmental applications: Future development trends. Pure Appl. Chem. 2004, 76, 723–752. [Google Scholar] [CrossRef]
- Belkin, S. Microbial whole-cell sensing systems of environmental pollutants. Curr. Opin. Microbiol. 2003, 6, 206–212. [Google Scholar] [CrossRef]
- Salinas, R.A.; Domínguez, M.A.; Orduña, A. Antibody Immobilization in Zinc Oxide Thin Films as an Easy-Handle Strategy for Escherichia coli Detection. ACS Omega 2020, 5, 20473–20480. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, M.A.; Flores, F.; Luna, A.; Martinez, J.; Luna-Lopez, J.A.; Alcantara, S.; Rosales, P.; Reyes, C.; Orduña, A. Impact of active layer thickness in thin-film transistors based on Zinc Oxide by ultrasonic spray pyrolysis. Solid-State Electron. 2015, 109, 33–36. [Google Scholar] [CrossRef]
- Xu, M.; Wang, R.; Li, Y. Electrochemical biosensors for rapid detection of Escherichia coli O157:H7. Talanta 2017, 162, 511–522. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez, R.A.S.; Hinostroza, O.O.; Díaz, A.O.; Jiménez, M.Á.D. Antibody Immobilization in ZnO-Thin Film Transistors for Low-Cost Biosensors Applications. Eng. Proc. 2021, 4, 23. https://doi.org/10.3390/Micromachines2021-09587
Domínguez RAS, Hinostroza OO, Díaz AO, Jiménez MÁD. Antibody Immobilization in ZnO-Thin Film Transistors for Low-Cost Biosensors Applications. Engineering Proceedings. 2021; 4(1):23. https://doi.org/10.3390/Micromachines2021-09587
Chicago/Turabian StyleDomínguez, Rafael Antonio Salinas, Ovier Obregón Hinostroza, Abdú Orduña Díaz, and Miguel Ángel Domínguez Jiménez. 2021. "Antibody Immobilization in ZnO-Thin Film Transistors for Low-Cost Biosensors Applications" Engineering Proceedings 4, no. 1: 23. https://doi.org/10.3390/Micromachines2021-09587





